Abstract
Information concerning the link between surface-water photochemistry and climate is presently very scarce as only a few studies have been dedicated to the subject. On the basis of the limited knowledge that is currently available, the present inferences can be made as follows: (1) Warming can cause enhanced leaching of ionic solutes from the catchments to surface waters, including cations and more biologically labile anions such as sulphate. Preferential sulphate biodegradation followed by removal as organic sulphides in sediment could increase alkalinity, favouring the generation of the carbonate radical, CO3 ·−. However, this phenomenon would be easily offset by fluctuations of the dissolved organic carbon (DOC), which is strongly anticorrelated with CO3 ·−. Therefore, obtaining insight into DOC evolution is a key issue in understanding the link between photochemistry and climate. (2) Climate change could exacerbate water scarcity in the dry season in some regions. Fluctuations in the water column could deeply alter photochemistry that is usually favoured in shallower waters. However, the way water is lost would strongly affect the prevailing photoinduced processes. Water outflow without important changes in solute concentration would mostly favour reactions induced by the hydroxyl and carbonate radicals (·OH and CO3 ·−). In contrast, evaporative concentration would enhance reactions mediated by singlet oxygen (1O2) and by the triplet states of chromophoric dissolved organic matter (3CDOM*). (3) In a warmer climate, the summer stratification period of lakes would last longer, thereby enhancing photochemical reactions in the epilimnion but at the same time keeping the hypolimnion water in the dark for longer periods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural systems such as the cryosphere, hydrosphere and biosphere are strongly influenced by climate, which is a major actor in determining the different ecosystem features. Accordingly, climate change is presently considered to be one of the most dangerous risks to the Earth's ecosystems.
A large number of studies have demonstrated that many physical and biological processes have already been affected by global and regional climate change. For example, physical phenomena such as sea-level rise and changes in run-off and some biological processes show the strong and widespread influence of the rise in temperature, which is the main climatic variable (Rosenzweig et al. 2007; Adrian 2009).
Limnological studies have recently demonstrated that freshwater systems and particularly lakes are good sentinels of climate change due to their ability to quickly modify their chemical, physical and biological features as a consequence of changes in their surrounding landscape and atmosphere (Carpenter et al. 2007; Pham et al. 2008; Williamson et al. 2008). This makes a large difference to the chemical properties of non-coastal oceanic waters, which respond slowly to anthropic impacts as a consequence of their enormous mass. On the contrary the physical–chemical properties of lakes, especially if small and located in regions largely impacted by climate change, can undergo measurable variations in relatively small time windows.
Lake physical parameters (e.g. fluctuations in water level and water temperature) and chemical features (dissolved organic carbon (DOC), pH, alkalinity, nutrient concentration, ion concentration, etc.), together with plankton composition, can act as measurable response variables for climate change, either directly or indirectly through the influence of climate on the catchment (Williamson et al. 2009; Rogora et al. 2003; Rosenzweig et al. 2007; Karst-Riddoch et al. 2005).
Physical, chemical and biological variables play a key role in a large number of science fields such as limnology, ecology and geochemistry. Therefore, such variables are monitored periodically by several laboratories that study the evolution over time of the ecological status of surface-water bodies (Evans et al. 2001; Hobbie et al. 2003). This explains the presence of many long-term data series that are essential for the monitoring of surface-water quality and health.
On the other hand, surface waters are the main receptors for (1) new classes of pollutants that are not biodegradable and/or cannot be eliminated in conventional waste water treatment plants (WWTPs) and (2) pesticide contamination from agricultural and urban use (Delpla et al. 2009). Modifications of chemical composition and physical properties of water bodies lead to an alteration of the fate and behaviour of dissolved organic compounds, thereby modifying their ability to affect the quality and health of the water systems.
The persistence in surface-water bodies of dissolved organic compounds, including both natural organic molecules and xenobiotic pollutants, is influenced by their transformation kinetics via abiotic and biological processes (Bucheli-Witschel and Egli 2001). Many organic pollutants such as polycyclic aromatic hydrocarbons, some pesticides, pharmaceuticals and their transformation intermediates are refractory to biological degradation. In such cases, the abiotic transformation processes can represent major removal pathways from surface waters.
Within the abiotic transformation reactions of xenobiotics, those induced by sunlight are receiving increasing attention because of their potential importance in the removal of the parent molecules and for the possible production of harmful secondary pollutants. Instances of photochemical generation of compounds that are of higher concern than the parent pollutant include the production of dioxins upon photolysis of triclosan (Latch et al. 2003) and the generation of acridine from carbamazepine (De Laurentiis et al. 2012a; Donner et al. 2013). The main photochemical pathways occurring in surface waters are direct photolysis and reaction with transients species that are photochemically generated, such as the triplet states of chromophoric dissolved organic matter (3CDOM*), the radicals · OH (hydroxy) and CO3 ·− (carbonate) as well as singlet oxygen, 1O2 (Hoigné 1990). Such species are produced upon absorption of sunlight by nitrate, nitrite and CDOM, in the presence of bicarbonate and carbonate as far as CO3 ·− generation is concerned.
