Abstract
Bioremediation using isolated anti-cyanobacterial microorganism has been widely applied in harmful algal blooms (HABs) control. In order to improve the secretion of activated anti-cyanobacterial substances, and lower the cost, a sequential optimization of the culture medium based on statistical design was employed for enhancing the anti-cyanobacterial substances production and chlorophyll a (Chl a) removal by Streptomyces sp. HJC-D1 in the paper. Sucrose and KNO3 were selected as the most suitable carbon and nitrogen sources based on the one-at-a-time strategy method, and sucrose, KNO3 and initial pH were found as major factors that affected the anti-cyanobacterial ability of the isolated stain via the Plackett–Burman design. Based on the response surface and canonical analysis, the optimum condition of culture medium was obtained at 22.73 g l-1 of sucrose, 0.96 g l-1 of KNO3, and initial pH 8.82, and the Chl a removal efficiency by strain HJC-D1 increased from 63 ± 2 % to 78 ± 2 % on the optimum conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Harmful algal blooms (HABs) occur frequently in lakes and reservoirs all over the world, which cause damage to the biodiversity and equilibrium of aquatic ecosystems via toxins release (Pitois et al. 2001; Qin et al. 2006; Rapala et al. 2002) and increase the risk in drinking water sources (Hitzfeld et al. 2000; Rapala et al. 2002; Chen et al. 2009). Chemical agents such as copper sulfate and aluminum sulfate, are often used as the emergency reagents for modifying contaminated aquatic ecosystem (Chow et al. 1999; Garcia-Villada et al. 2004). However, these methods induce potential secondary pollution. In recent years, researchers have paid much attention to eutrophication issues by applying biological and ecological technologies, which are economical and environmental friendly, especially in field of microbial algal inhibition such as bacteria (Lovejoy et al. 1998; Choi et al. 2005; Shi et al. 2006; Hua et al. 2009; Wang et al. 2010; Kong et al. 2013d) and fungi (Redhead and Wright 1978; Jia et al. 2011).
Numerous studies showed that the growth and activity of microorganisms were influenced by the composition of the substrate (C and N sources, inorganic salts) and growth conditions such as temperature, pH, and oxygen (Rao et al. 2007; Song et al. 2007; Purama and Goyal 2008; Sansonetti et al. 2010). As several parameters are involved, it is difficult to identify the critical factors and to optimize them for biotechnological processes. Compared with conventional methods such as one-at-a-time strategy, response surface methodology (RSM) is taken as an useful mathematical and statistical technique for searching optimum condition, as it could provide statistical model and design experiment for revealing the interaction among the relative factors (He et al. 2009; Gao et al. 2009; Bankar and Singhal 2010). It has been successfully applied in many aspects such as medium and fermentation conditions optimization for improving the biomass production (Song et al. 2007; Manikandan et al. 2009; Purama and Goyal 2008) and antimicrobial activity production (Tabbene et al. 2009), identifying the influential priority of factors governing anaerobic H2 production (Fang et al. 2010), as well as palm oil mill effluent treatment in an upflow anaerobic sludge fixed film bioreactor (Zinatizadeh et al. 2006). Therefore, RSM is preferred to enhance biological anti-cyanobacterial technology by optimizing the medium components of anti-cyanobacterial microorganism.
A bacterial strain identified as Streptomyces sp. HJC-D1, with remarkable anti-cyanobacterial activity against bloom-forming cyanobacterium Microcystis aeruginosa, was isolated from an eutrophic pond in Hangzhou, China (Kong et al. 2013a, b, c). In our previous study, the variations of cell integrity, physiological characteristics, and antioxidant enzymes activities of M. aeruginosa cells by Streptomyces sp. HJC-D1 were investigated (Kong et al. 2013a, b), and the biodegradation products of M. aeruginosa were illustrated (Kong et al. 2013c). In order to improve the secretion of activated anti-cyanobacterial substances and lower the cost, one-at-a-time strategy design, two stages of Plackett–Burman (PB) design and central composite design (CCD) were applied to study the effect of different medium composition and pH on the inhibition of M. aeruginosa by Streptomyces sp. HJC-D1, and it is expected to establish the optimizing cultivation strategy of strain HJC-D1 for efficient cyanobacterial inhibition.
