Abstract
Molecularly imprinted polymer adsorbent has been prepared to remove a group of recalcitrant and acutely hazardous (p-type) chemicals from water and wastewaters. The polymer adsorbent exhibited twofold higher adsorption capacity than the commercially used polystyrene divinylbenzene resin (XAD) and powdered activated carbon adsorbents. Higher adsorption capacity of the polymer adsorbent was explained on the basis of high specific surface area formed during molecular imprinting process. Freundlich isotherms drawn showed that the adsorption of p-type chemicals onto polymer adsorbent was kinetically faster than the other reference adsorbents. Matrix effect on adsorption of p-type chemicals was minimal, and also polymer adsorbent was amenable to regeneration by washing with water/methanol (3:1, v/v) solution. The polymer adsorbent was unaltered in its adsorption capacity up to 10 cycles of adsorption and desorption, which will be more desirable in cost reduction of treatment compared with single-time-use activated carbon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the All India Organization of Chemists and Druggists (AIOCD), sale of all types of medicines in India stands about at US$9.61 billion, which is projected to reach around US$31.59 billion by 2020 (Mathew and Unnikrishnan 2012). As a result of this growth, the pharmaceutical medicines/drugs are easily available at reasonable cost. This may be one of the reasons why people are just flushing off the unused pharmaceuticals and consuming drugs unnecessarily. Discharge from manufacturing units containing active ingredients of drugs in their effluent without proper treatment into the environment is also contributing to high concentrations of pharmaceutical chemicals. The recent report on sewage treatment plant at Patancheru (near Hyderabad), India claimed the highest levels of pharmaceuticals in surface and ground waters (Heberer 2002). In similar lines, occurrence of pharmaceuticals and personal care products in Illinois drinking water and even in ground waters is of great human health concern (Bureau of Water Illinois 2008). Many of the pharmaceutical chemicals are regulated under the Resource Conservation and Recovery Act (RCRA), enacted in 1976, is the principal federal law in the USA governing the disposal of solid waste and hazardous waste (Focazioa et al. 2008). The RCRA regulations include four lists of materials, designated with the letters F, K, P, and U. P-listed chemicals are considered “acutely hazardous” by the US EPA the worst of the worst. If a chemical on the P list is the sole active ingredient of a discarded product, it causes the entire product, including the solvent and container, to be contaminated and must be treated as a hazardous waste.
Presence of the pharmaceuticals residues in environment and their constant addition to water resources needs an improvement and upgradation of treatment facilities to remediate pharmaceutical pollution (Maurer et al. 2007). One issue of great concern is “treatment at trace level” of pharmaceutical chemicals in highly complex wastewaters. A typical pharmaceutical effluents treatment plant (ETP) consists of a three-tier system with primary, secondary, and tertiary treatment. In addition to the three-tier treatment system, in few circumstances, based on stipulations of regulatory agencies, industries intended to install advanced oxidation processes (AOPs) and membrane processes (activated carbon adsorption, microfiltration, ultrafiltration, nanofiltration, and reverse osmosis), multiple effective evaporators followed by incineration (Fontela et al. 2008). However, the advanced treatment methods are quite expensive and ETP operational costs are quite high. Recently reported research articles on carbon nanotubes, zeolites, activated carbon adsorbents showed an excellent adsorption removal towards pharmaceutical contaminants (Khalid et al. 2004; Pan et al. 2008; Reungoat et al. 2010). Although these materials and methods have merits of adsorption removal efficiency, their high cost and very limited reusability restricts their field application (Lin and Juang 2009). Adsorption is regarded as a promising method for the removal of micropollutants such as metals and organic compounds (Demirbas 2008; Ren et al. 2011; Shen et al. 2012). By the adsorption/desorption process, adsorbents in water can be recycled. So, it is of great importance to develop reusable high adsorption capacity adsorbents.
