Abstract
Bioaccumulation of perfluorooctane sulfonate (PFOS) in a restricted terrestrial food chain was investigated with the omnivorous wood mouse (Apodemus sylvaticus) on top of the studied food chain. The levels detected are very high compared with literature as a result of the presence of fluorochemical plant in the immediate vicinity of the study area. Soil, surface water, fruits of European elder and common blackberry, invertebrates, bank vole and wood mouse were collected at two sites, e.g. Blokkersdijk, adjacent to the fluorochemical plant, and Galgenweel, a reference site 2 km further away. In wood mouse, the highest PFOS concentrations were found in the liver followed by the pancreas, lungs and kidneys, with the spleen having the lowest levels. In the liver, the concentrations ranged from 787 to 22,355 ng/g ww at Blokkersdijk and these were significantly correlated with those detected in the kidneys (13.7–4,226 ng/g ww). If current results are compared to the findings of a previous study conducted in 2002 at the same sites, a significant decrease of PFOS in livers of wood mouse is observed. To the best of our knowledge, so far no studies reported levels of PFOS in terrestrial invertebrates under field conditions. At Blokkersdijk, PFOS was detected in all invertebrate species ranging from 28 to 9,000 ng/g. Soil and water were also contaminated with levels of respectively 68 ng/g and 22 ng/L. Biota-to-soil accumulation factors ranged from 0.11 to 68 for earthworms. Biomagnification factors (BMFs) of liver wood mouse/berries were as high as 302. BMFs for invertebrates were remarkably lower (up to 2).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perfluoroalkyl acids (PFAAs) are highly persistent substances which have been produced for more than five decades. These substances consist of a perfluoroalkyl chain where all hydrogen atoms are replaced with fluor atoms and a hydrophilic group. The strong C–F binding in combination with the hydrophobic and lipophobic character of PFAAs results in unique physiochemical properties which makes them suitable for a wide range of applications such as surface coatings for carpets, textile stain and soil repellents, food contact paper and fire fighting foams (Buck et al. 2011; Kissa 2001). The widespread use of poly- and perfluorinated substances (PFASs) has led to a global contamination of the environment, wildlife and humans as described in many publications. PFAAs are directly emitted to the environment or are formed indirectly from environmental degradation of precursors (Buck et al. 2011). Although the presence of these chemicals is well documented, the effects and environmental dynamics are not well understood yet.
Previous studies on the exposure of wild animals to PFASs showed that perfluorooctane sulfonic acid (PFOS) is occurring in the highest levels in animals (Kannan et al. 2002; Van de Vijver et al. 2003; Falk et al. 2012). The mean level of PFOS in liver of polar bears (Ursus maritimus), a top predator, from East Greenland was as high as 3,270 ng/g ww (Greaves et al. 2012). Also in smaller predators, for example the American mink (Mustela vison), comparable levels (up to 3,680 ng/g ww) have been measured (Giesy and Kannan 2001).
Many studies have been performed on the biomagnification and bioaccumulation of PFOS in food chains. Most of these studies were performed in either marine or freshwater ecosystems and studies on terrestrial ecosystems remain scarce. Bioaccumulation was shown to take place in marine mammals (Van de Vijver et al. 2003), a food web consisting of invertebrates and various fish species of Lake Ontario in Canada (Martin et al. 2004a) and in the Barents Sea food web (Haukås et al. 2007). Trophic magnification factors (TMFs) and biomagnification factors (BMFs) were reported for the bottlenose dolphin from the USA (Houde et al. 2006), for beluga whales in the Arctic (Kelly et al. 2009) and for fish and invertebrates in a subtropical food web in Hong Kong (Loi et al. 2011). Recently, Müller et al. (2011) determined BMFs and TMFs for several PFASs in a remote terrestrial food chain: lichen–caribou–wolf in Canada.
The persistence, potential health effects and widespread distribution of various PFAAs has led to (a) the voluntary phaseout of PFOS and related compounds by their major global manufacturer in 2002 (3M 2000), (b) directives restricting the marketing and use in the European Union (European Parliament 2006) and (c) the inclusion of PFOS, its salts and perfluorooctane sulfonyl fluoride in the Stockholm Convention on Persistent Organic pollutants (Stockholm Convention 2009).
In a previous study, the levels of PFOS in livers of wood mouse (Apodemus sylvaticus) living near a fluorochemical plant in Belgium was investigated (Hoff et al. 2004). Although these animals are relatively low in the food chain, measured levels of PFOS were the highest ever observed in wildlife. Other studies have also shown that the Antwerp region is a hotspot for PFOS contamination (Hoff et al. 2005; Dauwe et al. 2007). In the study of Hoff et al. (2004), the levels of PFOS in wood mouse were investigated but the biomagnification through their food chain has never been studied.
The aim of the present study was to characterise the levels of PFOS in a restricted terrestrial food chain in which the omnivorous wood mouse occupies the highest investigated trophic position. Wood mouse and various invertebrates, as well as fruit of the common blackberry (Rubus fruticosus) and European elder (Sambucus nigra), were collected from two sites in the Antwerp region nearby a known PFOS source. At one site, also bank vole (Myodes glareolus) was captured. The distribution of PFOS in different organs of wood mouse was evaluated. To determine the biota-to-soil accumulation factors (BSAFs), soil was also sampled. Furthermore, BMFs were assessed for wood mouse and bank vole. The levels of PFOS in the liver of the wood mouse were compared with a previous study conducted in the same area (Hoff et al. 2004) to investigate possible changes in levels of PFOS over time.
Materials and methods
Sample collection
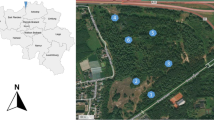
Sampling took place from September to November 2006 at two locations near Antwerp, Belgium (Fig. 1). The first location, Blokkersdijk, is a nature reserve protected by the European Council directive 79/409/EEC on the conservation of wild birds (1979) in the near vicinity of a fluorochemical plant. The control site, Galgenweel, is 3 km further away. Both areas are artificial sand dune habitats and have similar vegetation. Wood mice were captured alive in both Blokkersdijk (n = 20) and Galgenweel (n = 21) using Sherman Live traps. At Blokkersdijk, in addition six bank voles were captured. In the laboratory, the rodents were sacrificed by an overdose of diethyl ether. Gender and body weight were determined. The liver, pancreas, lungs, kidneys and spleen were collected from each individual wood mouse. From the bank vole, only the livers were dissected. The eye lenses of the wood mice were collected and fixed in 10 % formaldehyde for age determination.