Materials and methods
Target algae, microorganism, and culture condition
M. aeruginosa FACHB-905 was purchased from the Freshwater Algae Culture Collection of Institute of Hydrobiology (FACHB), Chinese Academy of Sciences (Wuhan, China). Before used as inoculants, it was cultured for 7 days to reach the log phase, and the culture condition was as follow: sterilized BG11 medium (Rippka et al. 1979); 2,000 lux white light, light:dark = 14 h:10 h; 25 °C.
The strain HJC-D1 used in this study was isolated from an eutrophication pond in Hangzhou, China, which had excellent anti-cyanobacterial activity (Kong et al. 2013a, b, c). Based on the 16S rRNA gene sequence analysis, it was identified as Streptomyces sp. The seed was maintained at 4 °C in a Gause's synthetic agar medium. The inoculum was prepared by incubating the seed culture at 28 °C with a shaking speed of 150 rpm for 72 h. For anti-cyanobacterial substances production, the prepared inoculum was transferred to a fermentation medium at a ratio of 1:20 and incubated at 28 °C with a shaking speed of 150 rpm for 4 days. The fermentation culture medium (g l-1) contained: 20 of soluble starch, 1.0 of KNO3, 0.5 of NaCl, 0.5 of K2HPO4 · 3H2O, 0.5 of MgSO4 · 7H2O and 0.002 of FeSO4 · 7H2O. The initial pH of the medium was adjusted to 7.0 by 1 M HCl or 1 M NaOH. In the experiment of medium optimization, the concentration of each component including pH value was changed according to what is required (see Tables 1 and 2). Each Erlenmeyer flask (250 ml) containing 100 ml medium was used for both seed culture and anti-cyanobacterial substances production.
Anti-cyanobacterial activity test
The anti-cyanobacterial effects were studied by adding 5 ml Streptomyces sp. HJC-D1 fermentation broth to a 250 ml sterilized conical beaker with 95 ml BG11 medium containing M. aeruginosa cells at a chlorophyll a (Chl a) concentration of 62.7 ± 7.4 μg l-1 for the control group, 5 ml Streptomyces sp. HJC-D1 fermentation broth was replaced by 5 ml Gause's synthetic medium. Before addition, the fermentation broth of Streptomyces sp. HJC-D1 was treated as the following procedure: centrifuged at 10,000 × g for 10 min; filtered through a 0.22 μm cellulose acetate membrane for cell-free filtrate acquisition. Finally, the cell-free filtrate was added into M. aeruginosa culture for cyanobactericidal activity test (Kong et al. 2013b, c, d). The whole experiments were performed in aseptic workbench, and all the samples and controls were incubated under the pre-set conditions described in “Target algae, microorganism, and culture condition” section. As Chl a is used as an index for Microcystis biomass and the changes in Microcystis biomass are used to demonstrate effects of inhibitory substances, the Chl a concentration was determined after 4 days' incubation. Each treatment was replicated three times, and the arithmetical means (±SD) were obtained.
The concentration of Chl a was determined by spectrophotometric method using 90 % acetone extraction (APHA 1998). The removal efficiency of Chl a was calculated according to the following equation:
where C 0 and C t are the Chl a concentrations of M. aeruginosa in the control and test groups at initial and time t, respectively (Kong et al. 2013a, c).
Experimental design and data analysis
Based on the advantages of one-at-a-time strategy design, PB design and CCD (Mao et al. 2005; Badhan et al. 2007; Fu et al. 2009), the present study combined all methods to optimize bioprocess. Three steps consisted: (1) The one-at-a-time strategy design was applied to select the best carbon and nitrogen sources for the growth of anti-cyanobacterial microorganism; (2) The preliminary analysis was applied to identify the proper range of the culture medium composition; and (3) the CCD aimed at determining the culture condition.
One-at-a-time strategy
Effects of carbon and nitrogen sources and culture temperature on the removal efficiency of Chl a by strain HJC-D1 were estimated using one-at-a-time strategy design. Various kinds of carbon sources (soluble starch, glucose, sucrose, sodium acetate, and glycerol) and nitrogen sources (potassium nitrate, ammonium sulfate, peptone, beef extract, yeast extract, and carbamide) were investigated for the inhibition effect of M. aeruginosa individually at 25 °C. The removal efficiency of Chl a was determined after cultivation for 4 days. Triplicate experiments were carried out for each treatment.