Highly selective molecularly imprinted polymers (MIPs) recently are being used widely in selective separation of targeted compounds from matrices such as environmental waters, seawater, biological fluids, and drug delivery systems (Haupt and Mosbach 2000; Wulff 2002; Sellergren 2010). In molecular imprinting, the target molecule acts as the template around which interacting and cross-linking monomers are arranged and co-polymerized in the presence of a porogen to form a cast-like shell. Initially, the monomers form a complex with the template through covalent or noncovalent interactions. Removal of the template leaves a vacant space with recognition sites with high affinity to the target molecule. The shape and size of the imprint and the special arrangement of the functional groups are complementary to the structure of the template molecule (Haupt 2003; Spivak 2005; Hoshino et al. 2010). There are quite a few studies reporting on application of MIPs for selective separation of pharmaceutical residues from water and wastewaters. A cefalexin-imprinted polymer prepared with the formulation acrylamide (as functional monomer) and trimethylpropanol trimethacrylate (TRIM) showed binding capacity of 29.9 μmol/g (Guo and He 2000). Ciprofloxacin and fleroxacin were found in primary and final sewage effluent samples with the contents in the range of 26–87 ng L−1. Duan et al. (2013) prepared a novel multi-templates molecularly imprinted polymer (MIP), using acidic pharmaceuticals mixture [ibuprofen (IBP), naproxen (NPX), ketoprofen (KEP), diclofenac (DFC), and clofibric acid (CA)] as the template, was prepared as solid-phase extraction (SPE) material for enrichment of acidic pharmaceuticals in environmental samples and off-line coupled with liquid chromatography–mass spectrometry (LC/MS/MS). Our earlier studies reported the selective binding of a group of carcinogenic polycyclic aromatic hydrocarbons from environmental samples onto methacrylic acid-co-ethylene glycol dimethacrylate formulated polymers showed analytical merits as well as removal of group of pollutants from environmental waters (Krupadam et al. 2010a, b). Mostly, the imprinted polymers reported in the literature are primarily focused on separation of single compound from a given solution; however, this approach restricts the application imprinted polymers for removal of more than one compound. Each compound requires one imprinted polymer, this causes higher cost of treatment and their wider environmental applications, in particular. In this article, three p-type chemicals were simultaneously imprinted and the merits and feasibility of the polymer in terms of binding capacity, selectivity, and reusability over traditionally used resin and activated carbon were examined.
Materials and methods
P-type pharmaceutical chemicals
The physicochemical properties of three p-type pharmaceutical chemicals imprinted in the polymer are listed in support information (Table SI-1). Nicotine (with purity of 99 %) and epinephrine (97 %) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and physostigmine (99 %) was procured from BOC Sciences (New York, NY, USA) and used as received. Nicotine is a hygroscopic, oily liquid which is miscible in water in its base form. As nicotine enters the body, it is distributed quickly through the bloodstream and crosses the blood-brain barrier reaching the brain within 10–20 s after inhalation. Nicotine acts on the nicotine acetylcholine receptors, specifically the ganglion-type nicotine receptor and central nervous system. Epinephrine is the primary drug of choice for treating bronchoconstriction and hypotension resulting from anaphylaxis as well as all forms of cardiac arrest. Physostigmine is a cholinesterase inhibitor that is rapidly absorbed through membranes and it is widely used in conjunctiva treatment. It has a potential to cross the blood-brain barrier and is used when central nervous system effects are desired, as in the treatment of severe anticholinergic toxicity.
Preparation of polymer adsorbent
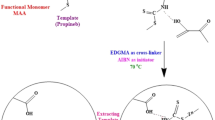
The multi-template imprinted polymer adsorbent was prepared using equimolar concentration of three p-type chemicals (1 mmol each) mixed as the template. The details of polymer composition are listed in Table 1. The polymer precursors, functional monomers itaconic acid and cross-linking monomer, and ethylene glycol dimethacrylate were dissolved in 10 mL of dichloromethane in a 30-ml glass tube. The sealed glass tube containing the reaction mixture was freeze–thaw degassed by submersing the tube in liquid nitrogen and holding the frozen tube under a vacuum of 100 Torr for a period of 15 min for each cycle. The sample was freeze–thaw gassed in this manner for three cycles. The tube was thawed and charged with a positive pressure of argon and was placed in ice-filled Dewar flasks, and temperature was equilibrated. The Dewar flask was then placed under water-cooled medium pressure mercury vapor lamp (550 W) for a period of 18 h. During this time, the tube was turned and the ice in the Dewar flask was changed three times to maintain a constant temperature of 0 °C during the entire polymerization. The polymer monolith formed in the glass tube was mechanically crushed and the templates were extracted with methanol till there were no more template residues in the extracted methanol. The polymer particles in the size range 25–40 μm were separated using sieves and then dried in vacuum at 30 °C for 6 h. The same procedure was followed without a template to prepare the non-imprinted polymer to know the effectiveness of imprinting.
