Abstract
Toxicity profiles of two soils (a brownfield in Legazpi and an abandoned iron mine in Zugaztieta; Basque Country) contaminated with several metals (As, Zn, Pb and Cu in Legazpi; Zn, Pb, Cd and Cu in Zugaztieta) and petroleum hydrocarbons (in Legazpi) were determined using a multi-endpoint bioassay approach. Investigated soils exceeded screening values (SVs) of regulatory policies in force (Basque Country; Europe). Acute and chronic toxicity bioassays were conducted with a selected set of test species (Vibrio fischeri, Dictyostelium discoideum, Lactuca sativa, Raphanus sativus and Eisenia fetida) in combination with chemical analysis of soils and elutriates, as well as with bioaccumulation studies in earthworms. The sensitivity of the test species and the toxicity endpoints varied depending on the soil. It was concluded that whilst Zugaztieta soil showed very little or no toxicity, Legazpi soil was toxic according to almost all the toxicity tests (solid phase Microtox®, D. discoideum inhibition of fruiting body formation and developmental cycle solid phase assays, lettuce seed germination and root elongation test, earthworm acute toxicity and reproduction tests, D. discoideum cell viability and replication elutriate assays). Thus, albeit both soils had similar SVs, their ecotoxicological risk, and therefore the need for intervening, was different for each soil as unveiled after toxicity profiling based on multiple endpoint bioassays. Such a toxicity profiling approach is suitable to be applied for scenario-targeted soil risk assessment in those cases where applicable national/regional soil legislation based on SVs demands further toxicity assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Europe, 3.5 million sites were estimated to be potentially contaminated in 2006 (EC 2006). These soils are potential reservoirs of pollutants that can induce ecosystem perturbation and adverse health effects on ecosystems and humans (Gleyzes et al. 2002; Asensio et al. 2013). Thus, the environmental risk of contaminated soils is a matter of concern for stakeholders and environmental managers, who currently require that soil contamination does not exceed screening values (SVs; Carlon 2007). As a general rule, SVs can be ranked into three types according to their role in decision-making (Carlon 2007): (1) “A” values represent a limit above which remediation interventions will be required; (2) “B” values indicate the need for further investigations; and (3) “C” values are references in support to the application of the site-specific risk assessment. Specifically, the Law for Prevention and Correction of Soil Pollution of the Basque Autonomous Community established SVs (IVA = Indicative Value for Assessment) on the basis of literature data derived from toxicity testing conducted with single pollutants (EJ/GV 2007; Ihobe 1998a, b; Urzelai et al. 2000). IVA-A refers to the background concentrations found in soils with little anthropogenic influence. Risk is acceptable when the concentrations of individual pollutants are below IVA-B values, and unacceptable when they exceed IVA-C values. If the concentration of one pollutant is between IVA-B and IVA-C values, then further toxicity assessment must be conducted before a decision for intervention is taken.
Though SVs are based on total concentrations of individual pollutants in soil, these concentrations do not necessarily reflect toxicity to biota (Wang et al. 2004; Harmsen et al. 2005). Environmental risk assessment in soils needs to be holistically conceived on the basis of the combination of chemical and toxicological approaches. First, toxicity largely depends on bioavailability. Second, both bioavailability and toxicity are influenced by the soil physicochemical properties and the chemical form of the pollutants (Bierkens et al. 1998; Beeby 2001). Pollutant toxicity and mobility largely depend on their specific chemical forms and on their binding state. For instance, key contaminants in soil such as metals can occur precipitated with primary or secondary minerals or complexed by organic ligands. Likewise, changes in environmental conditions such as acidity, redox potential or levels of available organic ligands can modify metal speciation and provoke mobilisation/retention of metals to render them more/less available (Giller et al. 1998; Gleyzes et al. 2002; Wang et al. 2004). Chemical extraction, which can be sequential or single stepped, allows the identification of metal pools, which differ in mobility, and thus, in potential behaviour and susceptibility to interact with biological receptors (Wang et al. 2004; Diatta et al. 2011). Third, though mixture effects are generally not considered in the derivation of SVs, they are generally accounted for in site-specific risk assessments (Carlon 2007). Last but not least, different species may be subject to different exposure pathways and may exhibit different sensitivity to toxicants. Thus, in order to assess environmentally relevant soil toxicity, a suite of organisms from different taxonomical groups and ecological levels is recommended, together with the consideration of diverse exposure routes and toxicity endpoints (Bierkens et al. 1998; Eisentraeger et al. 2004).
Within this context, toxicity profiling of contaminated soils can be achieved by determining soil physicochemical properties, total and available concentrations of pollutants, pollutant bioaccumulation and multiple endpoint toxic responses (including lethal and a variety of sublethal effects) for a suite of target organisms (Bierkens et al. 1998; Critto et al. 2007; Hamers et al. 2013). A toxicity profile is a toxicological “fingerprint” of a sample, ranging from a pure compound to a complex mixture, obtained by testing the sample or its extract for its activity using a battery of biological endpoints (Hamers et al. 2013).
This investigation aimed to determine the toxicity profile of two soils ascribed to the same rank of environmental risk according to SVs in force (Ihobe 1998b) using multiple endpoint bioassays. For this purpose, a suite of acute and chronic bioassays with bacteria (Vibrio fischeri), protists (Dictyostelium discoideum), plants (Lactuca sativa, Raphanus sativus) and invertebrates (Eisenia fetida) was applied to assess toxicity of the solid phase and elutriates of the two target soils. These soils corresponded to a brownfield and an abandoned iron mine that potentially exceed acceptable risks to the environment because the concentrations of several metals (As, Zn, Pb and Cu in the brownfield; Zn, Pb, Cd and Cu in the abandoned iron mine) and mineral oil (in the brownfield) were between their respective IVA-B and IVA-C values, and therefore, required further toxicity assessment (Ihobe 1998b; Asensio et al. 2013; Gascón et al. 2007). The investigation was restricted to a particular case study; however, it was conceived as a pilot exercise, so that an equivalent approach might be applied in those cases in which applicable national/regional soil legislation is based on SVs.
Material and methods
Soil collection
Control soils
OECD artificial soil (70 % quartz sand, 20 % kaolinite clay and 10 % sphagnum moss; pH 6.0 ± 0.5; OECD 1984) and Italian rural soil (IRS; texture = loam, OC = 1.4 %, CEC = 14 meq/100 g soil dry wt, pH = 7.9; Rapp Prova Analisi Terreno N°76/07/T, 03/07/2007, Cadir Lab SRL, UNIPM, Alessandria) were used as control soils to produce dilutions of the reference and test soils in different experimental sets as described by OECD (1984, 2004) and by INIA et al. (2007).
Reference and test soils
Soils were collected from three localities in the Basque Country (Spain): (a) a rural pristine site at Delika (42°, 57′ N, 2° 59′ W) that was used as reference site (DE soil); (b) a former industrial area in Legazpi (43°02′ N, 2°20′ W) (LG soil); and (c) an abandoned iron mine in Zugaztieta (43°15′ N, 3°04′ W) (ZU soil). Previously published reports revealed that LG soil presented metal concentrations (26.4 mg As/kg soil dry wt, 441 mg Zn/kg soil dry wt, 208 mg Pb/kg soil dry wt; and 198 mg Cu/kg soil dry wt; Gascón et al. 2007) between IVA-B and IVA-C values (Ihobe 1998b) and high levels of total petroleum hydrocarbons (14,479 mg total petroleum hydrocarbons (TPH)/kg soil dry wt; Gascón et al. 2007) according to INIA et al. (2007). ZU soil presented moderate levels of metal pollution (270 mg Zn/kg soil dry wt, 51 mg Pb/kg soil dry wt, 0.8 mg Cd/kg soil dry wt and 51 mg Cu/kg soil dry wt; Asensio et al. 2013), which were also between IVA-B and IVA-C values (Ihobe 1998b). Soil samples were collected from the top layer (0–15 cm) and sieved (6.3 mm mesh) immediately in the field to remove larger particles and organisms.
Soil characterisation
Upon arrival at the laboratory, DE, LG and ZU soils were again sieved through a 2-mm mesh, characterised and kept at 4 °C until use. Briefly, texture was determined by mechanical analysis of air-dried soil (Brown 2003). Humidity and organic content as loss on ignition (LOI) were measured by gravimetric method according to ISO 11465 and NEN 5754, respectively (ISO 1993; NEN 1994). Soil pH (ISO 10390; ISO 2005) and electrical conductivity (EC) were determined in a 1:5 soil:H2O slurry by potentiometric titration. Soil respiration (CO2 evolution) was measured by a NaOH trap (Alef and Nannipieri 1995). Briefly, 10–20 g wet sieved soil were placed in a jar together with 3 mL 1 M NaOH solution in a small glass bottle that hanged from the jar lid. The jar was closed airtight, and incubated for 3 days at 25 °C. Upon incubation, NaOH was determined by HCl titration.
Chemical analysis
Metals in soils
Total metals
Total concentration of metals were measured in soils, as well as in their experimental dilutions used in the adapted OECD 207 assay. Air-dried soil samples were sieved (<0.16 mm mesh), and digested with aqua regia in Teflon vessels in microwave oven. Samples were then taken to 50 mL, filtered (0.45 μm Teflon filter) and analysed by ICP/AES (Perkin Elmer Optima 2100 DV spectrophotometer, Shelton, CT, USA) for As, Zn, Pb, Cd, Fe, Cr, Cu, Al, Mo, Sb, Co, Ba, Mn, Ni and Ca. Quality control was maintained by including certified reference material (7004, Loam with elevated analyte levels, Czech Metrology Institute, Praha, Czech Republic) and by repetitive measurements of standard curves (USEPA 3052, USEPA 6010C; USEPA 1996a; USEPA 2007).