PB design
PB design was employed for screening the most significant medium compositions. Each independent variable was tested at two levels of high and low, which are denoted by (+) and (-), respectively. The levels of each factor are listed in Table 1. A 12-run experiment was generated by the software package of Design Expert 7.0 (Stat-Ease, Minneapolis, MN, USA; Table 2). All experiments were conducted in triplicate. Data were pooled and analyzed by Design Expert 7.0. The factors which were significant at 5 % level (P < 0.05) were considered to have significant effect on the removal efficiency of Chl a (Table 3).
Central composite design
PB design is usually used as the first step to screen significant factors from a number of variables, which is generally followed by CCD (Lakshmikanth et al. 2006; Fu et al. 2009; Gao et al. 2009; Liu et al. 2012). After the critical medium components were selected by PB design, RSM was employed to optimize the component concentrations for maximizing the anti-cyanobacterial effect of strain HJC-D1. Three components of sucrose, KNO3, and initial pH, were optimized by RSM with a three-factor, five-level CCD. The factors and respective codes and actual levels were given in Table 4. A 20-run experiment generated by Design Expert 7.0 was carried out with eight factorial points, six axial points, and six trials at the center point (shown in Table 5). All experiments were conducted in triplicate. The mean removal efficiency of Chl a was taken as the response. In order to correlate the response variable to the independent variables, the Chl a removal efficiency was fitted according to the following second-order polynomial model:
where Y represents the response variable (removal efficiency, %), X i and X j are the coded independent factors, b 0 is the interception coefficient, b i is the coefficient of linear effect, b ii is the coefficient of quadratic effect, and b ij is the coefficient of interaction effect when i < j and k, the numbers of involved variables. Statistical analysis of the model, each independent variable and interactions between the variables was performed to evaluate the analysis of variance (ANOVA) by Design Expert 7.0.
Results and discussion
Optimization of carbon and nitrogen sources
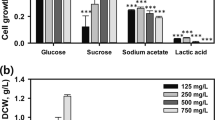
The effects of carbon and nitrogen sources on the growth of Streptomyces sp. HJC-D1 and the inhibition effect of Streptomyces sp. HJC-D1 on M. aeruginosa were determined for screening appropriate nutrient substrates. As shown in Fig. 1, the highest growth rate of M. aeruginosa (Chl a = 929.4 ± 25.6 μg l-1) and the lowest removal efficiency of 7 ± 2 % were achieved using glucose as the carbon source, while the lowest growth rate (Chl a = 430.2 ± 12.8 μg l-1) and the highest removal efficiency of 57 ± 3 % were obtained in the media containing sucrose. To assess the effect of nitrogen source on the inhibition effect of M. aeruginosa, various nitrogen sources including inorganic and organic compounds were tested at a final concentration of 0.1 % (w/v; Fig. 2). Results showed that there was no remarkable difference between various nitrogen sources. The effectiveness of these nitrogen sources may be due to their ability to serve as direct sources of amino acids for the synthesis of the antimicrobial metabolites and/or might be implicated as antibiotic precursors (Tabbene et al. 2009). In consideration of the application feasibility and cost, KNO3 was used in further studies.
Screening of the significant factors by PB design
Based on the above results, sucrose and KNO3 were selected to be the optimal carbon and nitrogen sources for the determining variables. Therefore, PB design was used to screen the significant factors responsible for algicidal activity of M. aeruginosa by strain HJC-D1. A seven-factor, 12-run PB design was conducted, and the response for each run was presented in Table 2. Regression analysis was performed to evaluate the significance of each factor. The results of PB experiments showed both positive and negative dispersion of values (Table 3). On the basis of the calculated F values and P values (Table 3), sucrose, KNO3, and initial pH were found to be the most significant variables affecting the inhibition of M. aeruginosa.
Optimization of significant factors by CCD
To examine the combined effects of sucrose, KNO3 and initial pH, 20 treatments with these independent variables were established using computer simulation with Eq. 3. The CCD of the experiment and respective experimental concentrations were presented in Table 4.
The following quadratic regression equation was obtained to describe the removal efficiency of Chl a:
where X 1, X 2, and X 3 are the coded values of sucrose, KNO3, and initial pH, respectively.