The reference adsorbents, powdered activated carbon (PAC), used in the study was manufactured in Mumbai, India, with a particle size of 10 mesh; and this PAC is used widely in drinking water purification and industrial effluent treatment plants in India. Resin XAD (with the surface area of 300–400 m2 g−1 and 40–60 mesh particle size) was procured from Aldrich (Milwaukee, WI, USA). Before use, resin XAD was thoroughly washed with 4.0 mol l−1 HCl, distilled water, 1.0 mol l−1 NaOH, and distilled water sequentially. Finally, the resin was washed with 20 ml of methanol and dried in vacuum before being used in adsorption experiments.
Surface area and pore distribution of polymer and other adsorbents
The surface area of the adsorbents was measured using surface area analyzer—JEOL JSM 6400 (MA, USA). The surface area measurements were repeated twice for each adsorbent and the experimental error of these measurements was found in the range of 2–5 %. The surface area measurements were performed with both the degassed and non-degassed adsorbents samples. Degassing was achieved by placing the sample under a flow of helium for 2 h at 100 °C. Pore size distribution was measured using a Micrometrics ASAP 2010 machine with nitrogen, utilizing the Barret, Joyner, and Halenda (BJH) methods (ASTM D4641-94).
Adsorption experiments
Adsorption experiments were conducted in batch mode where the adsorbent was allowed to reach equilibrium with p-type chemicals of initial concentrations (C o) ranged between 1 and 10 μg/L at 30 °C. A known quantity of the adsorbent (10 mg) was added to 25-mL glass vials each containing 10 mL of p-type chemicals mix solution. The vials were agitated in an orbital shaker (REMI-410, India) with a constant speed of about 300 rpm. After reaching equilibrium, the supernatant was separated and the concentration of p-type chemicals in the supernatant was measured using a liquid chromatograph coupled with mass spectrometer (LC/MS). Initial experiments showed that the adsorption equilibrium (C e) was achieved within 2 h; then, the content in the glass vials were centrifuged at 15,000 rpm to separate adsorbents and the solution. The concentration of p-type chemicals in solution was quantified using LC/MS and the amount of each p-type chemical bound (C b, millimolar) was determined by subtracting the amount of free each p-type chemical from the total amount of p-type chemical (i.e., C b = C T − Cf) added to the polymer solution. A plot of C b versus C f is often used to graph the adsorption isotherms for the adsorbents. Each experiment was repeated at least three times to establish adsorption properties of each adsorbent for p-type chemicals. The same experimental procedure was followed using the reference adsorbents—non-imprinted polymer, XAD, and PAC.
Regeneration of MIP
Ten milliliters of p-type chemical mix solution (10 μg/L of each p-type chemical) was incubated with 10 mg of imprinted polymer adsorbent at 30 °C while stirring with 300 rpm for 2 h. The contents in the glass vial were centrifuged at 15,000 rpm for 5 min to separate the adsorbent. Then, the supernatant was analyzed for p-type chemicals concentration by LC/MS. The polymer adsorbent settled at the bottom of the glass vial was washed twice with 10 mL of water followed by 10 mL of methanol/water (1/3, v/v), then adsorbent was dried in vacuum to reuse for adsorption of p-type chemicals. Twenty adsorption and desorption cycles were conducted for p-type chemicals to determine re-adsorption capacity of the polymer adsorbent. The similar experimental procedure was followed to determine reusability of other reference adsorbents used in this study.