Water-soluble fraction of metals
Soil elutriates were obtained following the German standard method DIN 38414-S4 (DIN 1984). EC, pH and the concentrations of Cd, Pb and Zn in the water-soluble fraction were determined as described above.
Exchangeable fraction of metals.
Unbuffered 0.1 M CaCl2 and 1 M NH4NO3 were used to extract the exchangeable fraction of soil contaminants by a single step (Gleyzes et al. 2002). First, 50 mL 1 M NH4NO3 were added to 20 g soil dry wt, constantly shaken at 20 rpm at room temperature for 2 h, decanted and filtered through a Teflon filter (0.45 μm) according to the German standard DIN 19730. Second, after adding 48 mL 0.1 M CaCl2 to 6 g soil, soils were thoroughly mixed at room temperature for 1 h and the supernatant was removed and filtered through a Teflon filter (0.45 μm). The concentrations of Cd, Pb and Zn in the exchangeable fraction were determined by ICP/AES as described above.
Sequential extraction of metals
In order to study metal speciation in DE, LG and ZU soils, the modified “Bureau Commune de Reference” (BCR) sequential extraction protocol was applied (Rauret et al. 1999).
Metals in earthworms
After 24 h of depuration, earthworms (five per treatment; see “Toxicity testing”) were rinsed in distilled water, frozen and freeze-dried. Digestion was carried out as described above. Earthworms exposed to LG soil were digested individually, and those exposed to ZU soil were digested in pools of five animals. Following digestion, samples were adjusted to 25 mL, filtered (0.45-μm Teflon filter) and metals (As, Zn, Pb, Ni and Ca for both, and Cd, Fe, Cr, Cu, Al, Mo, Sb, Co, Ba and Mn in earthworms exposed to ZU soil) were measured as described above.
Total petroleum hydrocarbons in soil
A portion of air-dried soil (DE, LG and ZU and dilutions of LG soil; see below “Earthworm Acute toxicity test (OECD 207)”) was sieved (<1 mm mesh). TPHs were extracted by sonication followed by centrifugation of 5–10 g soil dry wt in 25 mL dichloromethane according to USEPA3550B (USEPA 1996b). The extract was cleaned up with Florisil and brought up to 1 mL in hexane before measuring the sum of the fraction C10-C40 by FID-GC (Agilent Technologies SYS-GC-6890, Santa Clara, CA, USA) according to NEN 5733 (NEN 1997).
Total petroleum hydrocarbons in earthworms
After 24 h of depuration, five earthworms were pooled per exposure group, rinsed in distilled water, frozen and freeze-dried. Fat and TPH content were determined after Soxhlet extraction with dichloromethane (SM 5520D; SM 2001). Total fat content was determined by gravimetry. The recovered extract was cleaned up with Florisil, brought to 0.5 mL in hexane and measured as described above according to the NEN 5733 (NEN 1997). TPH concentrations were calculated relative to the total fat content.
Toxicity testing
The solid phase and the water-soluble fractions (DIN 38414) of DE, LG and ZU soils toxicity tests were used for toxicity testing with standard and novel biomarker-based tests. Wherever possible, EC50 values were calculated (see “Data treatment and statistical analysis”).
Test organisms
D. discoideum and E. fetida were obtained from our laboratory stocks. D. discoideum (AX2 strain) was grown in axenic medium (14.3 g/L peptone, 7.15 g/L yeast extract, 18 g/L maltose, 0.419 g/L Na2HPO4, 0.486 g/L KH2PO4, pH 6.6) with 10 μg/mL tetracycline. Cells were grown and developed in an orbital incubator (Sanyo Gallenkamp Plc., Loughborough, UK) at 21 °C/180 rpm. Cultures were maintained at logarithmic phase of growth, keeping the cell density at about 2–4 × 106 cell/mL. Healthy adult clitellated E. fetida of similar size (0.3–0.6 g fresh weight) were selected from a stock population reared under laboratory conditions (kept in tanks at 21 °C and 12:12 light/dark cycle with horse manure as food source ad libitum). Pesticide-free (Greene et al. 1989) lettuce and radish seeds were obtained from a local dealer.
Microtox®
Toxicity to V. fischeri was determined for water-soluble fractions (DIN 38414) and for the solid phase of DE, LG and ZU as well as for the experimental soil dilutions of LG and ZU soils used in the adapted OECD 207 assay. Microtox® was conducted according to the manufacturer’s standard procedures (Azur 1995a, b). IC50s and associated values for the 95 % confidence limits were normalised for moisture content and values were shown as mg/L (Environment 2002).
D. discoideum
D. discoideum inhibition of fruiting body formation (IFBF) solid phase assay
DE, LG and ZU soils were mixed with IRS to obtain a series of soil dilutions (0, 10, 25, 50, 75 and 100 %). Test soils (1 g) were placed into Petri dishes (3.5 cm ∅) and D. discoideum cells (2.7 × 107) were transferred. For this purpose, the needed volume of cell culture was retrieved from the stock culture and washed following Fey’s protocol (Rodríguez-Ruiz et al. 2013). The number of fruiting bodies was counted on the micrographs and used to calculate the percentage inhibition of fruiting body formation (IFBF; Balbo and Bozzaro 2008).
D. discoideum developmental cycle (DDDC) solid phase assay
DE, LG and ZU soils (2.5 g) were placed into Petri dishes (5.5 cm ∅) and uniformly mixed with distilled water to saturation (100 % water holding capacity—WHC) and air-dried. Then, 6.7 × 107 cells/Petri dish (resuspended in 80 % of soil WHC PAS/Petri dish) were uniformly spread over the soil surface following a spiral pattern (n = 2). Petri dishes were covered and kept in a humid dark chamber at 21 °C. After 24 h, at least 12 micrographs (190 mm2) were obtained per test soil (6 optical fields × 2 Petri dishes) using a stereo microscope at × 7.1 magnification. Aggregates (AG), slugs (migrating formsMF), culminants (CC) and fruiting bodies (FB) were counted. The percentage of multicellular units (MU%) (MU = AG + MF + CC + FB) and the fruiting body size factor (FBSF = r 3) were calculated. For each micrograph, the aggregation arrest index (AAI = AG/MU), the migration arrest index (MAI = MF/(MF + CC + FB + 1)), and the culmination arrest index (CAI = CC/(CC + FB + 1)) were calculated in order to evaluate the progress of the developmental cycle of D. discoideum through critical checkpoints (Rodríguez-Ruiz et al. 2013).
D. discoideum elutriate toxicity
Cells, which are incubated in orbital shaker at 21 °C/180 rpm, were taken during their logarithmic growth phase (2–4 × 106 cell/mL), washed with PAS, centrifuged (500 × g; 4 °C; 5 min) and diluted (in 15 mL Falcon tubes) to 0.75 × 106 cell/mL in exposure medium (DE, LG and ZU soil elutriates) together with 25 % AX2 medium with 10 μg/mL tetracycline. Cell viability, lysosomal membrane stability, endocytosis rate and cell replication rate were measured (Dondero et al. 2006; Sforzini et al. 2008).
Lettuce seed germination and root elongation test
Solid phase and elutriate effect on seed germination and root elongation was measured. Open-air-dried LG and ZU soils mixed with OECD artificial soil to obtain the same dilutions than in the above described OECD 207 assay (0, 0.3, 0.6, 1.25, 2.5, 25, 50 and 100 %; 6 g) were placed in 90-mm ∅ Petri dishes and covered with filter paper (n = 4). Distilled H2O (7 mL) was added to the soil and 15 lettuce seeds were spread on the filter. Besides, elutriate (2 mL) of DE, LG and ZU soils was added on filter paper placed on a 90-mm ∅ Petri dish (n = 4). Lettuce (L. sativa) (n = 15) or radish (R. sativus) seeds (n = 10) were spread on the filter. Procedure blank (distilled water processed like the elutriates) was used as control. Petri dishes were kept in darkness at 21 °C, and after 72 h, the number of germinated seeds was counted and the root length measured. Seed germination (%) was estimated relative to the initial number of seeds in each treatment. Root elongation was evaluated as the root length to the nearest mm and expressed as average mm per seed (Linder et al. 1989). The percentages of relative seed germination (RSG) and relative root growth (RRG) were determined to calculate the Germination Index (GI), expressed as GI = [RSG × RRG]/100 (Hoekstra et al. 2002).
Earthworm
Earthworm Acute toxicity test (OECD 207)
LG and ZU soils were homogenously mixed with OECD artificial soil to obtain eight dilutions of soil (0, 0.3, 0.6, 1.25, 2.5, 25, 50, 100 %) recommended by the Spanish methodological guidelines to calculate L(E)C50 values (OECD 1984; INIA et al, 2007). Undiluted DE soil served as a reference soil to compare to the effects of non-diluted LG, ZU and OECD soils. To each 1-L glass container, 400 g soil (dry wt) was added, moistened to 60 % WHC and then let equilibrate for 24 h before initiating the assay (n = 4). On days 7 and 14 external pathologies, behavioural changes (phototropism, atypical mobility events), loss of weight and mortality were recorded. Humidity, OM, EC, pH, metals and TPH in soils, and total metal and TPH tissue concentrations in earthworms were measured, according to the procedures described above.
Earthworm reproduction test (ISO 11268-2)
This test was performed in accordance with guideline ISO 11268-2 (ISO, 2012); an updated revision of the OECD test guideline 222 recommended by the Spanish methodological guidelines (INIA et al. 2007). LG soils were homogenously mixed with OECD artificial soil (0 %, 0.3 %, 0.6 %, 1.25 %, 2.5 %, 10 %, 30 % and 60 %), and EC50 values for mortality and juvenile number were calculated using Probit analysis. DE soil was used as reference soil. Soil (500 g soil dry wt) was added to each container, moistened to 60 % WHC and then equilibrated for 24 h before initiating the assay (n = 4). On day 28, adult individuals were removed and changes in weight and mortality were recorded (OECD 2004). On day 56, juveniles were counted. During the assay, the temperature was maintained at 22 ± 2 °C under natural summer photoperiod (July–August).