The location of the two study sites, Blokkersdijk and Galgenweel, near Antwerp, Belgium (taken from Hoff et al. 2004)
For the sampling of the earthworms (family Lumbricidae), a solution of mustard powder in water was poured on the ground (Gunn 1992) causing the worms to emerge from the soil. This procedure was done at several spots at each site, all nearby the locations of the Sherman live traps. Other invertebrates were hand collected over the entire site by lifting up stones and pieces of wood and included slugs (order Stylommatophora), millipedes (class Diplopoda) and woodlice (order Isopoda). The plant samples included the fruits of common blackberry and European elder. These berries were taken from several plants spread around the site. For all invertebrate species (n = 4) and berries (n = 2), at least three 50 mL tubes per site were filed and brought to the lab.
Water samples were collected in pre-cleaned PP bottles (0.5 L) at five different spots at the borders of the ponds in both Blokkersdijk and Galgenweel. Before filling the bottles, they were rinsed three times with the water of the pond. Soil was sampled over 10 cm depth and 5 cm wide with a stainless shovel after removal of the upper organic layer by hand. This was done at five places all over the sites, nearby the spots were the Sherman live traps were set.
All handlings were conducted in accordance with national and international guidelines (Directive 2007/526/EC of the European Commission) for the protection of animal welfare.
Age determination
The age of the wood mice was determined by the method of Vandorpe and Verhagen (1979). Briefly, the eye lenses were dried at 80 °C for 24 h and then weighted with an accuracy of 0.1 mg. The age of the animals was calculated using the (empirical) equation: age (in days) = exp ((weight of both lenses in mg + 15.213) / 6.568).
Sample preparation and analysis
Prior to extraction, the liver, kidney, spleen and lungs of wood mice and liver of bank voles were homogenised with an Ultra Turrax T8 homogenizer (IKA Werke, Germany). Between 0.5 and 1 g of the livers and kidneys were homogenised in a PP centrifuge tube. Due to the small size of the spleen, pancreas and lungs, these organs were pooled per sex (Blokkersdijk (Bl): 10m and 10f; Galgenweel (G): 16m and 5f) to create in total eight pooled samples of each site.
Homogenisation of invertebrates and berries was done with mortar and pestle. Individual invertebrates were pooled per species, i.e. slugs (n G = 28, n Bl = 12), millipedes (n G = 11, n Bl = 39), woodlice (n G = 25, n Bl = 39) and earthworms (n G = 5, n Bl = 7) which results in four pooled samples of each location. Of each pooled sample, three subsamples of approximately 1 g wet weight (ww) were extracted. For both elder- and blackberry, five subsamples (1 g ww) per site were prepared. Soil was sieved and of each location five times 2.5 g was taken for extraction. Samples were stored at −20 °C in PP tubes until extraction.
All the extraction procedures applied were based on methods described by Powley et al. (2005) with some adaptations and were performed in November 2006. For the invertebrates, berries and organs, a solvent extraction was used. Briefly, an isotopically labelled internal standard (13C4-PFOS) was added to the sample and mixed thoroughly. After adding 10 mL acetonitrile, the solution was sonicated and centrifuged. One millilitre of the non-concentrated supernatant was cleaned up with 50 mg Envi Carb® and 50 μL acetic acid. After centrifugation, the supernatant was retained and 70 μL extract was mixed with 130 μL reverse osmosis water and filtered through the PES membrane filter (0.2 μm) into a polypropylene injector vial. Concentrations are expressed in nanogram per gram ww.
For the soil, internal standard was added together with 1 mL NaOH (200 mM). After 30 min, 100 μL 2 M HCL and 9 mL acetonitrile were added. The samples were vortexed and put on a wrist action shaker (30 min). One millilitre of the supernatant was cleaned up and filtrated as described earlier.
For the water samples, solid phase extraction was applied. To a volume of 500 mL of sample, 2.5 mL of formic acid was added. After filtration with a Whatman filter, 80 mg of ammonium acetate and 20 μL of the internal standard were added. The samples were extracted on Oasis HLB cartridges, preconditioned with methanol and washed with methanol/water (40/60). Target analytes were eluted with 8 mL of methanol. The eluent was evaporated till 250 μL.
Before injection, 20 μL of an injection standard (3,5-bis(trifluromethyl) fenyl acetic acid), to monitor the injection reproducibility, and 250 μL ammonium acetate were added to the samples.
The analysis was performed using an HPLC (Cap LC system, Waters, USA) coupled to a Quattro II triple quadrupole mass spectrometer (Waters, USA) with an electrospray interface operating in negative ionisation mode (ES-MS/MS). Separation was performed on a fluophase column (50 mm × 1 mm, Thermo, USA). A pre-column (Optiguard, C18; 10 mm × 1 mm, All tecfh, USA) was inserted between the solvent mixer and the injector to remove any PFASs originating from the HPLC system. The injection volume was 5 μL and the flow rate was set to 40 μL min−1. A gradient program delivering mobile phases consisted of 4 mM ammonium acetate and methanol. The monitored transitions are 499 → 80 (PFOS), 499 → 99 (PFOS), 503 → 80 (13C4-PFOS) and 503 → 99 (13C4-PFOS).
Non-labelled standard of PFOS was used to construct ten-level calibration curves (r 2 > 0.99) encompassing the entire linear range. Prior to extraction, all samples were spiked with isotopically labelled internal standard (13C4-PFOS). Relative response factors were generated based on identically spiked calibration curve solutions, allowing percent recoveries to be calculated. PFOS concentrations were corrected for recovery.
Laboratory blanks were treated identically to all other samples, but without tissue. For each batch of ten samples, a laboratory blank was prepared to check for background contamination. Pure acetonitrile was injected after every eight samples to check for carry-over effects. A standard was injected after every eight samples to check the stability of the HPLC-MS/MS system.
Biota-to-soil-accumulation factors and biomagnification factors
BSAFs are calculated for the invertebrates and the berries because soil is the most important exposure route for invertebrates (ingestion of soil through the gut) and for plants (direct exposure of leaf surfaces or uptake via roots). The BSAFs were determined as follows:
Where [PFOS]X is the concentration of PFOS in the invertebrates or berries (in nanogram per gram ww) and [PFOS]soil is the concentration of PFOS in soil (in nanogram per gram ww).