The actual and predicted values of Chl a removal efficiency based on CCD experimental design were shown in Table 5, and the ANOVA test for response surface quadratic model was summarized in Table 6. The ANOVA of the quadratic regression model demonstrated that the model was highly significant, which was indicated by the F value of 30.22 and a low probability value of [(P > F) < 0.0001]. The fit goodness (R 2) of the model was 0.9654, which indicated 96.54 % of the variability in response, could be explained by this model. The adjusted R 2 value was also good at 93.26 %. At the same time, the coefficient of variation (CV = 2.44 %) indicated a better precision of the experiments. Nevertheless, the predicted R 2 value of 72.92 % was not as close to the adjusted R 2 value of 93.26 %. It was probably due to a large block effect.
The significance of each coefficient was determined by F value and P value. The larger the magnitude of the F value and smaller the P value, the more significant was the corresponding coefficient. As shown in Table 7, X 1, X 3, X 1 2, and X 3 2 were significant at the 99 % probability level (P < 0.0001), while X 2, X 2 × X 3, and X 2 2 were significant at the 95 % probability level (P < 0.05). In contrast, X 1 × X 2 and X 1 × X 3 had no significant influence on the removal efficiency of Chl a (P > 0.05).
To further assess the effect of independent variables on the removal efficiency of Chl a, three-dimensional response surface was used to demonstrate relationships between the response and experimental levels of each variable. Fig. 3 showed the response surface plots and their respective contour plots of Chl a removal. Each contour curve represented the effect of two test factors, while the other factor was held at zero level.
It could be seen in Fig. 3a that the removal efficiency of Chl a increased with the increase of sucrose concentration, while it was not obviously improved with the increase of KNO3 concentration. In Fig. 3b, the removal efficiency of Chl a depended on both sucrose and initial pH, which increased from 69 % to 86 % when the initial pH was from 6.0 to 9.0. Fig. 3c showed the removal efficiency as a function of KNO3 concentration and initial pH, and the maximum removal efficiency achieved in the middle range of the corresponding variables. As shown in the surface plots, only the interaction between KNO3 (X 2) and pH (X 3) was significant (P = 0.0113). Surprisingly, lower or higher concentration of sucrose (X 1) and KNO3 (X 2) had negative effect on Chl a removal efficiency, and the interaction between sucrose (X 1) and KNO3 (X 2) was not significant (P = 0.2431), indicating that the C/N ratio did not play a key role in the growth inhibitory effect of M. aeruginosa.
Determination of inhibitory effect in the optimized medium
According to the canonical analysis, the optimal levels of three variables determined by Design Expert 7.0 were 21.73 g l-1 of sucrose, 0.96 g l-1 of KNO3, and 8.82 of initial pH, corresponding to the maximum Chl a removal efficiency of 80 %. To evaluate the model applicability in the anti-cyanobacterial effect of strain HJC-D1, the experiment that using the calculated level of the variables was carried out in a cultured system. The growth inhibitory effect of M. aeruginosa by strain HJC-D1 in the optimized medium was compared to that in Gause's synthetic agar media (Fig. 4). In the Gause's synthetic agar media the removal efficiency of Chl a was 63 ± 2 % at the 4th day, while it reached 78 ± 2 % in the optimized media (15 % higher than the former). Results revealed that strain HJC-D1 had excellent anti-cyanobacterial activity by producing anti-cyanobacterial substances during short fermentation period in the optimized culture medium.
Biotic interactions are considered to be one of the potentially biological control in the dramatics termination of cyanobacterial bloom (Lovejoy et al. 1998; Qin et al. 2006; Tang et al. 2012), prompting the isolation and characterization of algicidal bacteria capable of controlling the bloom-forming cyanobacterium M. aeruginosa (Choi et al. 2005; Hua et al. 2009; Kong et al. 2013b, d). Several anti-cyanobacterial bacteria have been reported and the studies focused on the effects of anti-cyanobacterial bacteria on M. aeruginosa, and the factors which are essential for controlling the outbreak and disappearance of HABs (Lovejoy et al. 1998; Shi et al. 2006; Wang et al. 2010). However, there is not any work related to the optimization of anti-cyanobacterial substances production of anti-cyanobacterial microorganisms.