Data treatment and statistical analysis
Homogeneity of variances (Levene’s test) as well as normality of data (Kolmogorov-Smirnov’s test) were tested before statistical analysis. Normal datasets were subject to either Student’s t test or one-way ANOVA followed by Duncan’s test or to two-way ANOVA. Non-parametric Mann-Whitney U, Kruskal-Wallis followed by Mann-Whitney U or Scheirer Ray Hare’s tests were applied to non-normal data sets (e.g. heterogeneous variance). In all cases, statistically significant differences were established at p < 0.05. Statistical tests were performed using SigmaStat v2.03. The median effective (EC50) and lethal (LC50) concentrations were calculated according to the Probit method (SPSS Statistic v17.0). No observed effect concentration (NOEC) and lowest observed effect concentration (LOEC) were determined by comparison with the control experimental set through one-way ANOVA followed by Dunnett’s test as recommended by OECD (OECD 2004), using SigmaStat v2.03 (Systat Software, San Jose, CA, USA).
Results
Characterisation of the reference soil (Delika)
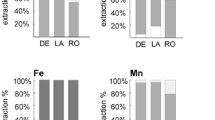
DE soil had high OM (10.8 %), high pH (8.0) and moderate levels of Cd, Co and Ni (Table 1). Zn, Pb, Co, Ba and Mn were associated with the reducible fraction or bound to oxides and hydroxides (Fig. 1). Ni, Cr and Cu were more or less equally distributed between the fraction associated with reducible phases and that of metals bound to sulphides and organic matter (Fig. 1). Cd was below detection limits in all fractions analysed. Zn, Pb and Cd were below detection limits in DIN 38414, DIN 19730 and CaCl2 extracts.
Zn, Pb, Ni, Co, Fe, Ba, Cr, Mn, Cu and Ca distributions in Delika (DE), Legazpi (LG) and Zugaztieta (ZU) soils, shown as the extractable element concentrations related to the total recovered elements (%) performed after the BCR protocol:  step 1 is extracted with acetic acid;
step 1 is extracted with acetic acid;  step 2 with hydroxylamine hydrochloride;
step 2 with hydroxylamine hydrochloride;  step 3 is extracted with in H2O2 and ammonium acetate; and
step 3 is extracted with in H2O2 and ammonium acetate; and  step 4 is the residual fraction
step 4 is the residual fraction
DE soil resulted to be non-toxic according to both solid phase and basic Microtox® tests performed on DIN 38414, DIN 19730 and CaCl2 extracts (Tables 2 and 3).
Properties and toxicity profile of LG and ZU soils
LG soil was silt loamy with a lower pH than DE soil, higher EC and similar soil respiration (Table 1). LG soil presented significantly higher concentrations of several metals (As, Zn, Pb, Fe, Cu and Ni), and, more markedly, TPHs than DE soil (Table 1). Particularly, Zn concentrations were 3–4 times higher, and Cu and Pb concentrations were 8–10 times higher than those of DE soil. However, 12–16 % of the recovered Zn (37.9 ± 22.2 mg Zn/kg soil dry wt), Pb (27.0 ± 39.3 mg Pb/kg soil dry wt) and Cu (4.2 ± 1.7 mg Cu/kg soil dry wt) were present in the easily soluble fractions, all below IVA-B values (Fig. 1). Over 15 % of the recovered Zn, Pb, Co, Ba and Cu was present in the easily soluble fraction. Only 4–12 % of Zn, Pb and Cu were recovered in the oxidable fraction. In contrast, the 76–82 % of the total metals (181.1 mg Zn/kg soil dry wt; 158.5 mg Pb/kg soil dry wt; 26.5 mg Cu/kg soil dry wt; all above IVA-B values) was recovered in the reducible fractions (Fig. 1). Cr and Mn total concentrations were slightly but significantly less in LG soil than in DE soil (Table 1). No difference was observed in the sequential extraction pattern of Cr, Mn and Ca with respect to the reference soil (Fig. 1). Zn, Pb and Cd were below detection limits of DIN 38414, DIN 19730 and CaCl2 extracts.
ZU soil was sandy with significantly higher humidity and pH than DE soil and similar soil respiration (Table 1). ZU soil presented significantly higher concentrations of several metals (As, Zn, Pb, Fe, Cr, Cu, Al, Sb, Co, Ba and Mn) than DE soil (Table 1); remarkably, 7-times higher for Cu and 4-times for As and Zn. In contrast, Cd concentrations were slightly but significantly less in ZU soil than in DE soil. In the easily soluble fraction, only 12.3 % of the recovered Zn (6.9 ± 0.2 mg Zn/kg soil dry wt; below IVA-B value) was present, and Pb, Cd and Cu were under detection limits (Fig. 1). Likewise, only the 5–17 % of the recovered Zn, Pb and Cu was present in the oxidable fraction (all below IVA-B values), where Cd was under detection limits. In contrast, 72–95 % (40.5 ± 0.9 mg Zn/kg soil dry wt; 47.2 ± 3.6 mg Pb/kg soil dry wt; 12.3 ± 0.7 mg Cu/kg soil dry wt) was in the reducible fraction, with Pb concentration remaining above its IVA-B value. The reducible fraction of Ni and Fe was over 50 % of the recovered metal. No difference was observed in the sequential extraction pattern of Cr and Co in comparison with the reference soil (Fig. 1). Zn, Pb, Cd and Cu were under detection limits in DIN 38414, DIN 19730 and CaCl2 extracts. In ZU soil, over 10 % of the recovered Zn was present in the easily soluble fraction (Fig. 1),
Toxicity screening
Solid phase toxicity
Microtox® showed an IC50 > 1000 mg/L after exposure to LG and ZU soils solid phase. However, according to the interim guidelines recommended by Environment Canada (2002), LG and ZU soils resulted to be toxic. 100 % LG soil resulted to be toxic to V. fischeri (NOEC = 50 % LG soil; LOEC = 100 % LG soil), 50 % ZU soil was weakly toxic (NOEC = 25 % ZU soil; LOEC = 50 % ZU soil). Regarding D. discoideum developmental cycle (DDDC) assay results, a significant reduction in MU%, MAI and CAI was found in LG soil in comparison with DE soil (Table 2). In contrast, FBSF was significantly elevated relative to DE soil. Overall, less aggregates, slugs and culminants were recorded in LG soil than in DE soil, which rendered the total number of multicellular units reduced beyond 50 % (Table 2). Regarding ZU soil, a significant increase in MU% and AAI, and a significant decrease in MAI and CAI were found in comparison with DE soil (Table 2). Overall, more aggregates and less slugs and culminants were recorded in ZU soil than in DE soil, which rendered unchanged the total number of multicellular units (Table 2).
Elutriate toxicity
The results of the basic Microtox® test on LG soil DIN 38414 elutriate (high EC values and basic pH; Table 3) and on ZU soil DIN 38414 (low EC values and basic pH; Table 3), did not reveal any significant toxicity. Likewise, LG soil elutriate did not produce significant effects on plant (lettuce and radish) germination and root elongation. ZU soil elutriate did not produce significant effects on radish germination and root elongation. But lettuce root elongation and GI (%) were significantly reduced. Besides, D. discoideum mortality was significantly lower than in the reference soil both in LG and ZU soil elutriates (Table 3).
Critical toxic values
D. discoideum toxicity
IRS, used as control soil for dilutions of LG soil, significantly inhibited fruiting body formation after 24 h in comparison with the reference soil (DE, Fig. 2a). In order to avoid the influence of confounding factors others than LG soil toxicity on the effects of LG soil dilutions, the toxicity of this soil was estimated relative to DE soil for each experimental concentration of IRS. Thus, a dose response curve was clearly obtained for IFBF, depending on LG dilutions (Fig. 2b). EC50 was 15.0 % LG soil and the estimated NOEC and LOEC values were <10 % and 10 % LG soil, respectively. The effect of ZU soil, estimated also relative to DE soil for each experimental dilution in IRS, a dose response curve was envisaged for IFBF depending on ZU soil dilutions (Fig. 2c). EC50 was 36.5 % ZU soil.
a Inhibition of fruiting body formation (IFBF; %) in D. discoideum grown on DE soil mixed with IRS. b–c IFBF (%) relative to DE soil for LG (b) and ZU (c) soils mixed with IRS. Values are shown as average ± standard deviation. Asterisks indicate significant differences (p ≤ 0.05) according to one-way ANOVA followed by Duncan’s test
Lettuce toxicity
Exposure of seeds to 50 % LG soil resulted in statistically significant reduction in seed germination, root elongation and GI (%) (Fig. 3a, c, e). However, exposure of seeds to ZU soil resulted in no statistically significant differences in seed germination and GI (%) (Fig. 4a, e). In contrast, root elongation significantly changed compared to control soil (Fig. 4c). When 100 % OECD (control), DE (reference) and LG (test) soils were compared, germination and root elongation in LG soil were significantly different from those recorded in OECD and DE soils (Fig. 3b, d), and GI (%) was significantly different among the three soils (Fig. 3f). The estimated EC50 values (as % LG soil) were >100 for germination, 99.5 for root elongation and 71.8 for GI (%) (NOEC = 25 % LG soil and LOEC = 50 % LG soil, for the three parameters). When OECD, DE and ZU soils were compared, germination and GI (%) in DE soil were significantly different from those recorded in OECD and ZU soils (Fig. 4b, f), whilst root elongation in OECD soil was different from those recorded in DE and ZU soils (Fig. 4d). EC50, NOEC and LOEC could not be calculated.