For the wood mouse and bank vole, BMFs were determined as food is the major exposure route. The exact diet of these mice is not known and therefore a diet-weighted BMF could not be calculated. The BMFs were calculated based on a single tissue concentration (kidney or liver) and the mean concentrations in the berries or invertebrates using the following equation:
The concentrations (in nanogram per gram ww) are not normalised for lipid content, like usually done for BMF calculations, as PFASs are not lipophilic. For the calculation of both BSAFs and BMFs, a steady state of body and environmental burdens is assumed. It should be stressed that for the calculation of BMFs the use of whole body or muscle tissue is preferred and that the use of liver and kidney will yield higher values.
Statistical analysis
General linear models (glm function with normal error) were applied to analyse the data, except where stated otherwise. The following models were fitted: effects of age, sex and site, and all two- and three-way interactions, on mass of, and PFOS concentrations in, liver and kidney; effects of taxon, site and their interaction on PFOS concentrations in invertebrates and berries; age differences between sites and sex and their interaction; differences in PFOS concentrations between Blokkersdijk and Galgenweel; change in PFOS concentration in wood mouse livers between 2002 and 2006 at both sample sites. Model selection started with the full model containing all main effects and interactions. Non-significant terms were removed with a backward stepwise method following Zuur et al. (2009). Significance of the effects was tested using χ 2 likelihood ratio tests.
Since there was only one pooled sample per sex and site for pancreas, lung and spleen, one sample t tests were applied to assess whether PFOS concentration differed from mean liver concentration. Correlations of PFOS concentrations between the liver and kidney were assessed with Pearson correlations.
To fulfil the normality assumptions of the tests, PFOS concentrations and age were log-transformed prior to analysis. All analyses were carried out using the R (version 2.15.3) statistical package (R core team 2013).
Results
In general, the recoveries ranged from 70 to 120 %, except for most of the liver and some kidney samples which had lower recoveries (45–80 %). The recoveries of the invertebrates and fruits (40–60 %) are also lower probably because the used extraction procedures were not optimised for these materials. Further adjustment and optimisation of the extraction method for these materials seems necessary. To the best of our knowledge at the time of the analyses, methods on the determination of PFOS in invertebrates and fruits had not been published before, and it was decided to use the same method as used in the past as poor recoveries had not been observed for other matrices thus far. The difference in extraction efficiencies between the different tissues could also be due to the differences in fat contents (Holmström et al. 2005) and the presence of an exoskeleton of some of the invertebrates analysed.
Wood mouse
At Blokkersdijk, the age of the wood mice ranged from 18 till 212 days (mean 99 days) and from 31 till 521 days (mean 172 days) at Galgenweel. The PFOS measurements revealed that 1 old male (age = 212 days) from Blokkersdijk had an aberrant, more than 10× lower PFOS concentration in the liver than expected (Fig. 2). If that male is omitted from the dataset, a significant site × age interaction (log-likelihood (χ 21 = 8.50, p = 0.004) is found. At Blokkersdijk, the PFOS concentrations in livers (average ± SE, 6,100 ± 1,340 ng/g) are higher than at Galgenweel (153 ± 37 ng/g) and increase with age (χ 21 = 8.01, p = 0.004) while there is no age effect at Galgenweel (χ 21 = 0.66, p = 0.416) (Fig. 2). There is no sex difference (χ 21 = 0.006, p = 0.937), nor is there a sex × age interaction (χ 21 = 0.34, p = 0.561) indicating that both sexes take up PFOS at the same rate. When the aberrant male is included in the analysis, the site × age interaction disappears (χ 21 = 2.24, p = 0.135) and there is no age effect anymore (χ 21 = 0.05, p = 0.824). The overall PFOS concentration in livers of Blokkersdijk animals (5,810 ± 1,300 ng/g), however, still remains higher than at Galgenweel (χ 21 = 57.12, p = 0.001).
In the kidneys, a significantly higher mean concentration of PFOS is observed at Blokkersdijk (885 ± 237 ng/g) compared to Galgenweel (24.1 ± 5.6 ng/g) (χ 21 = 43.17, p < 0.001). Neither an effect of sex (χ 21 = 0.81, p = 0.368) nor age (χ 21 = 0.61, p = 0.435) was determined.
In the current study, a significant correlation between levels of PFOS in kidneys and livers in Blokkersdijk (males: r = 0.80 ± 0.21, t8 = 3.83, p = 0.005; females: r = 0.70 ± 0.27, t7 = 2.61, p = 0.035) is observed. In Galgenweel, there is only a trend for males (r = 0.45 ± 0.24, t14 = 1.87, p = 0.083) while there is no correlation for females (r = 0.54 ± 0.49, t3 = 1.11, p = 0.348). The latter might be a result of a very low sample size and two kidney measurements that were below the detection limit. Within a site, concentrations in livers are on average 8.09 ± 1.51 times higher than in kidneys (Fig. 3).
Concentrations of PFOS (in nanogram per gram ww) in different organs of wood mouse (female (F), male (M)) for Blokkersdijk (Bl., top panel) and Galgenweel (G., bottom panel). PFOS concentrations in the liver and kidney in mice of Blokkersdijk are higher compared to Galgenweel (liver χ 21 = 57.12, p < 0.001; kidney χ 21 = 43.17, p < 0.001). Note difference in y-axis scales. Error bars represent standard errors (SE)
Because samples were pooled for spleen, pancreas and lungs of the wood mice, it was impossible to run a test for these organs. However, from the graph (Fig. 3), it is clear that PFOS concentrations are higher at Blokkersdijk than at Galgenweel.
Based on the data sampled, no differences between pancreas and liver (one-sample t test, all p > 0.063) and between lung and liver (one-sample t test, all p > 0.255) were found for both sexes and both sites. Levels in spleen are significantly lower than in liver (one sample t test, all p < 0.037).
Since the female bank vole mice were all pregnant, only males were used to compare between the two species. Mean body mass did not differ between the species (χ 21 = 0.06, p = 0.803). The concentrations of PFOS in the livers of wood mouse and bank vole did not differ significantly (χ 21 = 0.02, p = 0.887). In wood mouse, an average concentration of 5,810 ± 1,300 ng/g was detected, in bank vole 3,890 ± 750 ng/g.