It is showed that statistical designs, one-factor-at-a-time design followed by statistical designs of the PB design and CCD have been effectively for screening the significant factors and obtaining the optimal medium in shaker flask cultivation (Lakshmikanth et al. 2006; Fu et al. 2009; Gao et al. 2009; Liu et al. 2012). It was found that yeast extract was the optimal nitrogen source for agarase production by Agarivorans albus YKW-34 (Fu et al. 2009), while NaNO3 was the optimal nitrogen source for agarase production by Acinetobacter sp. AG LSL-1 (Lakshmikanth et al. 2006), which was consisted with the conclusion that KNO3 was selected as the nitrogen source in our present study (Fig. 2).
Based on the PB design, sucrose, KNO3 and initial pH were selected as the most significant variables that influence the anti-cyanobacterial substances production. It was also reported that agar, yeast extract, and initial pH had a significant effect on agarase production by A. albus YKW-34 (Fu et al. 2009), and sucrose, ammonium nitrate, and NaH2PO4 had significant effects on C15-surfactin production from Bacillus amyloliquefaciens (Liu et al. 2012). However, PB design could only screen significant factors, it was not adequate to determine the optimum levels of these factors (Fu et al. 2009; Gao et al. 2009). Thus, further optimization was carried out by CCD in this study, and it was noteworthy that Streptomyces sp. HJC-D1 produced anti-cyanobacterial substances efficiently with a 15 % higher anti-cyanobacterial activity in the optimized fermentation medium.
Our present study focused on anti-cyanobacterial substances production not only due to its effective biological activities on bloom-forming cyanobacterium M. aeruginosa, but also because the anti-cyanobacterial strain Streptomyces sp. HJC-D1 was isolated from an eutrophic pond which could be a good potential for natural eutrophication control. It takes the first step in enhancing anti-cyanobacterial effect and improving anti-cyanobacterial substances production secreted by Streptomyces sp. HJC-D1. Nevertheless, regardless of medium and culture condition optimization, the ecological safety and anti-cyanobacterial ability of Streptomyces sp. HJC-D1 in natural environment should be fully considered. In this respect, the present study is useful for the further investigation of the industrial production of anti-cyanobacterial substances and purification of the anti-cyanobacterial compounds. With a long-term goal of developing an effective method for controlling HABs, further studies will focus on identifying the active anti-cyanobacterial compounds and revealing the inhibition mechanism by Streptomyces sp. HJC-D1.
Conclusions
Application to optimize the major components of culture medium for improving the secretion of activated anti-cyanobacterial substances of strain Streptomyces sp. HJC-D1, the one-at-a-time strategy design, PB design and CCD design were used. The optimized composition of the medium for Streptomyces sp. HJC-D1 fermentation was as follows (g l-1): sucrose 22.73, KNO3 0.96, NaCl 0.5, K2HPO4 0.5, MgSO4 0.5, FeSO4 0.002, and initial pH of 8.82. Under this optimization strategy, the removal efficiency of Chl a obtained by Streptomyces sp. HJC-D1 reached 78 ± 2 %. Results showed that the use of optimized medium improve the anti-cyanobacterial substances production by Streptomyces sp. HJC-D1 obviously, and the anti-cyanobacterial ability of strain HJC-D1 on M. aeruginosa is enhanced.
References
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association (APHA)
Badhan AK, Chadha BS, Kaur J, Saini HS, Bhat MK (2007) Production of multiple xylanolytic and cellulolytic enzymes by thermophilic fungus Myceliophthora sp IMI 387099. Bioresour Technol 98(3):504–510. doi:10.1016/j.biortech.2006.02.009
Bankar SB, Singhal RS (2010) Optimization of poly-epsilon-lysine production by Streptomyces noursei NRRL 5126. Bioresour Technol 101(21):8370–8375. doi:10.1016/j.biortech.2010.06.004