Lettuce seed germination (% initial seed number, a), root elongation (mm/seed, c) and Germination Index (GI%, e) in Legazpi (LG) soil mixed with OECD artificial soil in a dilution series/gradient. Germination (b), root elongation (d) and GI (e) in 100 % OECD soil (control soil), 100 % DE soil (reference soil) and 100 % LG soil (test soil). Values are shown as average ± standard deviation. Asterisks indicate significant differences (p ≤ 0.05) according to one-way ANOVA followed by Duncan’s test
Lettuce seed germination (% of initial seed number, a), root elongation (mm/seed, c) and Germination Index (GI%, e) in Zugaztieta (ZU) soil mixed with OECD artificial soil in a dilution series/gradient. Germination (b), root elongation (d) and GI (e) in 100 % OECD soil (control soil), 100 % DE soil (reference soil) and 100 % ZU soil (test soil). Values are shown as average ± standard deviation. Asterisks indicate significant differences (p ≤ 0.05) according to one-way ANOVA followed by Duncan’s test
Earthworm acute toxicity
Marked differences in physicochemical characteristics were found among the different experimental soils (Online Resource 1). OM varied from 5.76 % in OECD soil to 12.1 % in LG soil, with DE soil in between. pH also rose at increasing dilutions of LG soil, the pH value recorded in 100 % LG being similar to the one of DE soil. The levels of metals were significantly higher in the reference soil (DE) than in control soil (OECD) (acute toxicity test data in Online Resource 3).
E. fetida weight loss was evident in all experimental dilutions (>20 %) as well as in the reference soil (14.7 %; Online Resource 2). No obvious physical or pathological symptoms or distinct changes in behaviour were observed in the test organisms. However, after 14 days exposure, they accumulated pollutants from soils, especially Pb and TPHs (Online Resource 3). Mortality in control animals, as well as in those exposed up to 50 % LG soil, did not exceed 10 % at the end of the test (Fig. 5a; Online Resource 2). Each experimental dilution resulted in bioaccumulation of Pb and TPHs after 14 days (Fig. 5a). LC50 was 65.5 % LG soil (NOEC = <0.3–0.6 % LG soil; LOEC = 0.3–1.25 % LG soil) whereas the lowest concentration causing 100 % mortality was 100 % LG soil. Likewise, there were significant differences in the mortality recorded for OECD (control), DE (reference) and 100 % LG (test) soils (Fig. 5b).
a Cumulative mortality (%) in E. fetida after 14-day exposure to LG soil (test soil) and its dilutions in OECD soil (control soil) with indication of Pb and PAH concentrations at each experimental dilution. b Cumulative mortality after 14-day exposure to 100 % OECD soil (control soil), 100 % DE soil (reference soil) and 100 % LG soil (test soil). c E. fetida juvenile numbers after 56-day exposure to LG soil (test soil) and its dilutions in OECD soil (control soil). d Juvenile numbers after 56-day exposure to 100 % OECD soil, 100 % DE soil and 60 % LG soil. Values are shown as average ± standard deviation. Asterisks indicate significant differences (p ≤ 0.05) according to one-way ANOVA followed by Duncan’s test
OM varied from 5.80 % in OECD soil to 6.12 % in ZU soil (Online Resource 4). pH increased at increasing concentrations of ZU soil, and was similar in both DE and 50 % ZU soils (Online Resource 4). The levels of metals were significantly higher in DE soil than in OECD soil (acute toxicity test data in Online Resource 6).
E. fetida statistically significant weight loss (>10 %) was observed in all experimental dilutions as well as in the reference soil (Online Resource 5). No obvious physical or pathological symptoms or distinct changes in behaviour were observed in the test organisms. However, after 14 days exposure, bioaccumulation of Pb, Cr and Cu was observed (7.2 μg Pb/g dry wt tissue, 7.3 μg Cr/g dry wt tissue and 20.9 μg Cu/g dry wt tissue at day 14 vs. <2.5 μg Pb/g dry wt tissue, <2.5 μg Cr/g dry wt tissue and 9.15 μg Cu/g dry wt tissue at day 0; Online Resource 6). No significant mortality occurred in any experimental group (NOEC = 100 % ZU soil).
V. fischeri toxicity
After applying Microtox® Solid Phase test to the dilutions of the LG soil in presence of earthworms for 0 and 14 days (in parallel with the earthworm acute toxicity test), there were significant effects at 100 % LG soil at day 0 and at 25, 50 and 100 % LG soil at day 14 (Online Resource 2). Thus, estimates of NOEC and LOEC were 50 and 100 % LG soil, respectively, at day 0; and 2.5 and 25 % LG soil, respectively, at day 14. The estimated EC50 was 30.8 % LG soil at day 0 and 34.1 % LG soil at day 14. The reference soil was not toxic to V. fischeri neither at day 0 nor at day 14.
Significant toxicity effects were found on V. fischeri at 50 and 100 % ZU soil (NOEC = 25 % ZU soil; LOEC = 50 % ZU soil; Online Resource 5) at day 0 of the acute toxicity test. The estimated EC50 was 11.7 % ZU soil.
Earthworm reproduction test
No difference was found in humidity along the experimental time for each experimental group (Online Resource 1). Although not all the replicates in the control produced at least 30 juveniles by the end of the test and the coefficient of variation was higher than 30 %, no adult mortality was recorded after 4 wk and these validity conditions were fulfilled by the reference soil (>30 juveniles; CV = 19 %; no mortality). Thus, the test results were considered valid. In addition, neither obvious pathological symptoms nor distinct changes in behaviour were recorded. A significant decrease in juvenile numbers occurred subsequent to 50 % LG soil exposure (Fig. 5c; Online Resource 2). EC50 for juvenile numbers was 6.7 % LG soil (NOEC = 10 % LG soil; LOEC = 30 % LG soil). Juvenile number values recorded in OECD and LG 60 % soils were significantly lower than in DE soil (Fig. 5d).
Discussion
It was confirmed that the total concentrations of As, Zn, Pb and Cu were above their respective IVA-B values in LG soil (Tables 1 and 5), in agreement with previous reports (Gascón et al. 2007). In contrast, the concentration of TPHs (32,300 mg TPH/kg soil dry wt) was higher than previously reported (Gascón et al. 2007). While to date IVA values have not been assigned to TPHs (Ihobe 1998b), TPH concentrations found in LG soil were higher than in heavily polluted soils that had previously been demonstrated to be toxic by a range of other studies (Table 4). Therefore, there was a need for further toxicity assessment of the LG soil.
In the case of ZU soil Zn, Pb, Cd and Cu reached their respective IVA-B values (Tables 1 and 5), and high concentrations of Mn (~10× Mn concentration in DE soil), for which IVA values are not available, were recorded. Therefore, further toxicity assessment of the ZU soil was required, which seems to be particularly important in historically contaminated soils because the use of total concentration of metals for regulatory purposes may overestimate their risk (Diez et al. 2009).
DE soil resulted to be suitable to be used as “reference soil” to evaluate the effects of LG and ZU soils (INIA et al. 2007), although it must be mentioned that OM% was lower in ZU soil than in DE soil.
Availability of metals
Physicochemical characteristics of a soil such as the pH and organic matter content may influence metal bioavailability and toxicity (Giller et al. 1998; Diatta et al. 2011). Both LG and DE (reference) soils had a high organic matter content (>10 %) and were basic. Therefore, the solubility of metal ions was low in these soils (Giller et al. 1998; Martínez and Motto 2000). Accordingly, Zn, Pb and Cd in LG soil DIN 19730 and DIN 38414 extracts were below detection limits. Likewise, sequential extraction of LG soil showed that only 12–16 % of the recovered Zn, Pb and Cu were present in the easily soluble fractions, all below IVA-B values. Most of the total Zn, Pb and Cu concentrations (76–82 %) were found in the reducible fractions, which include metal oxides/hydroxides soluble under slightly acidic pH and metals associated with reducible amorphous Fe-Mn oxyhydroxides (Velimirovic et al. 2010) Besides, they were all found in concentrations above IVA-B values. Therefore, the release of the metals from this matrix will most likely be affected by the redox potential and pH, although the Fe-Mn oxide and organic matter have a scavenging effect and may provide a sink for heavy metals. The trace metal content in the Fe/Mn oxide phase is sensitive to anthropogenic inputs in sediments (Singh et al. 2005).
In ZU soil, mobile Zn, Pb, Cd and Cu (DIN 19730) were at concentrations below their detection limits, and of these metals, only Zn was recovered in the easily soluble fraction 6.9 ± 0.2 mg Zn/kg soil dry wt, which is below IVA-B value. As in LG soil, most of the recovered Zn, Pb and CU were found in the reducible fraction, 72–95 %, with Pb concentration remaining above its IVA-B value.
Overall, the non-residual fractions of Zn, Pb and Cu in both soils (LG and ZU soils) were ~100 %, which, according to Velimirovic et al. (2010), is an indication that these metals may be potentially available for exchange and/or release into the environment. Indeed, following the Risk Assessment Code (RAC; Velimirovic et al. 2010), LG soil would exhibit a medium risk for Zn, Pb and Cu, and ZU soil would exhibit a medium risk for Zn but no risk at all for the other metals that were present in ZU soil at concentrations above their IVA-B values (Cd, Pb and Cu).