Invertebrates and fruit
At Blokkersdijk, the median concentrations of PFOS in isopods, millipedes, slugs and worms are 497, 2,570, 3,090 and 2,410 ng/g, respectively. At Galgenweel, median concentrations of 269, 280, 125 and 65 ng/g are found in the respectively species. The concentrations of PFOS in the different invertebrate species at Blokkersdijk are significantly higher than those at Galgenweel (Fig. 4: χ 23 = 20.54, p < 0.001). There is a significant difference among taxa in Blokkersdijk (χ 23 = 20.54, p < 0.001) where the PFOS content in isopods is significantly lower than in the other taxa (Tukey t, all p < 0.041). The levels do not differ among the other taxa (all p > 0.869). In Galgenweel, there is no difference among taxa (χ 23 = 5.16, p = 0.161).
Concentrations of PFOS (in nanogram per gram) in invertebrates collected at Blokkersdijk and Galgenweel. Concentrations at Blokkersdijk are higher compared to those at Galgenweel (χ 23 = 20.54, p < 0.001). In Blokkersdijk, the PFOS concentration in isopods is lower than in the other taxa (Tukey t, all p < 0.041). Error bars represent standard errors (SE)
For the fruits, the highest concentrations are again observed at Blokkersdijk (Fig. 5: χ 21 = 12.17, p < 0.001). The concentrations at Blokkersdijk are also higher in elderberry than in blackberry (χ 21 = 5.35, p = 0.021) with median concentrations of 25 and 7.7 ng/g, respectively. The median levels at Galgenweel for both fruit species are below the limit of detection (2.0 ng/g).
Concentrations of PFOS in berries (in nanogram per gram) of Blokkersdijk and Galgenweel. At Blokkersdijk, the concentrations of PFOS are higher compared to those at Galgenweel (χ 21 = 12.17, p < 0.001). Within Blokkersdijk, the levels in elderberry are higher compared to blackberry (χ 21 = 5.35, p = 0.001). Error bars represent standard errors (SE)
Soil and water
The concentration of PFOS observed in soil from Blokkersdijk (68.7 ± 9.0 ng/g ww) is significantly higher (χ 21 = 49.69, p < 0.001) than in soil from Galgenweel (<LOD, LOD = 2.4 ng/g). In water, again the concentration of PFOS observed at Blokkersdijk (21.9 ng/L) is higher than in Galgenweel (1.03 ng/L). It should be noted that at each location only one (pooled) sample was analysed so a standard error could not be calculated.
BSAFs and BMFs
In Table 1, an overview of the levels determined in the different samples and the BSAFs is presented. The lowest levels were observed in water and berries. The BSAFs could only be calculated for Blokkersdijk as the level of PFOS in soil from Galgenweel was below the LOD. The largest BSAFs are observed for the earthworms (68) and slugs (60) and are twice as high as the BSAF for millipedes (35). The isopods show a lower BSAF of 7.1. The lowest BSAFs are found in the fruits (blackberry 0.11, elderberry 0.45).
The BMFs for liver of wood mouse and bank vole (Table 2) are similar for both berries (302 and 202, respectively) and invertebrates (2.0 and 0.3, respectively). The BMF based on the concentration in the kidneys of wood mouse are lower than the liver-based BMF for both plants (46) and invertebrates (0.3).
Effect of time
Comparison of the PFOS concentrations in the livers of wood mouse from the current study with those of the study of Hoff et al. (2004), who collected wood mice at the same locations in 2002, reveals a significant decrease in concentrations of PFOS (χ 21 = 14.46, p < 0.001) both at Blokkersdijk and Galgenweel (Table 3). Levels decreased evenly for both places as there is no significant site × time interaction (χ 21 = 0.49, p = 0.483).
Discussion
Wood mouse
At Blokkersdijk, the concentrations of PFOS in liver of wood mice increased with age. This correlation was not found at Galgenweel. The elimination half-life of PFOS in mice (laboratory animals) is 30–43 days after a single oral dose (Chang et al. 2012) but the authors assume that the magnitude of PFOS exposure will not decrease during the life span of the mice and therefore this elimination will not have a great impact on the levels in the mice. In other words, the intake of PFOS at Blokkersdijk will be higher compared to the elimination and therefore the older mice could accumulate higher levels. This age effect is not observed at Galgenweel, and this could possibly be explained by a lower uptake of PFOS whereby the effect of the elimination is noticeable. Current levels could also indicate that at Galgenweel the pre- and postnatal exposure of PFOS is higher than the exposure of older individuals. It should be noted that in the present study the contribution from precursors such as perfluoroalkyl sulfonamides which can degrade to PFOS (Buck et al. 2011) is not known. The inclusion of these indirect sources would possibly influence the results. Some studies confirmed this increased PFOS concentration with age (Smithwick et al. 2005; Butt et al. 2007), but in general, no clear pattern between age and PFOS levels is observed (Lau et al. 2007). While the concentrations of PFOS in both liver and kidneys observed in the Blokkersdijk mice are among the highest ever reported, those from the reference site Galgenweel are also high compared to the literature. Harrad (2010) concluded that the levels of PFOS in the livers of wood mice from Blokkersdijk in the study from Hoff et al. were the highest ever reported. Although the present levels are lower, the concentrations of PFOS in Blokkersdijk remain, with the exception of the data from Hoff et al. (2004), the highest ever reported in wildlife. In the liver of polar bears, a mean PFOS concentration of 3,270 ± 290 ng/g was reported by Greaves et al. (2012). Blokkersdijk is a nature reserve next to a fluorochemical plant which is or has been a direct point source of both PFOS and precursors which can degrade to PFOS and forms a worst case emission scenario for the direct environment. Mice will be directly exposed via the consumption of contaminated water, inhalation of the air and ingestion of fine particles. Indirect exposure via the consumption of plants and invertebrates which already contain accumulated levels of PFOS results in a high exposure scenario for all the living species including the wood mouse and the bank vole. Earlier studies carried out in this area confirm the high concentrations of PFOS, e.g. in livers of great tit (Parus major) and blue tit (Cyanistes caeruleus) nestlings (Hoff et al. 2005; Dauwe et al. 2007) and in feathers of barn owl (Tyto alba) (Jaspers et al. 2013). The literature on PFOS in wild terrestrial mammals is very limited. Martin et al. (2004b) reported mean levels of PFOS in livers of Artic fox (Alopex lagopus) and mink (Mustela vison) from the Canadian Artic of respectively 250 and 8.7 ng/g. Müller et al. (2011) analysed livers from wolves (Canis lupus) and caribou (Rangifer tarandus groenlandicus) in Canada. Livers from caribou contained average concentrations of PFOS between 0.67 and 2.2 ng/g depending on the sampling year and location. In the livers of wolves, a range of 1.4–1.7 ng/g PFOS was detected. These levels are about 2 orders of magnitude below the levels detected in livers of mice from our reference site Galgenweel. Only the study of Falk et al. (2012) investigated terrestrial mammals in Europe. This study focused on the temporal and spatial trends of PFAS in liver of roe deer (Capreolus capreolus) in Germany and reported levels of PFOS up to 67 ng/g in deer liver.