Chen FZ, Song XL, Hu YH, Liu ZW, Qin BQ (2009) Water quality improvement and phytoplankton response in the drinking water source in Meiliang Bay of Lake Taihu, China. Ecol Eng 35(11):1637–1645. doi:10.1016/j.ecoleng.2008.01.001
Choi HJ, Kim BH, Kim JD, Han MS (2005) Streptomyces neyagawaensis as a control for the hazardous biomass of Microcystis aeruginosa (Cyanobacteria) in eutrophic freshwaters. Biol Control 33(3):335–343. doi:10.1016/j.biocontrol.2005.03.007
Chow CWK, Drikas M, House J, Burch MD, Velzeboer RMA (1999) The impact of conventional water treatment processes on cells of the cyanobacterium Microcystis aeruginosa. Water Res 33(15):3253–3262
Fang F, Zeng RJ, Sheng GP, Yu HQ (2010) An integrated approach to identify the influential priority of the factors governing anaerobic H2 production by mixed cultures. Water Res 44(10):3234–3242. doi:10.1016/j.watres.2010.03.001
Fu XT, Lin H, Kim SM (2009) Optimization of medium composition and culture conditions for agarase production by Agarivorans albus YKW-34. Proc Biochem 44(10):1158–1163. doi:10.1016/j.procbio.2009.06.012
Gao H, Liu M, Liu JT, Dai HQ, Zhou XL, Liu XY, Zhuo Y, Zhang WQ, Zhang LX (2009) Medium optimization for the production of avermectin B1a by Streptomyces avermitilis 14-12A using response surface methodology. Bioresour Technol 100(17):4012–4016. doi:10.1016/j.biortech.2009.03.013
Garcia-Villada L, Rico M, Altamirano M, Sanchez-Martin L, Lopez-Rodas V, Costas E (2004) Occurrence of copper resistant mutants in the toxic cyanobacteria Microcystis aeruginosa: characterisation and future implications in the use of copper sulphate as algaecide. Water Res 38(8):2207–2213. doi:10.1016/j.watres.2004.01.036
He J, Zhen QW, Qiu N, Liu ZD, Wang BJ, Shao ZZ, Yu ZN (2009) Medium optimization for the production of a novel bioflocculant from Halomonas sp V3a' using response surface methodology. Bioresour Technol 100(23):5922–5927. doi:10.1016/j.biortech.2009.06.087
Hitzfeld BC, Hoger SJ, Dietrich DR (2000) Cyanobacterial toxins: removal during drinking water treatment, and human risk assessment. Environ Health Pers 108:113–122. doi:10.2307/3454636
Hua XH, Li JH, Li JJ, Zhang LH, Cui Y (2009) Selective inhibition of the cyanobacterium, Microcystis, by a Streptomyces sp. Biotechnol Lett 31(10):1531–1535. doi:10.1007/s10529-009-0051-0
Jia Y, Han GM, Wang CY, Guo P, Jiang WX, Li XN, Tian XJ (2011) The efficacy and mechanisms of fungal suppression of freshwater harmful algal bloom species. J Hazard Mater 183(1–3):176–181. doi:10.1016/j.jhazmat.2010.07.009
Kong Y, Chen J, Xu XY, Zhu L (2013a) Spectra characteristic of degradation products and inhibition mechanism of Streptomyces sp HJC-D1 on Microcystis aeruginosa. Spectrosc Spectr Anal 33:167–171
Kong Y, Xu XY, Zhu L (2013b) Cyanobactericidal effect of Streptomyces sp HJC-D1 on Microcystis auruginosa. PLoS ONE 8(2):e57654
Kong Y, Zhu L, Zou P, Qi JQ, Yang Q, Song LM, Xu XY, Miao LH (2013c) Isolation and characterization of dissolved organic matter fractions from antialgal products of Microcystis aeruginosa. Environ Sci Pollut Res. doi:10.1007/s11356-013-2114-y
Kong Y, Zou P, Yang Q, Xu XY, Miao LH, Zhu L (2013d) Physiological responses of Microcystis aeruginosa under the stress of antialgal actinomycetes. J Hazard Mater 262:274–280
Lakshmikanth M, Manohar S, Souche Y, Lalitha J (2006) Extracellular b-agarase LSL-1 producing neoagarobiose from a newly isolated agar-liquefying soil bacterium, Acinetobacter sp. AG LSL-1. World J Microbiol Biotechnol 22:1087–1094
Liu XY, Ren B, Gao H, Liu M, Dai HQ, Song FH, Yu ZY, Wang SJ, Hu JC, Kokare CR, Zhang LX (2012) Optimization for the production of surfactin with a new synergistic antifungal activity. PLoS ONE 7(5):e34430. doi:10.1371/journal.pone.003443
Lovejoy C, Bowman JP, Hallegraeff GM (1998) Algicidal effects of a novel marine Pseudoalteromonas isolate (class Proteobacteria, gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Appl Environ Microbiol 64(8):2806–2813