Bioaccumulation in earthworms
Metals
After 14-day exposure to LG soil, unfed E. fetida seemingly regulated Zn, As and Ni tissue levels. Zn and Ni are known to be regulated in earthworms (Beeby 1991; Lock and Janssen 2001b; Maleri et al. 2007). As can be metabolised to produce organic As compounds (i.e. arsenobetaine) and metabolites (i.e. arsenosugars) (Langdon et al. 2003; Watts et al. 2008). Nevertheless, the absence of bioaccumulation does not necessarily mean that exposure to these metals was harmless to earthworms, since metal regulation may provoke extra energetic demand and result in oxidative stress (Beeby 1991; Maity et al. 2008; Watts et al. 2008). In E. fetida, Zn toxicity is documented both after single exposure and in mixtures (Lock and Janssen 2001a; Maity et al. 2008; Asensio 2009; Rodríguez-Ruiz 2010). Inorganic As is known to be lethal after exposure to ~100 mg/kg soil dry wt for 56 days (Langdon et al. 2003) and genotoxic (Button et al. 2010). Ni seems to be less toxic (Lock and Janssen 2002), albeit lethal toxicity in agar medium and reproduction toxicity (>85 mg Ni/kg soil dry wt) have been reported in earthworms (Scott-Fordsmand et al. 1998; Maleri et al. 2007).
Pb uptake was negligible in E. fetida exposed to LG soil, except at Pb concentrations of 13.9 mg Pb/kg soil dry wt. Similarly, the concentration of Pb in E. andrei tissues did not increase at soil dilutions below 88 mg Pb/kg soil dry wt (Luo et al. 2014). As a general rule, Pb is not taken up by earthworms when the metal occurs at low concentrations in soil, especially when the organic matter content and the pH are high (Scaps et al. 1997; Davies et al. 2003; Luo et al. 2014), which is the case of LG soil. Furthermore, Pb seems to be toxic to earthworms only at very high soil concentrations. In E. fetida, the 14-day LC50 is as high as 2,827–7,000 mg Pb/kg soil dry wt (Spurgeon et al. 1994; Davies et al. 2003), and the EC50 for body weight loss is 6,670 ± 162 mg Pb/kg soil dry wt (Nahmani et al. 2007). In E. andrei, weight loss and reproduction are significantly affected at soil total Pb concentrations >2,000 mg/kg (Luo et al. 2014). Consequently, unless interactions between pollutants in mixture occurred, Pb would not be expected to exert toxic effects in the present case.
Tissue Zn concentration was low and seemed to be regulated in all ZU soil concentrations, as when E. fetida was exposed to LG soil. E. fetida bioaccumulated Ba, Pb, Ni, Cu, Cr and, more markedly, Fe and Mn after 14-day exposure to ZU soil. Mn can be very toxic, especially in combination with Fe (Roth and Garrick 2003). Likewise, accumulation and toxicity of Mn have been reported in earthworms (Morgan et al. 2007; Reinecke and Reinecke 1996).
Total petroleum hydrocarbons
The concentrations of TPHs in E. fetida fat ranged from 14,564 μg THP/g fat on exposure to 0 % LG soil to 35,685 μg THP/g fat on exposure to 25 % LG soil. E. fetida can accumulate, metabolise and/or excrete hydrocarbons (Tang et al. 2002; Komiyama et al. 2003; Gastaldi et al. 2007; Asensio 2009). Hydrocarbon bioaccumulation depends primarily on body lipid content, soil organic matter and the octanol-water partition coefficient (Markwell et al. 1989). Thus, hydrocarbon uptake is enhanced by the presence of high levels of organic matter in soil (Belfroid et al. 1994; Eijsackers et al. 2001). It is therefore conceivable that, due to the high OM% present in LG soil, hydrocarbons were taken up partially metabolised and retained in fat tissues; which would led to body concentrations lower than in soil. The Biological Soil Accumulation Factor (BSAF; normalised BCF according to the organic matter in soil and the fat content in earthworms; Ma et al. 1998) in earthworms exposed to 2.5–100 % LG was in the range of 0.02–0.61. In E. fetida, an average BSAF value 0.02 was reported on exposure to PAHs (Matscheko et al. 2002). Likewise, BSAF values ranged between 0.24 and 1.21 for individual PAHs after exposure to creosote (Juhasz et al. 2010) and between 0.20 and 0.35 after exposure to different concentrations of kerosene (Asensio 2009). The present moderate-to-low BSAF values would suggest that hydrocarbon compounds present in LG soil have been at least partially metabolised. In any case, both accumulated hydrocarbons and the metabolites resulting from their degradation in tissues could cause toxic effects. Indeed, hydrocarbons are known to be genotoxic and to produce general and oxidative stress as well as impairment of certain enzyme functions in earthworms (Venier and Zampieron 2005; Eom et al. 2007; Asensio 2009).
Toxicity
Microbes
Soil basal respiration is a measure of the total biological activity in soil and results from the degradation of organic matter, where the formation of CO2 is the last step of carbon mineralization (Margesin et al. 2003). Taking into account the high levels of TPHs in LG soil, a high soil respiration would be expected (Margesin et al. 2000), but respiration was similar in LG and DE (reference) soils. Metals found in LG soil might also have a toxic effect on soil microorganisms (Giller et al. 1998) and hamper biological activity. Indeed, LG soil toxicity to V. fischeri increased after bioremediation in biopiles (Gascón et al. 2007).
LG soil was found to be toxic to V. fischeri (IC50 = 1,797 mg/L; eq. 0.18 % LG soil), according to Environment Canada (2002) interim guidelines; toxicity being seemingly linked to the presence of hydrocarbons. First, similar estimates of solid phase toxicity were recorded in various sites polluted with organochemicals (Table 4). Second, toxicity could not be attributed to water-soluble pollutants since, even though Zn is highly toxic to V. fischeri (IC50 = 0.6–2.2 mg/Azur 1995a), LG soil DIN 38414 elutriate resulted to be non-toxic (IC50 > 3,000 mg/L; Environment 2002). Finally, hydrocarbon toxicity, which is determined by the soil properties, is known to be more perceptible after the Microtox® solid phase test than after the basic test (Harkey and Young 2000). Moreover, hydrocarbon extractability also depends on soil properties. Presently, a weak extracting agent (DIN 38414) was applied to a soil with high OM% (>10 %); which might explain the lack of responsiveness of the Microtox® basic test. Additional extraction procedures should be used in the future to obtain a more feasible indication of hydrocarbon toxicity in soils. Indeed, 0.001 M Ca(NO3)2 elutriates of oil-polluted soils with low OM% (<4 %) were shown to be toxic to V. fischeri (Van Gestel et al. 2001), even if they had lower concentrations of TPHs than LG soil and similar concentrations of Zn and Pb (Tables 1 and 4).
ZU soil was found to be toxic to V. fischeri, where 50 % ZU soil was weakly toxic (NOEC = 25 % ZU soil; LOEC = 50 % ZU soil), although Zn and Pb concentrations in this soil dilution were below IVA-B values. Besides, and as in LG soil, DIN 38414 ZU elutriate was not toxic to V. fischeri.
Although LG and ZU soil toxicity to V. fischeri seemed to be low, the potential toxicity might have been underestimated by the application of Microtox® solid phase test to real soils, because it does not consider the influence of biological activity on pollutant toxicity and mobility (Wen et al. 2004; Contreras-Ramos et al. 2006). Accordingly, our results revealed that LG soil toxicity was enhanced by the presence of E. fetida. Whilst dilutions other than 100 % LG soil were not toxic to V. fischeri (NOEC = 50 % LG soil; LOEC = 100 % LG soil), after 14 days in presence of earthworms the toxicity of LG soil increased markedly (NOEC = 2.5 % LG soil; LOEC = 25 % LG soil).
Protists
Marked toxic effects were elicited in the slime mould, D. discoideum, exposed to LG soil with significant reduction in the number of multicellular units (lowered MU%), stepping up of the migration and culmination stages and enlargement of the fruiting bodies, which as a whole resulted in IFBF after 24 h. Since the number of multicellular units was severely reduced, LG soil seemed to affect amoeba viability in soil and/or their aggregation (i.e. cAMP signalling or cell movement; Gross, 1994). Moreover, LG soil DIN 38414 elutriate affected amoeba cell replication, which besides being an environmentally relevant toxicological endpoint, would lead to less colonies being established (Dondero et al. 2006; Sforzini et al. 2008). This suggests that cell viability, and thus the vegetative life cycle, was compromised by the water-soluble fraction of pollutants. In contrast, successful multicellular units progressed more quickly into migration and culmination phases and produced enlarged fruiting bodies. These effects can be either the result of less competition among colonies at lower densities of these or an adaptation mechanism to compensate the impact of LG soil in colony formation (Rodríguez-Ruiz et al. 2013).
Overall, as previously reported for other polluted soils (Balbo and Bozzaro 2008), fruiting body formation is drastically affected by LG soil (IFBF EC50 = 15.0 % LG soil; NOEC = <10 % LG soil; LOEC = 10 % LG soil). Therefore, the ecological fitness of this social amoeba, in terms of its capacity to resist starvation and start a new vegetative cycle in presence of food, would be severely reduced at concentrations of individual pollutants below their respective IVA-B values (e.g. in 15 % LG soil). These results imply that considering D. discoideum as the toxicological endpoint, current IVA-B values would need to be revised to be suitable as SVs.
DIN 38414 ZU elutriate was not toxic to slime mould amoebae. Conversely, significant effects were elicited in D. discoideum developmental cycle (augmented MU% and AAI, and reduced MAI and CAI). The arrest in the aggregate phase together with speeding up the migration and culmination phases might indicate alterations in the progressing from aggregates to slugs (Rodríguez-Ruiz et al. 2013). Overall, IFBF values revealed toxicity (EC50 = 36.5 % ZU soil) although the metals in this soil dilutions were low. Thus, for example, toxicity could not be attributed to the Zn levels in 36.5 % ZU soil (39 mg Zn/kg soil dry wt; Online Resource 6), since 522 mg Zn/soil dry wt are needed to provoke a 50–75 % reduction in IFBF (Balbo and Bozzaro 2008). Other pollutants, their combination in a mixture or either the influence of soil properties (Rodríguez-Ruiz et al. 2013) might account for the observed toxicity instead.