LC/MS analysis of p-type chemicals
The concentration of p-type chemicals in aqueous solution was determined using LC/MS where the LCMS-2010A system (Shimadzu; Kyoto, Japan) consisting of a LC-10ADvp solvent delivery pump, CTO-10Avp column oven, FCV-12H two-position flow changeover value, and a FCV-13AL six-port flow selection valve were installed. LCMS-2010A single-stage quadrupole MS detection unit, SPD-M10A photo-diode array detector (PDA), SCL-10Avp system controller, and LC/MS solution workstation software were present in the instrument. The mass spectrometer was operated in negative mode electrospray ionization (ESI). The operating conditions of LC/MS were as follows: mobile phase, water/acetonitrile = 70/30 (v/v); flow rate, 0.2 mL/min for analysis; concentrated volume 10 mL; analytical column, Inertsil ODS-3 (150 mm × 2.1 mm).
Results and discussion
Surface properties of the polymer adsorbent
The adsorption of organic pollutants is primarily controlled by the physical and chemical interactions between the adsorbates (p-type chemicals) and the adsorbent. The physical characteristics of the adsorbent control the access of molecules to the finer pores inside the polymer. The accessible adsorbent surface is especially important for the adsorption of high molecular weight pollutants such as p-type chemicals, synthetic dyes, and polycyclic aromatic hydrocarbons.
In the present study, a new polymer adsorbent was prepared using molecular imprinting where the specific binding site (imprinted site) formation leads in improvement of surface area- and molecule-specific pore formation. The pre-polymer complex between template and functional monomer provides information about the nature of imprinted sites formed in the polymer. Based on combinatorial screening and computer simulation procedures, it was concluded that the best polymer precursors for the p-type chemicals imprinted polymers were itaconic acid (functional monomer), ethylene glycol dimethacrylate (cross-linking monomer), and the solvent dichloromethane (please refer Support Information SI-1). After polymerization, the template (p-type chemicals) was extracted from the polymer to leave out specific binding sites which were molecular size- and shape-specific.
The scanning electron microscope and high-resolution transmission electron microscope data showed that the formation of pores in the polymer (Fig. 1a, b). Nitrogen sorption measurement data showed that the polymer has a BET surface area of 510–540 m2 g−1 and cumulative pore volume 0.47–0.53 cm3 g−1. The nitrogen adsorption capacity of the polymer was 0.53 ± 0.4 cm3 g−1. About 90 % of total surface area (500 m2 g−1) of polymer had distribution of nano/microcavities of diameter between 0.2 and 0.4 nm (Fig. 1c). It was found that during nitrogen desorption, the release of nitrogen from the pores of certain size range (20–40 Å) a lower relative pressure could be the formation of pores formed in lower nitrogen saturation pressure (P 0). The wide pore size distribution and lower average pore diameter derived from adsorption plot indicates existence heterogeneous continuum of pores (Fig. 1d). This pore distribution data reveals the size of the pores formed in the polymer had a greater role in p-type chemicals specific adsorption.
Adsorption isotherms
Adsorption isotherms were drawn for the p-type chemicals (Fig. 2a–d), where the adsorption data was well fitted to the Freundlich model, q = K F C W n, by nonlinear regression (weighed on 1/q), where q (milligrams per gram) and C W (micrograms per liter) are the adsorbed quantity and aqueous concentration, respectively. The regression equation with the constants K F, n, and the correlation coefficient R are listed in Table 2. In all equations, the correlation factors (R) were higher than 0.91, represents the relationships are reliable for polymer adsorbents, i.e., P-Mix and XAD. The value of K F, a relative indicator of adsorption capacity in the Freundlich theory, indicates the adsorption capacity of P-Mix polymer was higher than those of XAD and PAC for all the three p-type chemicals. The exponent n was larger than 1 in all cases, represents favorable adsorption of p-type chemicals onto the polymer (Table 3).