Under laboratory conditions, Bogdanska et al. (2011) determined the tissue distribution in male mice after a 1–5-day dietary exposure to environmentally relevant levels of 35S-PFOS. They observed that the highest levels were found in the liver, followed by lungs and kidney and pancreas. In general, Bogdanska et al. (2011) found no indication for saturation of PFOS binding or uptake for most of the tissues with the possible exceptions of the spleen and fat pads (after 5 days, high exposure). This saturation of the spleen could be an explanation of the relative low PFOS levels in the present study. Only a few studies focus on the distribution of PFASs in terrestrial organisms in wildlife. The study of Greaves et al. (2012) on polar bears determined PFAS levels in liver, blood, brain, muscle and adipose tissue. Unfortunately, except for the liver, these tissues were not analysed in the present study.
Müller et al. (2011) analysed muscle, liver and kidney from caribou. The kidneys contained an average concentration of PFOS of 0.02 ng/g ww. The ratio liver/kidney observed in caribou was 33 which is much higher than the ratios found in the current study of 6.84 ± 0.79 and 9.14 ± 2.72 at Blokkersdijk and Galgenweel, respectively. Borg et al. (2010) determined the tissue distribution of PFOS in laboratory mice (C57Bl/6) and found a ratio of 4.7 in the dams after oral gavage or intravenous injection of 35S-PFOS, which is in the same range of the current study. Ratios of 6.2 and 4.4 (liver/kidney) were observed in, respectively, a low (environmental) and high daily dose exposure study of male mice (C57Bl/6) to 35S-PFOS via food (Bogdanska et al. 2011). Many studies have confirmed that liver is the major target organ for PFOS. Contrary to other persistent pollutants, PFASs do not accumulate in fat tissue but are predominantly distributed to liver and blood as they bind to proteins (Lau et al. 2007).
There is an overlap in the diet of bank vole and wood mouse but in the autumn, when our study took place, bank voles eat more berries and fungi whereas the diet of the wood mouse includes more acorns and animal material (Abt and Bock 1998). The invertebrates in our study had higher levels of PFOS than the berries but these differences in diet are not reflected in a difference between the concentrations in the livers of these two species. Inclusion of more dietary items such as grass spikes would possibly change this outcome.
Invertebrates and fruit
To date, no publications on levels of PFOS in terrestrial invertebrates in natural environments are available. On the other hand, several laboratory studies have focused on accumulation and potential toxicity of PFOS to some insects, i.e. Odonata (Van Gossum et al. 2009), Diptera (Van Gossum et al. 2010) and Bombus terrestris (Hymenoptera, Mommaerts et al. 2011). These studies revealed a reduction in the number of offspring, a reduced behavioural performance and detrimental effects on reproduction capacity, respectively. These negative effects were already observed when exposed to PFOS levels lower than the current levels detected in the pond of Blokkersdijk. One study investigated the effect of exposure of PFOS on earthworms. Xu et al. (2013) exposed Eisenia foetida to PFOS via soil and found several negative effects on growth, antioxidant defence systems and DNA damage even with the lowest exposure scenario of 10 mg/kg. In the study of Xu et al. (2013), the accumulation of PFOS was not determined and the used exposure dose is 1,000 times higher than the levels in soil of Blokkersdijk which makes comparison with the present study difficult.
Data on forest fruits are not available but a European research project analysed several commercial available fruit items which included stone fruit, berries, citrus fruit and exotic fruit collected in several European countries (EU FP 7 PERFOOD project). The levels found in these items are in the picogram per gram range with the highest levels found in Belgian apples (320 pg/g) and Czech pears (539 pg/g). These levels are similar to the levels found in elderberry and blackberry from Galgenweel but are at least 1 magnitude lower than the levels found at Blokkersdijk.
Soil and water
Literature data on PFASs in soil are limited and therefore the current results can only be compared with studies conducted in Asia. Wang et al. (2010) analysed soil samples around two PFAAs manufacturing plants in Wuhan, China. These plants were considered to be point emission sources of PFASs and samples were taken with increasing distance from the point sources. Samples were taken in 2009, 3 to 4 years after the production of PFOS in China peaked. China began with the production of PFASs in 2000 but before 2003 the production was relatively low. With the phaseout of PFOS in America and Europe, the production in China increased rapidly with maximum production volumes in 2006. In 2007, it dropped sharply due to the ban of the import of PFOS and related products by the European Union (Wang et al. 2010; Zhang et al. 2012). Albeit at a lower level, the production in China is still continuing. In the Wang et al. study, a mean of 2,583 ng/g in soil samples, which were taken within 0–0.5 km from the point source, was found which is 35 times higher than the current level at Blokkersdijk. The levels decreased exponentially with distance whereby the levels at 2 km approximating the levels found at the reference site (0.65 ng/g, Wang et al. 2010). Unfortunately, the LOQ of the extraction procedure in the present study was too high to detect such low levels at our reference site. Another study carried out in China (Wang et al. 2011) collected soil samples in the Guanting reservoir area. This reservoir is Beijing’s second largest source of water for agricultural and industrial purposes and was also used for drinking water production until 1997. Since then, contamination from industry and farming has substantially degraded the quality of the water. At seven locations in the surrounding area of this reservoir, soil samples were taken and in only one location PFOS was found (0.86 ng/g).