Plants
L. sativa is sensitive to metal and hydrocarbon pollution (Plaza et al. 2005). However, LG soil was found to be only weakly toxic to lettuce, affecting elongation and, to a lesser extent, seed germination, and ZU soil was only found to significantly reduce lettuce root elongation. Although there is some controversy, root elongation seems to be more sensitive than seed germination in several plant species (Saterbak et al. 1999; Plaza et al. 2005). Some metals (e.g. Pb) affect only root elongation (Chang et al. 1997; Plaza et al. 2005) and some hydrocarbons do not inhibit germination but provoke reduced root length (e.g. PAHs in lettuce and Chinese cabbage; Eom et al. 2007). Lettuce root elongation is inhibited at concentrations of individual metals such as 2.5 mg Ni/L, 5 mg Cu/L, 7 mg Cd/L, 18 mg Zn/L or 70 mg Mn/L (Wang 1987). Overall, LG and ZU soils cannot be considered highly toxic to lettuce (NOEC and LOEC, 25 and 50 % LG soil, respectively, for the three parameters) albeit a potential sublethal toxicity cannot be fully disregarded.
LG DIN 38414 elutriate was non-toxic to lettuce and radish, unlike in previous studies (Table 4) and in ZU DIN 38414 elutriate, which was non-toxic to radish but lettuce root elongation and GI (%) were significantly reduced. Presently, the concentration of individual pollutants in 71.8 % LG soil (Online Resource 3) would be just below (As and Pb) or over (Zn, Cu, TPHs) their respective IVA-B values. Thus, IVA values would be adequate to prevent toxicity to L. sativa, even in presence of mixtures suitable to provoke interactive effects. Soils with similar Zn concentrations in their elutriates compared to ZU elutriate were reported to inhibit germination in L. sativa (Schultz et al. 2004).
Earthworms
E. fetida was the most sensitive test organism among those presently employed to investigate LG soil toxicity. Earthworm bioassays are known to be more sensitive to hydrocarbon mixtures than Microtox® and plant germination and growth tests (Asensio 2009; Dorn et al. 1998). In contrast, after toxicity testing of incineration ash (metals), waste wood (metals, mainly Cu) and gaswork soil (PAHs), it was concluded that the sensitivity of plant tests was greater in comparison with the earthworm acute toxicity test, although the earthworm reproduction test was equally or more sensitive than plant tests (Foerster et al. 2009). Likewise, E. fetida did not show toxic responses when exposed to ZU soil in the acute toxicity test. In the present study, mortality, growth and reproduction were significantly affected by LG soil, which caused 100 % mortality. In addition, E. fetida reproduction was affected even in highly diluted LG soil (28-d EC50 = 6.7 % LG soil). Thus, LG soil toxicity should be considered at least moderately toxic in comparison with previous studies in sites polluted with mixtures of hydrocarbons and metals (Table 4). However, LG soil does not comply with the condition (LC50 < 1 % soil in earthworm acute toxicity test) to be declared polluted according to the Spanish regulation criteria, although it should be subject to environmental risk assessment because it elicited toxic effects (INIA et al. 2007). On the other hand, the concentration of Zn, Cu and TPHs in 65.5 % LG soil (14-d LC50) would exceed IVA-B values (Online Resource 3). Consequently, applicable SVs do not seem to ensure environmental protection.
Toxicity profile and risk assessment
Legazpi brownfield soil
Earthworms are recognised biomonitors and a potential bioavailability of pollutants may be envisaged for other species on the basis of bioaccumulation data in earthworms (Beeby 2001). Thus, though bioavailability may differ between species inhabiting the same soil, the fact that As, Zn, Ni, Pb and TPHs were taken up and either regulated/metabolised or bioaccumulated in earthworms reflects that these pollutants were potentially available for soil biota, which is in agreement with the sequential extraction results.
IVA values seem to be adequate to prevent toxicity to plants since LG soil was not very toxic to lettuce and radish. However, LG soil was toxic to V. fischeri, though the toxicity was not attributed to water-soluble pollutants but to TPHs. Interestingly, this toxicity was enhanced by the presence of earthworms in the soil. Likewise, LG soil caused toxic effects on D. discoideum and E. fetida. Ecologically relevant endpoints (fruiting body formation and cell replication in D. discoideum; and mortality, growth and reproduction in E. fetida) were significantly affected at soil dilutions of individual pollutants below their respective IVA-B values. Consequently, the present toxicity assessment clearly evidences that remediation interventions would be required in this soil, even though the soil dilutions of individual contaminants were not over their respective IVA-C values (Type A SVs; Carlon 2007).
Zugaztieta derelict mine soil
Overall, ZU soil seems to be only moderately toxic. However, caution is required since some toxicity values recorded for to V. fischeri, D. discoideum and L. sativa cannot be explained by the contaminants presently found at soil dilutions beyond applicable SVs (IVA-B values). This suggests that compliance with SVs does not necessarily ensure soil protection. In contrast, elutriates from a mining area polluted with Cd, Cu, Pb and Zn above the applicable SVs were found to be practically no toxic to V. fischeri and cress germination and root elongation (Alvarenga et al. 2008). It is worth mentioning that the environmental significance of SVs is controversial (Carlon 2007), especially in historically contaminated soils (Diez et al. 2009). The risk of these soils results from a trade-off between overestimated risks (e.g. by using metal total concentrations; Diez et al. 2009) and risks neglected by the SV approach (pollutants in combination and potential pollutants not measured or without an established SV).
Interestingly, ZU soil contains high levels of Mn, which lacks IVA-B value assignment (Ihobe 1998b). Mn is an essential metal that serves as cofactor of numerous enzymes essential for metabolic homeostasis (i.e. Mn superoxide dismutase) and can substitute Mg in many kinases (Gonzalez-Oreja et al. 2008). This metal has been reported to be practically non-toxic to V. fischeri (Teodorovic et al. 2009) and weakly toxic to lettuce (Wang 1987). In earthworms, Mn accumulation and toxicity studies have observed that even though earthworms are able to colonise successfully Mn polluted soils (Morgan et al. 2007)., reduced growth, cocoon production (EC20 = 629 mg Mn/kg soil dry wt; Reinecke and Reinecke 1996), and alterations in spermatozoa (Reinecke and Reinecke 1997) have occurred. Therefore, it is plausible that the extremely high concentrations of Mn found in ZU soil might have caused sublethal toxicity to E. fetida. Indeed, Mn tissue concentration was high. Moreover, Mn toxicity is enhanced in combination with Fe (Roth and Garrick 2003), which was the most abundant element in ZU soil. Finally, altered gene expression patterns and pathways in earthworms exposed to ZU soil were associated to Mn toxicity in a previous study (Asensio et al. 2013). As a result, there is a need to establish SVs for Mn and to accomplish specific investigations on the risk associated to this metal in ZU soil.
Conclusions
The application of a battery of toxicity bioassays with a selected set of test species (V. fischeri, D. discoideum, L. sativa, R. sativus and E. fetida) in combination with chemical analysis of soils, elutriates and earthworm tissues, LG and ZU soils revealed different toxicity profiles and severity. Zugaztieta is a historically contaminated soil that showed very little or no toxicity. However, there might be some risk associated to Mn, a potential pollutant without an established SV. On the other hand, LG soil exhibited significant toxicity according to the majority of the endpoints investigated (solid phase Microtox®, D. discoideum inhibition of fruiting body formation and developmental cycle solid phase assays, lettuce seed germination and root elongation test, earthworm acute toxicity and reproduction tests, D. discoideum cell viability and replication elutriate assays). Consequently, for similar SVs, the risk posed to the environment, and therefore the need for intervening, was different, which was unveiled after toxicity profiling based on multiple endpoint bioassays. An equivalent approach is suitable to be applied for scenario-targeted soil risk assessment in those cases where applicable national/regional soil legislation is based on SVs.