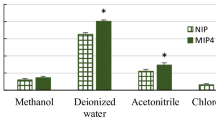
The adsorption capacity of adsorbents was determined at different initial concentrations of p-type chemicals, i.e., 1–10 μg L−1. It was found that when the p-type chemicals concentration was 10 μg/L, the adsorption capacities of the polymer for all three p-type chemicals were about 60 and 40 % higher than the XAD and PAC, respectively, while the p-type chemicals initial concentration was 1 μg/L. The adsorption capacity of the polymer was about 70 % higher than the other adsorbents. The goodness of fit was validated by obtaining correlation constant values (R 2) in the range of 0.968 to 0.981. The standard deviation calculated using SigmaPlot 7 was reported as the error in the fitting parameters; and the fit of the data to Freundlich isotherm showed the heterogeneity of the polymer adsorbent surface. An interesting aspect in molecular imprinting is the specific binding site formation in the polymer. The average pore diameter in the polymer was 24 nm which is quite smaller than the pore diameter measured in XAD (i.e., 92 nm). However, the significant difference in adsorption capacity was not related to the specific surface areas only. Contrary to the P-Mix polymer, it has predominant contribution of micro/nanopores, which favors a solute-solute interaction for p-type chemicals according to the capillary condensation theory (Neimark et al. 2003). Besides, the macro- and mesopores in XAD and PAC could be facilitating adsorbate diffusion process inside polymer particles. In contrast, for p-type chemicals, commercial microporous activated carbon is not a suitable adsorbent, suffering from low adsorption affinity due to the size exclusion effect and slow adsorption kinetics caused by highly disordered pore structure. In this study, the commercially used activated carbon was meso/microporous (40/60 %) in nature. The comparison of pore dimension of PAC with the cross-sectional area of p-type chemicals restricts the binding capacity of the PAC. The unique, ordered, and well-constructed 3-D structures of template-synthesized polymer makes the polymer adsorbent prepared in this study superior in adsorption capacity and fast adsorption kinetics compared with other reference adsorbents.
Apart from the surface area and pore properties of the polymer, the stacking of p-type chemicals on the polymer surface would be one of the reasons for higher adsorption capacity of polymer. When unsaturated organic groups are involved in non-covalent interactions, the “π-π stacking”, or more generally “p–p interactions” are more prevalent. Larger structures consisting of nitrogen atoms favors π-π stacking results in adsorbing more molecules for one binding site of the adsorbent. Experimental observations in this study indicate that the pore structure of the polymers play a crucial role in p-type chemicals adsorption. Therefore, the adsorption capacity and affinity to the polymer adsorbent (P-Mix) can be even further improved by precisely adjusting the adsorbent pore size specific to the targeted pollutants.
Adsorption mechanism between p-type chemicals and polymer adsorbents
The interactions between the p-type chemicals and the functional monomers are primarily responsible for formation of binding sites in the molecularly imprinted polymers. Selectivity for molecules arises from constraints imposed by the pore size and shape; molecules larger than a pore diameter are effectively excluded from the internal spaces. Molecules sufficiently small to enter the pore spaces interact with the polymer to varying extents based on van der Waals and other electrostatic interactions that will be a function of the size, shape and binding site functionality. It was theoretically found (based on ab initio computations) that there was a strong H-bonding and π-electron-dependent polarizable interactions were predominant, even though they are not as strong as the interaction associated with pyridine and its derivatives (Fig. 3). The workstation used to simulate p-type chemicals and the polymer functionalities was a DELL Precision T7500 running a Windows XP operating system, CPU—Intel (R) Xeon (R) 2.80 GHz/2.79 GHz Dual Processors, and 24 GB of RAM (memory), and a 4-TB hard disk. This system was used to run the software Gaussian 4.1 ver. and HyperChem Rel. 8.0. The geometric optimization was carried out on the semi-empirical (SE) quantum mechanical approach (PM3 method) to obtain optimized energy structures with aid of the Polak–Ribiere algorithm until the root mean square gradient was 0.01; which is acceptable in molecules optimization. The features of parameterization and calculation based on valence electrons, makes it fast and reliable for computational studies. Single-point calculations were performed on the optimized molecules. The single-point calculation gives the static properties of a molecule. The properties include potential energy, derivatives of the potential energy, electrostatic potential, molecular orbital energies, and the coefficient of molecular orbital for ground or excited state. The input molecular structure for a single-point calculation usually reflects the coordinates of a stationary points on the potential energy surface, typically a minimum or transition state. The binding energies of the molecules were then noted. The nature of bonds between the functional monomer and the p-type chemicals that were described based on computer simulations are as follows:
-
1.