In the Scheldt River, which runs next to the perfluorochemical plant and the nature reserve (Fig. 1) investigated in the present study, a mean concentration of 154 ng/L of PFOS has been reported (Eschauzier et al. 2011) which is an order of magnitude higher than the level currently found in the pond of Blokkersdijk. In general, the levels found in Central Europe are lower but other contaminated regions in Europa contain similar PFOS concentrations. The rivers Rhone, Rhine, Danube and Po have the highest discharges of PFAAs of the European rivers and consequently contain high PFAAs concentrations (McLachlan et al. 2007; Eschauzier et al. 2011). Between 2006 and 2009, mean concentrations of PFOS between 4 and 35 ng/L have been detected in the river Rhine (Germany and The Netherlands, reviewed by Eschauzier et al. 2011). It should be noted that these literature levels were measured in rivers and not in standing water as in the present study.
Giesy et al. (2010) calculated water quality criteria for the protection of avian species, i.e. an avian wildlife value (AWV) for PFOS of 47 ng/L. This value is in the same magnitude as the level observed in the Blokkersdijk pond (22 ng/L) in the present study in a sample that was collected 4 years after the phaseout. Therefore, it cannot be excluded that the concentration of PFOS in the pond has exceeded this AWV value in the past and thereby caused possible negative effects on the wide variety of birds in this area.
BSAFs and BMFs
Loi et al. (2011) investigated trophic magnification PFASs in a subtropical food web. The calculated BAF and biota–sediment accumulation factors for, respectively, phytoplankton and three worm species were 169 and 1.7–24 for PFOS. Very recently, Naile et al. (2013) published BCFs of PFAAs in an aquatic food web. The BCF was defined as log BCF = log (concentration in biota (ng/kg ww) / concentration in water (ng/L)). The calculated BCFs ranged from 1.7 (crabs/water) till 4.5 (liver fish/water). Like most of the studies that investigate bioconcentration/bioaccumulation of PFASs, these studies were conducted in aquatic environments which makes comparison with the current study difficult as in water the exposure routes are totally different. Thus, far no BCF/BSAF field studies on PFAAs in terrestrial ecosystems were found. However, Zhao et al. (2013) determined the bioaccumulation of several PFASs by earthworms in soil under laboratory conditions. Uptake and elimination rate constants, half-lives, time to steady-state as well as BSAF were determined by fitting the kinetics data with generic bioaccumulation models. PFOS was already detected in the earthworms after 2 days of exposure and it took approximately 60 days before an equilibrium had been reached. A BSAF for PFOS of 0.145 was found in the 100 ng/g exposure scenario. This value decreased with increasing exposure (0.097 with exposure of 500 ng/g). As the level of exposure observed in our study is below 500 ng/g and the BSAFs observed are higher, these numbers agree with the trend observed in the exposure experiment. Müller et al. (2011) conducted one study on BMFs and TMFs in a terrestrial food chain: lichen–caribou–wolf. The BMFs (C consumer/C diet) were calculated based on two different approaches, i.e. on single tissue concentrations and on estimated whole body concentration (“whole”) for caribou and wolf. For PFOS, the authors estimated BMFs of 3 to 4 for the “caribou liver/lichen” and 5 to 9 and for “caribou whole body/lichen”. A BMF of 0.8–2.1 was found for wolf liver/caribou liver and was similar to the BMF of 1.2–3.3 for the wolf whole/caribou whole. The authors mentioned that tissue-specific BMFs overestimate the biomagnification as these tissues have only minor contributions to the total body burden. This is confirmed by Houde et al. (2006) who determined BMFs in the bottlenose dolphin food web. Consequently, the liver/kidney-based BMFs in the current study will probably overestimate the bioaccumulation. The whole body-based BMFs would provide a more realistic estimation (Müller et al. 2011). The BMFs from Müller et al. (2011) are comparable with the BMF for wood mouse liver/invertebrates in the current study but are much lower than those determined for the wood mouse liver/plants. The BMFs for wolves (carnivorous) are lower compared to these from the caribou (herbivorous). So it seems that the magnification from “plants to consumer” is higher than from “plant consumers to wolves” and “invertebrates to wood mice”.
In the present study, the average concentration of PFOS in invertebrates was used to calculate the BMFs for the individual mice. The mean concentration of PFOS in the invertebrates was so high that it results in a mean BMF <1. However, some BMFs reach up to 5 which indicates that the BMFs are not only species dependent but also vary considerably among the individual mice.
The observed decrease in BSAFs and BMFs with an increasing exposure can be explained by saturation of the binding sites. This hypothesis is described by Zhao et al. (2013) as follows: If the exposure concentrations are low, more free binding sites for PFASs adsorption are available. With increasing exposure, the binding sites are already occupied with PFAS and become gradually saturated. If the exposure concentrations increase even more, there are no sites available anymore and the accumulation will decrease (Zhao et al. 2013). This decline in BSAF with increasing exposure scenarios have also been observed in other studies for other compounds (Morikawa et al. 2006; Zhao et al. 2013).
As Müller et al. (2011) pointed out, there are no other reported mammal to mammal BMFs but based on the data reported by Martin et al. (2004b) the authors calculated a BMF of 177 for polar bear liver/seal liver. This value is in the same magnitude than the current BMFs for mice liver/plants.
Effect of time
The observed decrease over 4 years in the concentration of the wood mouse livers cannot be explained by a difference in food source, as the sampling took place in the same season in both studies. Since the variety in food sources must have been similar, the concentrations of PFOS in the food will probably have decreased to the same extent or even more pronounced. Another important remark on the temporal trend is that for this comparison age, weight and sex of the mice were not taken into account as these individual parameters of the mice collected by Hoff et al. (2004) were not available. The average age of the wood mouse collected in 2002 at the two sites was 70 ± 36 days at Blokkersdijk and 71 ± 22 at Galgenweel. In the current study, the average age of the populations was higher (Blokkersdijk 99 ± 11, Galgenweel 173 ± 25 days) so if the age–liver concentration relationship for PFOS observed at Blokkersdijk (see “Wood mouse” section) was taken into account, the decrease in concentrations of PFOS in livers would even be more pronounced. The decrease is most probably due to the phaseout of PFOS and related products in 2002 (3M 2000) by the neighbouring fluorochemical plant. This phaseout reduced the release of these chemicals into the direct environment of the plant and consequently in the levels in soil, water, invertebrates and plants which, in turn, will have resulted in lower levels in the liver of the wood mice. The consequences of the phaseout are not only visible at (or really close to) the production site but also in the reference area (Galgenweel) a clear decrease is observed. To our knowledge, only one other study conducted in Europa confirmed a decrease of PFOS in terrestrial mammals; Falk et al. (2012) observed a significant decrease in concentrations of PFOS in liver of roe deer collected from 2001 to 2010 in Germany. Samples collected between 1989 until 2001 did not show significant decreases and confirm that the observed declines in the total time frame are directly related to the phaseout of PFOS and related substances in 2002 in Europe. Several studies observed a diminishing of the levels of PFOS in bird eggs from Northern Europe (Holmström et al. 2005; Ahrens et al. 2011) and in aquatic ecosystems (Rüdel et al. 2011).