References
Alef K, Nannipieri P (1995) Estimation of microbial activities. In: Kassem A, Paolo N (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London, pp 193–270
Alvarenga P, Palma P, Gonçalves A, Fernandes R, de Varennes A, Vallini G, Duarte E, Cunha-Queda A (2008) Evaluation of tests to assess the quality of mine-contaminated soils. Environ Geochem Health 30:95–99
Asensio V (2009) Health assessment of polluted soils after Eisenia foetida ex-situ bioassays based on conventional and in vitro cellular biomarkers and microarray technology. Ph.D.Thesis, University of the Basque Country
Asensio V, Rodríguez-Ruiz A, Garmendia L, Andre J, Kille P, Morgan AJ, Soto M, Marigómez I (2013) Towards an integrative soil health assessment strategy: a three tier (integrative biomarker response) approach with Eisenia fetida applied to soils subjected to chronic metal pollution. Sci Tot Environ 442:344–365
Azur Environmental (1995a) Microtox acute toxicity basic test procedures. Carlsbad, CA, USA
Azur Environmental (1995b) Microtox basic solid-phase test (basic SPT). Carlsbad, CA, USA
Balbo A, Bozzaro S (2008) A novel bioassay for evaluating soil bio-hazards using Dictyostelium as biosensor: validation and application to the Bio-Bio Project. Fresenius Environ Bull 17:1137–1143
Beeby A (1991) Toxic metal uptake and essential metal regulation in terrestrial invertebrates: a review. In: Newman MC, McIntosh AW (eds) Metal ecotoxicology: concepts and applications. Lewis, Chelsea, MI, USA, pp 65–89
Beeby A (2001) What do sentinels stand for? Environ Pollut 112:285–298
Belfroid A, Sikkenk M, Seinen W, Hermens J, Van Gestel K (1994) The toxicokinetic behavior of chlorobenzenes in earthworm (Eisenia andrei) experiments in soil. Environ Toxicol Chem 13:93–99
Bierkens J, Klein G, Corbisier P, Van Den Heuvel R, Verschaeve L, Weltens R, Schoeters G (1998) Comparative sensitivity of 20 bioassays for soil quality. Chemosphere 37:2935–2947
Bogan BW, Beardsley KE, Sullivan WR, Hayes TD, Soni BK (2005) Effect of volatile hydrocarbon fractions on mobility and earthworm uptake of polycyclic aromatic hydrocarbons from soils and soil/lampblack mixtures. Environ Toxicol Chem 24:181–189
Brown RB, (2003). Soil texture. Univ Florida, IFAS Extension. Available: http://ufdc.ufl.edu/IR00003107/00001 (last access, 11/03/2014)
Button M, Jenkin GRT, Bowman KJ, Harrington CF, Brewer TS, Jones GDD, Watts MJ (2010) DNA damage in earthworms from highly contaminated soils: assessing resistance to arsenic toxicity by use of the Comet assay. Mutat Res 696:95–100
Carlon C (ed.) (2007) Derivation methods of soil screening values in Europe. A review and evaluation of national procedures towards harmonization. European Commission, Joint Research Centre, Ispra, EUR 22805-EN, 306 pp
Chang LW, Meier JR, Smith MK (1997) Application of plant and earthworm bioassays to evaluate remediation of a lead-contaminated soil. Archiv Environ Contam Toxicol 32:166–171
Contreras-Ramos SM, Alvarez-Bernal D, Dendooven L (2006) Eisenia fetida increased removal of polycyclic aromatic hydrocarbons from soil. Environ Pollut 141:396–401
Critto A, Torresan S, Semenzin E, Giove S, Mesman M, Schouten AJ, Rutgers M, Marcomini A (2007) Development of a site-specific ecological risk assessment for contaminated sites: Part I. A multi-criteria based system for the selection of ecotoxicological tests and ecological observations. Sci Tot Environ 379:16–33
Davies NA, Hodson ME, Black S (2003) The influence of time on lead toxicity and bioaccumulation determined by the OECD earthworm toxicity test. Environ Pollut 121:55–61
Diatta J, Wirth S, Chudzinska E (2011) Spatial distribution of Zn, Pb, Cd, Cu, and dynamics of bioavailable forms at a polish metallurgical site. Fresenius Environ Bull 20:976–982
Diez M, Simon M, Martin F, Dorronsoro C, Garcia I, Van Gestel CAM (2009) Ambient trace element background concentrations in soils and their use in risk assessment. Sci Tot Environ 407:4622–4632
DIN (1984) DIN 38414-S4 Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung-Schlamm und Sedimente-Bestimmung der Eluierbarkeit mit Wasser. Deutsches Institut für Normung e.V. Berlin, Germany
Dondero F, Jonsson H, Rebelo M, Pesce G, Berti E, Pons G, Viarengo A (2006) Cellular responses to environmental contaminants in amoebic cells of the slime mould Dictyostelium discoideum. Comp Biochem Physiol 143C:150–157
Dorn PB, Vipond TE, Salanitro JP, Wisniewski HL (1998) Assessment of the acute toxicity of crude oils in soils using earthworms, microtox, and plants. Chemosphere 37:845–860
EC (2006) Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions—Thematic strategy for soil protection. COM(2006)231
Eijsackers H, Van Gestel CAM, De Jonge S, Muijs B, Slijkerman D (2001) Polycyclic aromatic hydrocarbon-polluted dredged peat sediments and earthworms: a mutual interference. Ecotoxicology 10:35–50
Eisentraeger A, Rila JP, Hund-Rinke K, Roembke J (2004) Proposal of a testing strategy and assessment criteria for the ecotoxicological assessment of soil or soil materials. J Soils Sed 4:123–128
EJ/GV (2007) Plan de suelos contaminados del País Vasco (2007-2012). Eusko Jaurlaritza/Gobierno Vasco, Vitoria-Gasteiz, Autonomous Community of the Basque Country (Spain) (in Spanish)
Environment Canada (2002) Biological test method: reference method for determining the toxicity of sediment using luminescent bacteria in a solid-phase test EPS 1/RM/42. Environment Canada, Ottawa, Ontario, Canada
Eom IC, Rast C, Veber AM, Vasseur P (2007) Ecotoxicity of a polycyclic aromatic hydrocarbon (PAH)-contaminated soil. Ecotox Environ Safety 67:190–205
Fernández MD, Cagigal E, Vega MM, Urzelai A, Babin M, Pro J, Tarazona JV (2005) Ecological risk assessment of contaminated soils through direct toxicity assessment. Ecotoxicol Environ Safety 62:174–184
Foerster B, Firla C, Junker T (2009) Plant tests Ecotoxicological characterization of waste. Results and experiences of an international ring test. Springer, New York, pp 117–128
Gascón JA, Arce M, Unzueta I, Susaeta I (2007) BIOSOIL project for the sustainable management of polluted soils. Residuos 97:76–85 (in Spanish)
Gastaldi L, Ranzato E, Capri F, Hankard P, Peres G, Canesi L, Viarengo A (2007) Application of a biomarker battery for the evaluation of the sublethal effects of pollutants in the earthworm Eisenia andrei. Comp Biochem Physiol 146C:398–405
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414
Gleyzes C, Tellier S, Astruc M (2002) Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures. Trac-Trend Anal Chem 21:451–467
Gonzalez-Ora JA, Rozas MA, Alkorta I, Garbisu C (2008) Dendroremediation of heavy metal polluted soils. Rev Environ Hlth 23:223–234
Greene JC, Bartels CL, Warren-Hicks WJ, Parkhurst BR, Linder GL, Peterson SA, Miller WE (1989) Protocols for short term toxicity screening of hazardous waste sites, USEPA, Corvallis, OR, 1989, EPA 600/3-88/029
Hamers T, Legler J, Blaha L, Hylland K, Marigómez I, Schipper CA, Segner H, Vethaak AD, Witters H, de Zwart D, Leonards PE (2013) Expert opinion on toxicity profiling—report from a NORMAN expert group meeting. Integr Environ Assess Manag 9:185–191
Harkey GA, Young TM (2000) Effect of soil contaminant extraction method in determining toxicity using the Microtox® assay. Environ Toxicol Chem 19:276–282
Harmsen J, Rulkens W, Eijsackers H (2005) Bioavailability: concept for understanding or tool for predicting? Land Cont Recl 13:161–171
Hoekstra NJ, Bosker T, Lantinga EA (2002) Effects of cattle dung from farms with different feeding strategies on germination and initial root growth of cress (Lepidium sativum L.). Agr Ecosyst Environ 93:189–196
Ihobe SA (1998a) Valores indicativos de evaluación (VIE-A). Nivel de referencia. Eusko Jaurlaritza/Gobierno Vasco, Vitoria-Gasteiz, Autonomous Community of the Basque Country (Spain), 45 pp (in Spanish)
Ihobe SA (1998b) Valores indicativos de evaluación (VIE-B, VIE-C) para la protección de los ecosistemas. Eusko Jaurlaritza/Gobierno Vasco, Vitoria-Gasteiz, Autonomous Community of the Basque Country (Spain), 104 pp. (in Spanish)
INIA, IGME, MMA (2007) Versión Web de la Guía Metodológica de aplicación del RD 9/2005 (14/01/2005) por el que se establece la relación de actividades potencialmente contaminantes del suelo y los criterios y estándares para la declaración de suelos contaminados (BOE Núm. 15, 18 enero 2005). Ministerio de Medio Ambiente, Madrid, Spain, pp. 114-119 (in Spanish)
ISO (1993) Soil quality—determination of dry matter and water content on a mass basis—Gravimetric method. ISO 11465
ISO (2005) Soil quality—determination of pH. International Standard. 10390, 1–7
ISO (2012) Soil quality—Effects of pollutants on earthworms -- Part 2: Determination of effects on reproduction of Eisenia fetida/Eisenia andrei. 11268–2
Juhasz AL, Smith E, Waller N, Stewart R, Weber J (2010) Bioavailability of residual polycyclic aromatic hydrocarbons following enhanced natural attenuation of creosote-contaminated soil. Environ Pollut 158:585–591
Knoke KL, Marwood TM, Cassidy MB, Liu D, Seech AG, Lee H, Trevors JT (1999) A comparison of five bioassays to monitor toxicity during bioremediation of pentachlorophenol-contaminated soil. Water, Air, Soil Pollut 110:157–169
Komiyama K, Okaue M, Miki Y, Ohkubo M, Moro I, Cooper EL (2003) Non-especific cellular function of Eisenia fetida regulated by polycyclic aromatic hydrocarbons. Pedobiologia 47:717–723