Manikandan M, Pasic L, Kannan V (2009) Optimization of growth media for obtaining high-cell density cultures of halophilic archaea (family Halobacteriaceae) by response surface methodology. Bioresour Technol 100(12):3107–3112. doi:10.1016/j.biortech.2009.01.033
Mao XB, Eksriwong T, Chauvatcharin S, Zhong JJ (2005) Optimization of carbon source and carbon/nitrogen ratio for cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Proc Biochem 40(5):1667–1672. doi:10.1016/j.procbio.2004.06.046
Pitois S, Jackson MH, Wood BJB (2001) Sources of the eutrophication problems associated with toxic algae: an overview. J Environ Health 64(5):25–32
Purama RK, Goyal A (2008) Screening and optimization of nutritional factors for higher dextransucrase production by Leuconostoc mesenteroides NRRL B-640 using statistical approach. Bioresour Technol 99(15):7108–7114. doi:10.1016/j.biortech.2008.01.032
Qin BQ, Yang LY, Chen FZ, Zhu GW, Zhang L, Chen YY (2006) Mechanism and control of lake eutrophication. Chin Sci Bull 51(19):2401–2412. doi:10.1007/s11434-006-2096-y
Rao YK, Tsay KJ, Wu WS, Tzeng YM (2007) Medium optimization of carbon and nitrogen sources for the production of spores from Bacillus amyloliquefaciens B128 using response surface methodology. Proc Biochem 42(4):535–541. doi:10.1016/j.procbio.2006.10.007
Rapala J, Lahti K, Rasanen LA, Esala AL, Niemela SI, Sivonen K (2002) Endotoxins associated with cyanobacteria and their removal during drinking water treatment. Water Res 36(10):2627–2635
Redhead K, Wright SJL (1978) Isolation and properties of fungi that lyse blue-green-algae. Appl Environ Microbiol 35(5):962–969
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111(Mar):1–61
Sansonetti S, Curcio S, Calabro V, Iorio G (2010) Optimization of ricotta cheese whey (RCW) fermentation by response surface methodology. Bioresour Technol 101(23):9156–9162. doi:10.1016/j.biortech.2010.07.030
Shi SY, Liu YD, Shen YW, Li GB, Li DH (2006) Lysis of Aphanizomenon flos-aquae (Cyanobacterium) by a bacterium Bacillus cereus. Biol Control 39(3):345–351. doi:10.1016/j.biocontrol.2006.06.011
Song XJ, Zhang XC, Kuang CH, Zhu LY, Guo N (2007) Optimization of fermentation parameters for the biomass and DHA production of Schizochytrium limacinum OUC88 using response surface methodology. Proc Biochem 42(10):1391–1397. doi:10.1016/j.procbio.2007.07.014
Tabbene O, Ben Slimene I, Djebali K, Mangoni ML, Urdaci MC, Limam F (2009) Optimization of medium composition for the production of antimicrobial activity by Bacillus subtilis B38. Biotechnol Progr 25(5):1267–1274. doi:10.1002/btpr.202
Tang XQ, Wu M, Yang WJ, Yin W, Jin F, Ye M, Currie N, Scholz M (2012) Ecological strategy for eutrophication control. Water Air Soil Pollut 223:723–737
Wang X, Li ZJ, Su JQ, Tian Y, Ning XR, Hong HS, Zheng TL (2010) Lysis of a red-tide causing alga, Alexandrium tamarense, caused by bacteria from its phycosphere. Biol Control 52(2):123–130. doi:10.1016/j.biocontrol.2009.10.004
Zinatizadeh AAL, Mohamed AR, Abdullah AZ, Mashitah MD, Isa MH, Najafpour GD (2006) Process modeling and analysis of palm oil mill effluent treatment in an up-flow anaerobic sludge fixed film bioreactor using response surface methodology (RSM). Water Res 40(17):3193–3208. doi:10.1016/j.watres.2006.07.005
Acknowledgments
This work was financially supported by the National Key Technology R&D Program (Nos. 2006BAJ08B01/2012BAJ25B07), the National Key Science and Technology Project: Water Pollution Control and Treatment (No. 2012ZX07101-012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Kong, Y., Zou, P., Miao, L. et al. Medium optimization for the production of anti-cyanobacterial substances by Streptomyces sp. HJC-D1 using response surface methodology. Environ Sci Pollut Res 21, 5983–5990 (2014). https://doi.org/10.1007/s11356-014-2532-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2532-5