Each molecule of p-type chemical has potential to form at least two H-bonds with each functional site of the polymer
-
2.
H-bonding between nicotine and polymer was strongest while physostigmine and polymer was weakest; however, the H-bonds are sufficiently enough strength to bind p-type chemicals onto the polymer.
-
3.
Among the three p-type chemicals tested, nicotine is most hydrophobic and has the highest adsorption capacities on polymer. This is in agreement with the previous report (Krupadam et al. 2007), where high hydrophobicity was found to favor the adsorption of organic compound onto the nonpolar and moderate polar polymeric adsorbents from aqueous solutions.
Reusability of the polymer adsorbents
Treatment cost is a major concern in water purification and wastewater treatment. The reusable adsorbents will reduce the cost of the treatment drastically. The reversibility of adsorption depends on whether there is a strong binding bond such as ionic or covalent or weak binding forces such as van der Waals forces or a dipole-dipole interaction formed between the adsorbent and the adsorbate. Based on adsorption assay and computer simulations discussed above, it was found that the weak binding forces (hydrogen bonding and π-π interactions) exist between the p-type chemicals and the polymer adsorbent. These types of interactions favor easy adsorption/desorption phenomena. The reusability experiments demonstrated that the polymer adsorbent has potential to reuse at least 10 times for p-type chemicals in aqueous solutions without losing adsorption capacity (Fig. 4). The polymer was regenerated by washing with water/methanol (9/1, v/v). Although the polymer adsorbent was regenerated 10 times, the removal efficiencies for the three p-type chemicals were maintained at more than 90 %, and no decreasing trend was observed. These experiments further demonstrated that the polymer adsorbent retains molecule-specific memory for selective extraction of the p-type chemicals after washing with water/methanol solution. The reusability of the polymer adsorbent was one of the most desirable properties of the adsorbents which reduces the energy and cost of the treatment. The polymer adsorbent has merits such as adsorption capacity and selectivity over single-use activated carbon and other commercially used materials.
Conclusions
The research work reports on the application of multi-template MIPs for the selective adsorption of three p-type pharmaceutical chemicals simultaneously from aqueous solutions. Computer simulations were used to understand the nature of binding between the functional monomer and the p-type chemicals during adsorption. The merits of the adsorbent over the conventional adsorbents were listed below:
-
(a)
The adsorption capacity of the polymer adsorbent was 11.32 mg g−1; which is four times higher than the conventional adsorbents such as resin XAD and PAC.
-
(b)
The adsorption kinetic experiments proved that the p-type chemicals adsorption is quite faster than the conventional adsorbents. About 60 % of the p-type chemicals were adsorbed on the polymer adsorbent within 10 min. This would be beneficial in handling high volume of wastewater in real-world applications.
-
(c)
Compared to one-time-use conventional adsorbents, the polymer adsorbent showed reusability about 10 times without losing any adsorption capacity, and the polymer was regenerated by washing water/methanol solution.
Although several experimental variables can affect the adsorption/desorption process, once operating parameters were optimized, the use of polymer adsorbents offers significant practical applications in water purification and wastewater treatment.