Conclusions
This study indicates that bioaccumulation of PFOS occurs in the terrestrial food chain of wood mice. Based on the levels in soil, a BSAF of 68 was found for earthworms. For wood mouse liver/plants, a BMF up to 300 was determined at the site near the fluorochemical plant. To the best of our knowledge, this is the first study that reports levels of PFOS in the diet of mice, e.g. several terrestrial invertebrates and wild fruit species. Furthermore, the distribution of PFOS to several organs of the mice has been determined. The highest levels are observed in the livers, followed by the pancreas, lungs, kidneys and spleen. A significant decline between 2002 and 2006 in concentrations of PFOS in liver of wood mouse was observed, but the levels found in 2006 can still be qualified as high compared to literature. Due to the short half-life of PFOS in rodents, these mice could be an interesting species to monitor temporal changes of PFOS and other PFAAs over time. The near vicinity of a fluorochemical plant, a former potential point source of PFOS and related products, still results in high levels in soil and water and consequently in the higher trophic levels.
References
3M Company (2000) Phase-out plan for POSF-based products. USEPA Administrative Record AR226-0600. Available from www.regultations.gov, as document EPA-HQ-OPPT-2002-0051-0006
Abt KF, Bock WF (1998) Seasonal variations of diet composition in farmland field mice Apodemus spp. and bank voles Clethrionomys glareolus. Acta Theriol 43:39–389
Ahrens L, Herzke D, Huber S, Bustnes JO, Bangjord G, Ebinghaus R (2011) Temporal trends and pattern of polyfluoroalkyl compounds in Tawny Owl (Strix aluco) eggs from Norway, 1986–2009. Envrion Sci Technol 45:8090–8097
Bogdanska J, Borg D, Sundström M, Bergström U, Halldin K, Abedi-Valugerdi M, Begman Å, Nelson B, Depierre JW, Nobel S (2011) Tissue distribution of 35S-labelled perfluorooctane sulfonate in adult after oral exposure to a low environmentally relevant dose or a high experimental dose. Toxicology 284:54–62
Borg D, Bogdanska J, Sundström M, Nobel S, Håkansson H, Bergman Å, Depierre JW, Halldin K, Bergström U (2010) Tissue distribution of 35S-labelled perfluorooctane sulfonate (PFOS) in C57Bl/6 mice following late gestational exposure. Reprod Toxicol 30:558–565
Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7:513–541
Butt CM, Muir DC, Stirling I, Kwan M, Mabury SA (2007) Rapid response of Artic ringed seals to changes in perfluoroalkyl production. Environ Sci Technol 41:42–49
Chang S-C, Noker PE, Gorman GS, Hibson SJ, Hart JA, Ehresman DJ, Butenhoff JL (2012) Comparitve pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice and monkeys. Reprod Toxicol 33:428–440
Dauwe T, Van de Vijver K, De Coen W, Eens M (2007) PFOS levels in the blood and liver of a small insectivorous songbird near a fluorochemical plant. Environ Int 33:357–361
Eschauzier C, de Voogt P, Brauch H-J, Lange FT (2011) Polyfluorinated chemicals in European surface waters, ground- and drinking waters. Handbook of Environ Chem 73–102
EU FP 7 PERFOOD project grant agreement no. 227525: scientific coordinator Prof. Pim de Voogt, University of Amsterdam-IBED, The Netherlands
European Parliament (2006) Directive 2006/122/EC of the European parliament and of the council of 12 December 2006. Official Journal of the EU, 27 December 2006, L372/32-L372/34
Falk S, Brunn H, Schröter-Kermani C, Failing K, Georgii S, Tarricone K, Stahl T (2012) Temporal and spatial trends of perfluoroalkyl substances in liver of roe deer (Capreolus capreolus). Environ Poll 171:1–8
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35:1339–1342
Giesy JP, Naile JE, Khim JS, Jones PD, Newsted JN (2010) Aquatic toxicology of perfluorinated chemicals. Rev Environ Contam Toxicol 202:1–52
Greaves AK, Letcher RJ, Sonne C, Dietz R, Born W (2012) Tissue-specific concentrations and patterns of perfluoroalkyl carboxylates and sulfonates in East Greenland polar bears. Environ Sci Technol 46:11575–11583
Gunn A (1992) The use of mustard to estimate earthworm populations. Pedobiologoa 36:65–67
Harrad S (2010) Persistent organic pollutants. Wiley, Chichester
Haukås M, Berger U, Hop H, Gulliksen B, Gabrielsen GW (2007) Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ Pollut 148:360–371
Hoff PT, Scheirs J, Van de Vijver K, Van Dongen W, Esmans EL, Blust R, De Coen W (2004) Biochemical effect evaluation of perfluorooctane sulfonic acid-polluted wood mice (Apodemus sylvaticus). Environ Health Perspect 112:1–6
Hoff PT, Van de Vijver K, Dauwe T, Covaci A, Maervoet J, Eens M, Blust R, De Coen W (2005) Evaluation of biochemical and organismal effects related to perfluorooctane sulfonic acid exposure in organohalogen-contaminated great tit (Parus major) and blue tit (Parus caeruleus) nestlings. Chemosphere 61:1558–1569
Holmström KE, Järnberg U, Bignert A (2005) Temporal trends of PFOS and PFOA in guillemot eggs from the Baltic Sea. Environ Sci Technol 39:80–84
Houde M, Bujas TAD, Small J, Well RS, Fair PA, Bossart GD, Solomon KR, Muir DCG (2006) Biomagnification of perfluoralkyl compounds in the bottlenose dolphin (Tursiops trunctatus) food web. Environ Sci Technol 40:4138–4144
Jaspers VLB, Herzke D, Eulaers I, Gillespie BW, Eens M (2013) Perfluoroalkyl substances in soft tissues and tail feathers of Belgian barn owls (Tyto alba) using statistical methods for left-censored data to handle non-detects. Eniviron Int 52:9–16
Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP (2002) Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ Sci Technol 36:3210–3216