Photochemical reactions are strongly dependent on sunlight irradiance, water chemistry and depth. Irradiance fixes incident sunlight photons, while water chemistry affects the concentration values of photoreactants (CDOM, nitrate and nitrite) and of the scavengers of reactive transients (largely DOM for · OH and CO3 ·−). Finally, depth is important because the deep layers of a water body are poorly illuminated by sunlight. Therefore, photochemical reactions are favoured in shallow water bodies. Climate change can affect water chemistry and depth in several ways (Delpla et al. 2009; Minella et al. 2011). For instance, a change in run-off and weathering rate can modify the concentration of cationic and anionic species in the receiving water environments. The biological lability of anions like sulphate would cause an increase of alkalinity (Schindler 2009), which would alter the concentration values of bicarbonate and carbonate and modify the formation rate of CO3 ·− upon oxidation of carbonate and bicarbonate by · OH.
To our knowledge, the effects of climate on surface-water photochemistry are largely unknown at the present moment, such that it can be argued that the topic does not even exist as a research field. Therefore, this contribution is intended to present the results of some pioneering works and our personal view about the potential impact that climate change can have on the photochemical processes in freshwater.
Photochemical processes in surface waters
Abiotic transformation reactions can be important for the degradation of bio-refractory organic pollutants in surface waters and of bio-refractory intermediates deriving from microbial processes (Oliveira et al. 2006). Interestingly, some bio-refractory organic compounds can become bioavailable after some degree of abiotic processing. Therefore, the combination of abiotic and biotic degradation can lead to the complete mineralisation of organic matter (Brinkmann et al. 2003). An example is the addition of hydroxyl groups to bio-refractory aromatic rings upon reaction between organic compounds and photochemically produced hydroxyl radicals. Usually, the biotransformation kinetics of hydroxylated substrates is orders of magnitude faster than that of the original compounds (Walker et al. 2006).
The abiotic transformation processes in surface waters include a large variety of reactions such as hydrolysis and oxidation mediated by dissolved species or by metal oxides such as Fe(III) and Mn(III,IV) (hydr)oxides. Hydrolysis will often produce bond cleavage, which in many cases results in the loss of a lateral functional chain. Hydrolytic reactions are usually acid- or base-catalysed, but at the pH values around neutrality that are typical of surface waters, the effects of catalysis may be limited (Comoretto et al. 2007).
Among the abiotic processes, light-induced reactions play a key role in the degradation of non-biodegradable compounds (Kuivila and Jennings 2007). Photochemical reactions can be divided into direct and indirect (or sensitised) photolysis. In the first pathway, compounds undergo direct transformation upon absorption of sunlight that can promptly induce photoionisation or the break of a chemical bond. In this case, the rate of degradation of a given molecule (R S ) depends on its absorption spectrum in the environmentally significant wavelength range and on the photolysis quantum yield φ S (Eq. 1: polychromatic photolysis quantum yield):
where the absorbed photon flux P a of a generic compound S (if alone in solution) is (Eq. 2: photon flux absorbed by a generic substrate S.)
In Eq. 2, p°(λ) is the incident spectral photon flux density of sunlight, ε(λ) is the molar absorption coefficient of the substrate S, b is the optical path length in the solution and [S] is the concentration of the substrate. The radiation absorption by dissolved compounds in the water body as well as the extinction of radiation by scattering phenomena may affect the rate of photolysis.
Sensitised photolysis is triggered by the absorption of radiation by photoactive compounds called photosensitisers, the main ones in surface waters being nitrate, nitrite and most notably CDOM. Dissolved organic matter (DOM) consists of water-dissolved organic compounds that may be derived from microbiological transformation of animal and plant spoils. DOM is composed of clusters of primary smaller moieties (100–200 Da) that are organised in supramolecular structures. These aggregates are composed of aromatic and aliphatic hydrocarbon structures with various functional groups (e.g. -CONH2, -COOH, -OH, -CO) (Leavitt et al. 2003). A very important role is played by aromatic/quinone moieties that are able to absorb sunlight. They are contained in humic and fulvic acids that arise from the biodegradation of lignin or from the oligomerisation/supramolecular association of smaller compounds, triggered by photo-oxidation (Leenher and Croue 2003). The fraction of DOM that is able to absorb sunlight is called CDOM (Boule et al. 2005). Radiation absorption by CDOM causes the formation of excited singlet states (1CDOM*) that are transformed by inter-system crossing (ISC) into excited triplet states (3CDOM*). The reactivity of dissolved compounds with 3CDOM* is more likely than that with 1CDOM* due to the much longer lifetime of the former (Canonica et al. 1995; Halladja et al. 2007).