References
Bureau of Water, Illinois EPA. Report on pharmaceuticals and personal care products in Illinois Drinking water, June 2008
Demirbas A (2008) Heavy metals and organic pollutants adsorption onto agro-based waste materials: A review. J Hazard Mater 157:220–229
Duan YP, Dai CM, Zhang YL, Chen L (2013) Selective trace enrichment of acidic pharmaceuticals in real water and sediment samples based on solid phase extraction using multi-templates molecularly imprinted polymers. Anal Chim Acta 758:93–100
Focazioa MJ, Kolpinb DW, Barnesb KK, Furlongc ET, Meyerd MT, Zauggc SD, Barbere LD, Thurman ME (2008) A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States—II): untreated drinking water sources. Sci Total Environ 402:201–216
Fontela MH, Galceran MT, Ventura F (2008) Stimulatory drugs of abuse in surface waters and their removal in a conventional drinking water treatment plant. Environ Sci Technol 42:6809–6816
Guo H, He X (2000) Study of the binding characteristics of molecularly imprinted polymer selective for cefalexin in aqueous media. Fres J Anal Chem 368:461–465
Haupt K (2003) Imprinted polymers—tailor-made mimics of antibodies and receptors. Chem Commun 171–178
Haupt K, Mosbach K (2000) Molecularly imprinted polymers and their use in biomimetic sensors. Chem Rev 100:2495–2504
Heberer T (2002) Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131:5–17
Hoshino Y, Haberaecker WW III, Kodama T, Zeng Z, Okahata Y, Shea KJ (2010) Affinity purification of multifunctional polymer nanoparticles. J Am Chem Soc 132:13648–13650
Khalid M, Joly G, Renaud A, Magnoux P (2004) Removal of phenol from water by adsorption using zeolites. Ind Eng Chem Res 43:5275–5280
Krupadam RJ, Ahuja R, Wate SR (2007) Benzo[a]pyrene imprinted polyacrylate nanosurfaces: adsorption and binding characteristics. Sensors Actuators B: Chem 124:444–451
Krupadam RJ, Khan MS, Wate SR (2010a) Removal of probable human carcinogenic polycyclic aromatic hydrocarbons from contaminated water using molecularly imprinted polymers. Water Res 43:681–688
Krupadam RJ, Bhagat B, Khan MS (2010b) Highly sensitive determination of polycyclic aromatic hydrocarbons in ambient air dust by gas chromatography-mass spectrometry after molecularly imprinted polymer extraction. Anal Bioanal Chem 397:3097–3106
Lin S-H, Juang R-S (2009) Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manag 90:1336–1349
Mathew G, Unnikrishnan MK (2012) The emerging environmental burden from pharmaceuticals. Econ Polit Wkly 5:31–34
Maurer M, Eschera BD, Richle P, Schaffner C, Alder AC (2007) Elimination of ß-blockers in sewage treatment plants. Water Res 41:1614–1622
Neimark AV, Ravikovitch PI, Vishnyakov A (2003) Bridging scales from molecular simulations to classical thermodynamics: density functional theory of capillary condensation in nanopores. J Phys: Cond Matter 15:347–353
Pan B, Mashayekhi H, Xing B (2008) Adsorption and hysteresis of bisphenol A and 17α-ethinyl estradiol on carbon nanomaterials. Environ Sci Technol 42:5480–5485
Ren X, Chen C, Nagatsu M, Wang X (2011) Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem Engg J 170:395–410
Reungoat J, Macova M, Escher BI, Carswell S, Mueller JF, Keller J (2010) Removal of micropollutants and reduction of biological activity in full scale reclamation plant using ozonation and activated carbon filtration. Water Res 44:625–637
Sellergren B (2010) Shaping enzyme inhibitors. Nat Chem 2:7–8
Shen X, Zhu L, Wang N, Ye L, Tang H (2012) Molecular imprinting for removing highly toxic organic pollutants. Chem Commun 48:788–798
Spivak DA (2005) Optimization, evaluation, and characterization of molecularly imprinted polymers. Adv Drug Del Rev 57:1779–1794
Wulff G (2002) Enzyme-like catalysis by molecularly imprinted polymers. Chem Rev 102:1–28
Acknowledgments
Financial support from Council of Scientific & Industrial Research (CSIR), New Delhi is gratefully acknowledged under Molecular Environmental Science and Engineering Research Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 646 kb)
Rights and permissions
About this article
Cite this article
Venkatesh, A., Chopra, N. & Krupadam, R.J. Removal of acutely hazardous pharmaceuticals from water using multi-template imprinted polymer adsorbent. Environ Sci Pollut Res 21, 6603–6611 (2014). https://doi.org/10.1007/s11356-014-2566-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2566-8