Kelly BC, Ikonomou MG, Bliar JD, Surridge B, Hoover D, Grace R, Gobas FAPC (2009) Perfluoroalkyl contaminants in an Artic marine food web: trophic magnification and wildlife exposure. Environ Sci Technol 43:4037–4043
Kissa E (2001) Fluorinated surfactants and repellents, vol 97, 2nd edn. Marcel Dekker, New York
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99:366–394
Loi EIH, Yeung LWY, Taniyasu S, Lam PKS, Kannan K, Yamashita N (2011) Trophic magnification of poly- and perfluorinated compounds in a subtropical food web. Environ Sci Technol 45:5506–5513
Martin JW, Whittle DM, Muir DCG, Mabury SA (2004a) Perfluoroalkyl contaminants in a food web from Lake Ontario. Environ Sci Technol 38:5379–5385
Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DCG, Mabury SA (2004b) Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ Sci Technol 38:373–380
McLachlan MS, Holmstrom KE, Reth M, Berger U (2007) Riverine discharge of perfluorinated carboxylates from the European continent. Environ Sci Technol 41:7260–7265
Mommaerts V, Hagenaars A, Meyer J, De Coen W, Swevers L, Mosallanejad H, Smagghe G (2011) Impact of a perfluorinated organic compound PFOS on the terrestrial pollinator Bombus terrestris (Insecta, Hymenoptera). Ecotoxicology 20: 447–456
Morikawa A, Kamei N, Harada K, Inoue K, Yoshinaga T, Saito N, Koizumi A (2006) The bioconcentration factor of perfluorooctane sulfonate is significantly larger than that of perfluorooctane in wild turtles (Trachemys scripta elegans and Chinemys reevesii): an Ai River ecological study in Japan. Ecotox Environ Saf 65:14–2
Müller CE, De Silva AO, Small J, Williamson M, Wang X, Morris A, Katz S, Gamberg M, Muir DCG (2011) Biomagnification of perfluorinated compounds in a remote terrestrial food chain: lichen–caribou–wolf. Environ Sci Technol 45:8665–8673
Naile JE, Khim JS, Hong S, Park J, Kwon B-O, Ryu JS, Hwang JH, Jones P, Giesy JP (2013) Distrubutions and bioconcentration characteristics of perfluorinated compounds in environmental samples collected from the west coast of Korea. Chemosphere 90:387–394
Powley R, George W, Ryan W, Buck C (2005) Matrix effect free analytical methods for determination of perfluorinated carboxylic acids in environmental matrixes. Anal Chem 77:6353–6358
R core team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (http://www.r-project.org/)
Rüdel H, Müller J, Jürling H, Bartel-Steinbach M, Koschorreck J (2011) Survey of patterns, levels, and trends of perfluorinated compounds in aquatic organisms and bird eggs from representative German ecosystems. Environ Sci Pollut R 1:1457–1470
Smithwick M, Maburay SA, Solomon KR, Sonne C, Martin JW, Born EW, Dietz R, Derocher AE, Letcher RJ, Evans TJ, Gabrielsen GW, Nagy J, Stirling I, Taylor MK, Muir DCG (2005) Circumpolar study of perfluoroalkyl contaminants in polar bears (Ursus maritimus). Environ Sci Technol 39:5517–5523
Stockholm Convention (2009) Report of the conference of parties of the Stockholm Convention on persistent organic pollutants on the work of its fourth meeting. UNEP/POPS/COP.4/38. 8 May 2009
Van de Vijver IK, Hoff PT, Das K, Van Dongen W, Esmans EL, Jauniaux T, Bouquegneau J-M, Blust R, De Coen W (2003) Perfluorinated chemicals infiltrate ocean waters: link between exposure levels and stable isotope ratios in marine mammals. Environ Sci Technol 37:5545–5550
Van Gossum H, Bots J, Snijkers T, Meyer J, Van Wassenbergh S, De Coen W, De Bruyn L (2009) Behaviour of damselfly larvae (Enallagma cyathigerum) (Insecta, Odonata) after long-term exposure to PFOS. Environ Poll 157:1332–1336
Van Gossum H, Audenaert B, De Bruyn L (2010) Perfluorooctane sulfonic acid contamination reduced fitness in Drosophila hydei (Diptera: Drosophilidae). Ann Entomol Soc 103:247–251
Vandorpe E, Verhagen R (1979) An age reference model for the wood mouse, Apodemus sylvaticus (Linnaeus, 1758), by use of the lens technique. Ann Soc Roy Zool Bel 109:133–140
Wang Y, Fu J, Wang T, Liang Y, Pan Y, Cai Y, Jiang G (2010) Distribution of perfluorooctane sulfonate and other perfluorochemicals in the ambient environment around a manufacturing facility in China. Environ Sci Technol 44:8062–8067
Wang T, Chen C, Naile JE, Khim JS, Giesy JP, Lu Y (2011) Perfluorinated compounds in water, sediment and soil form Guanting reservoir, China. Bull Environ Contam Toxicol 87:74–79
Xu D, Chandan L, Yuezhong W, Weiping L (2013) Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate (PFOS). Environ Poll 174:121–127
Zhao S, Zhu L, Liu L, Liu Z, Zhang Y (2013) Bioaccumulation of perfluoroalkyl carboxylates (PFCAs) and perfluoroalkane sulfonates (PFSAs) by earthworms (Eisenia fetida) in soil. Environ Poll 179:45–52
Zhang L, Liu J, Hu J, Liu C, Guo Wn Wang Q, Wang H (2012) The inventory of sources, environmental releases and risk assessment for perfluorooctane sulfonate in China. Environ Poll 165:193–198
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
Walter Van Dongen of the Nucleoside Research and Mass Spectrometry Unit from the University of Antwerp is acknowledged for assistance with LC-MS/MS measurements. Furthermore, the authors would like to thank Willy Verschueren, the conservator of Blokkersdijk, for his permission to allow us working in the nature reserve.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Leif Kronberg
Rights and permissions
About this article
Cite this article
D’Hollander, W., De Bruyn, L., Hagenaars, A. et al. Characterisation of perfluorooctane sulfonate (PFOS) in a terrestrial ecosystem near a fluorochemical plant in Flanders, Belgium. Environ Sci Pollut Res 21, 11856–11866 (2014). https://doi.org/10.1007/s11356-013-2449-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2449-4