Langdon CJ, Piearce TG, Meharg AA, Semple KT (2003) Interactions between earthworms and arsenic in the soil environment: a review. Environ Pollut 124:361–373
Linder G, Greene J, Ratsch H, Nwosu J,Smith S, Wilborn D (1989) Seed germination and root elongation toxicity tests in hazardous waste site evaluation: methods development and applications. U.S. Environmental Protection Agency, Washington, D.C., EA/600/D-89/109 (NTIS PB90113184)
Lock K, Janssen CR (2001a) Modelling zinc toxicity for terrestrial invertebrates. Environ Toxicol Chem 20:1901–1908
Lock K, Janssen CR (2001b) Zinc and cadmium body burdens in terrestrial oligochaetes: use and significance in environmental risk assessment. Environ Toxicol Chem 20:2067–2072
Lock K, Janssen CR (2002) Ecotoxicity of nickel to Eisenia fetida, Enchytraeus albidus and Folsomia candida. Chemosphere 46:197–200
Luo W, Verweij RA, van Gestel CAM (2014) Determining the bioavailability and toxicity of lead contamination to earthworms requires using a combination of physicochemical and biological methods. Environ Pollut 185:1–9
Ma WC, van Kleunen A, Immerzeel J, de Maagd PGJ (1998) Bioaccumulation of polycyclic aromatic hydrocarbons by earthworms: assessment of equilibrium partitioning theory in in situ studies and water experiments. Environ Toxicol Chem 17:1730–1737
Maity S, Roy S, Chaudhury S, Bhattacharya S (2008) Antioxidant responses of the earthworm Lampito mauritii exposed to Pb and Zn contaminated soil. Environ Pollut 151:1–7
Maleri RA, Reinecke AJ, Reinecke SA (2007) A comparison of nickel toxicity to pre-exposed earthworms (Eisenia fetida, oligochaeta) in two different test substrates. Soil Biol Biochem 39:2849–2853
Margesin R, Walder G, Schinner F (2000) The impact of hydrocarbon remediation (diesel oil and polycyclic aromatic hydrocarbons) on enzyme activities. Acta Biotechnol 20:313–333
Margesin R, Labbe D, Schinner F, Greer CW, Whyte LG (2003) Characterization of hydrocarbon-degrading microbial populations in contaminated and pristine alpine soils. Appl Environ Microbiol 69:3085–3092
Markwell RD, Connell DW, Gabric AJ (1989) Bioaccumulation of lipophilic compounds from sediments by oligochaetes. Water Res 23:1443–1450
Martínez CE, Motto HL (2000) Solubility of lead, zinc and copper added to mineral soils. Environ Pollut 107:153–158
Matscheko N, Lundstedt S, Svensson L, Harju M, Tysklind M (2002) Accumulation and elimination of 16 polycyclic aromatic compounds in the earthworm (Eisenia fetida). Environ Toxicol Chem 21:1724–1729
Morgan AJ, Pleasance B, Kinsey H, Murphy D, Davies S (2007) The manganese relationships of ecophysiologically contrasting earthworm species (Lumbricus rubellus and Aporrectodea caliginosa) inhabiting manganese-mine soils. Eur J Soil Biol 43:S297–S302
Nahmani J, Hodson ME, Black S (2007) Effects of metals on life cycle parameters of the earthworm Eisenia fetida exposed to field-contaminated, metal-polluted soils. Environ Pollut 149:44–58
NEN (1994) Determination of organic matter content in soil as loss-on-ignition. NEN 5754
NEN (1997) Soil—determination of mineral oil content in soil and sediments with gas chromatography. NEN 5733
OECD (1984) Earthworm, acute toxicity tests. OECD guideline for the testing of chemicals No. 207
OECD (2004) Earthworm reproduction test (Eisenia fetida/andrei). OECD guideline for the testing of chemicals No. 222
Plaza G, Nalecz-Jawecki G, Ulfig K, Brigmon RL (2005) The application of bioassays as indicators of petroleum-contaminated soil remediation. Chemosphere 59:289–296
Rauret G, Lopez-Sanchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Reinecke SA, Reinecke AJ (1996) The influence of heavy metals on the growth and reproduction of the compost worm Eisenia fetida (Oligochaeta). Pedobiologia 40:439–448
Reinecke SA, Reinecke AJ (1997) The influence of lead and manganese on spermatozoa of Eisenia fetida (Oligochaeta). Soil Biol Biochem 29:737–742
Rodríguez-Ruiz A (2010) Risk assessment in real soils from the Basque Country after soil health screening through toxicity profiles based on standard and novel multiple endpoint bioassays. Ph.D. Thesis, University of the Basque Country
Rodríguez-Ruiz A, Marigómez I, Boatti L, Viarengo A (2013) Dictyostelium discoideum developmental cycle (DDDC) assay: a tool for Hg toxicity assessment and soil health screening. Sci Tot Environ 450–451:39–50
Roth JA, Garrick MD (2003) Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol 66:1–13
Saterbak A, Toy RJ, Wong DCL, McMain BJ, Williams MP, Dorn PB, Brzuzy LP, Chai EY, Salanitro JP (1999) Ecotoxicological and analytical assessment of hydrocarbon-contaminated soils and application to ecological risk assessment. Environ Toxicol Chem 18:1591–1607
Sayles GD, Acheson CM, Kupferle MJ, Shan Y, Zhou Q, Meier JR, Chang L, Brenner RC (1999) Land treatment of PAH-contaminated soil: performance measured by chemical and toxicity assays. Environ Sci Technol 33:4310–4317
Scaps P, Grelle C, Descamps M (1997) Cadmium and lead accumulation in the earthworm Eisenia fetida (Savigny) and its impact on cholinesterase and metabolic pathway enzyme activity. Comp Biochem Physiol 116C:233–238
Schultz E, Joutti A, Räisänen ML, Lintinen P, Martikainen E, Lehtto O (2004) Extractability of metals and ecotoxicity of soils from two old wood impregnation sites in Finland. Sci Tot Environ 326:71–84
Scott-Fordsmand JJ, Weeks JM, Hopkin SP (1998) Toxicity of nickel to the earthworm and the applicability of the neutral red retention assay. Ecotoxicology 7:291–295
Sforzini S, Dagnino A, Torrielli S, Dondero F, Fenoglio S, Negri A, Boatti L, Viarengo A (2008) Use of highly sensitive sublethal stress responses in the social amoeba Dictyostelium discoideum for an assessment of freshwater quality. Sci Tot Environ 395:101–108
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges, India. J Hydrol 312:14–27
SM (2001) Oil and Grease. 5520D
Spurgeon DJ, Hopkin SP, Jones DT (1994) Effects of cadmium, copper, lead and zinc on growth, reproduction and survival of the earthworm Eisenia fetida (Savigny): assessing the environmental impact of point-source metal contamination in terrestrial ecosystems. Environ Pollut 84:123–130
Tang J, Liste HH, Alexander M (2002) Chemical assays of availability to earthworms of polycyclic aromatic hydrocarbons in soil. Chemosphere 48:35–42
Teodorovic I, Planojevic I, Knezevic P, Radak S, Nemet I (2009) Sensitivity of bacterial vs. acute Daphnia magna toxicity tests to metals. Eur J Biol 4:482–492
Urzelai A, Vega M, Angulo E (2000) Deriving ecological risk-based soil quality values in the Basque Country. Sci Tot Environ 247:279–284
USEPA (1996a) Microwave assisted acid digestion of siliceous and organically based matrices. Method 3052:1–20
USEPA (1996b) Ultrasonic extraction. Method 3550B:1–11
USEPA (2007) Inductively coupled plasma-atomic emission spectrometry. Method 6010C:1–34
Van Gestel CAM, Van der Waarde JJ, Derksen JGM, van der Hoek EE, Veul MFXW, Bouwens S, Rusch B, Kronenburg R, Stokman GNM (2001) The use of acute and chronic bioassays to determine the ecological risk and bioremediation efficiency of oil-polluted soils. Environ Toxicol Chem 20:1438–1449
Velimirovic MB, Prica MD, Dalmacija BD, Roncevic SD, Dalmacija MB, Becelic MD, Trickovic JS (2010) Characterisation, availability, and risk assessment of the metals in sediment after aging. Water, Air, Soil Pollut 214:219–229
Venier P, Zampieron C (2005) Evidence of genetic damage in grass gobies and mussels from the Venice lagoon. Environ Int 31:1053–1064
Wang W (1987) Root elongation method for toxicity testing of organic and inorganic pollutants. Environ Toxicol Chem 6:409–414
Wang XP, Shan XQ, Zhang SZ, Wen B (2004) A model for evaluation of the phytoavailability of trace elements to vegetables under the field conditions. Chemosphere 55:811–822
Wang SJ, Yan ZG, Guo GL, Lu GL, Wang QH, Li FS (2009) Ecotoxicity assessment of aged petroleum sludge using a suite of effects-based end points in earthworm Eisenia fetida. Environ Monit Assess 1–12
Watts M, Button M, Brewer T, Jenkin G, Harrington C (2008) Quantitative arsenic speciation in two species of earthworms from a former mine site. J Environ Monit 10:753–759
Wen B, Hu XY, Liu Y, Wang WS, Feng MH, Shan XQ (2004) The role of earthworms (Eisenia fetida) in influencing bioavailability of heavy metals in soils. Biol Fertility Soils 40:181–187
Wong DCL, Chai EY, Chiu KK, Dorn PB (1999) Prediction of ecotoxicity of hydrocarbon-contaminated soils using physicochemical parameters. Environ Toxicol Chem 18:2611–2621
Acknowledgments
Authors are indebted to Dr. Lara Boatti and Prof. Aldo Viarengo (DISIT, University of Piemonte Orientale) for their excellent support and valuable scientific discussions. This research was funded by Basque Government (UE09+/58, IE03-110 and IE06-179 Research Projects; Grant to Consolidated Research Group, GIC07/26-IT-393-07), UPV/EHU Research and Formation Unit in “Ecosystem Health Protection” (UFI 11/37) and Spanish Ministry of Science and Education (C6L-2006-06154). ARR was a recipient of a pre-doctoral fellowship from Fundación Centros Tecnológicos Iñaki Goenaga.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 113 kb)
Rights and permissions
About this article
Cite this article
Rodriguez-Ruiz, A., Asensio, V., Zaldibar, B. et al. Toxicity assessment through multiple endpoint bioassays in soils posing environmental risk according to regulatory screening values. Environ Sci Pollut Res 21, 9689–9708 (2014). https://doi.org/10.1007/s11356-014-2915-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2915-7