The radical·OH is produced by irradiation of nitrate, nitrite and CDOM. In the latter case, the details of the process are still unclear, with a significant but not exclusive role of H2O2 that could for instance be involved in Fenton reactions (Page et al. 2011; Vermilyea and Voelker 2009). Moreover, there is wide evidence of an H2O2-independent pathway of·OH generation by irradiated CDOM (Page et al. 2011; Dong and Rosario-Ortiz 2012), which might possibly be accounted for by triplet-sensitised oxidation of OH− and/or H2O (Sur et al. 2011). The radical CO3 ·− is produced upon oxidation of CO3 2− and HCO3 − by·OH and through oxidation of carbonate by 3CDOM*. The triplet states 3CDOM* can degrade pollutants on their own, but they can also react with O2 to form the reactive transient 1O2 that is also involved in pollutant degradation. The reactions that can take place in natural water systems (apart from the still unclear formation of·OH from irradiated CDOM) are reported as follows (Canonica 2007):
The transients species·OH, CO3 ·−, 1O2 and 3CDOM* can all induce the degradation of xenobiotics but with important differences. In particular, the radical·OH is mostly involved in depollution with usually limited formation of harmful intermediates. Compared to·OH, the probability to form harmful intermediates is considerably higher in the case of 1O2, CO3 ·− and 3CDOM*. Differences among the transients largely depend on each single xenobiotic. Moreover, the photochemical pathway that is most likely to form harmful intermediates is the direct photolysis, which is for instance able to form cyclic compounds from acyclic precursors through photoinduced ring closure (Boreen et al. 2003) or through restructuring. For instance, in freshwater, the antiepileptic drug carbamazepine with a seven-member aromatic ring can evolve by direct photolysis into mutagenic acridine with a six-member pyridine ring (De Laurentiis et al. 2012a). Other radical transients such as·NO2, Cl2 ·−, and Br2 ·− could also be involved in the generation of secondary pollutants because they are nitrating and halogenating species (Vione et al. 2006).
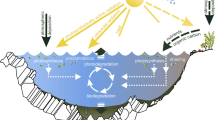
The photochemical reactions involving·OH, CO3 ·−, 1O2 and 3CDOM* strongly depend on water chemistry and depth. For instance, processes induced by·OH are favoured by elevated nitrate and nitrite (·OH sources) and are inhibited by DOM that is the main·OH scavenger in natural waters. The same conditions favour or inhibit processes that depend on CO3 ·−, because·OH is by far the main CO3 ·− source and DOM is its main scavenger. Additionally, the formation of CO3 ·− is enhanced by elevated carbonate and bicarbonate (reactions 3, 4, and 6). The processes induced by 1O2 and 3CDOM* are enhanced by elevated (C)DOM levels because CDOM irradiation is required to produce both 3CDOM* and 1O2. Moreover, CDOM is usually higher at higher DOM. Note that the scavenging of 1O2 and 3CDOM* is completely different from that of·OH and CO3 ·−. Indeed, 3CDOM* is mainly consumed by thermal deactivation and reaction with O2. In turn, 1O2 mainly undergoes inactivation by collision with the solvent. A picture of the main photochemical processes that take place in surface waters and that involve photosensitisers, photoactive transients and scavengers is reported (Fig. 1) (Minella et al. 2011).
Depth effect is mostly caused by water-dissolved absorbing substances and most notably by CDOM. The latter is the main radiation absorber in surface waters between 300 and 500 nm, which is the most significant wavelength interval for photoinduced processes. Sunlight absorption by CDOM plays a major role in decreasing the intensity of solar radiation in the water column. Therefore, the bottom layers of a water body are less illuminated than the surface, which causes photochemical processes to be faster in shallow-water bodies compared to deep ones. In the latter, high photoactivity in the surface layer is offset by lack of photoactivity at depth. Such an effect reduces the significance of the photochemical reactions when the water column depth increases, but it also protects the aquatic life from exposure to harmful UV radiation (Dattilo et al. 2005).
CDOM absorption shows an exponential decay with increasing wavelength, and absorption in the different spectral ranges decreases as UVB > UVA > visible. Accordingly, column penetration of radiation is in the order UVB < UVA < visible. Nitrate mostly absorbs UVB radiation, and its photochemistry is highly inhibited with depth. A lesser degree of inhibition is observed with nitrite that absorbs in the UVA, while CDOM that also absorbs in the visible is least affected by depth. Nitrite and nitrate are direct·OH sources and indirect ones of CO3 ·−, while 1O2 and 3CDOM* are only produced by CDOM. Therefore, the relative importance of 1O2 and 3CDOM* versus·OH and CO3 ·− increases with increasing depth (Minella et al. 2013a).
Links between climate and surface-water photochemistry
Effects of climate change on water chemistry and depth
Global warming can considerably affect the chemical composition and depth of surface waters. As far as water chemistry is concerned, a warmer climate will often enhance the transfer of solutes (sulphates, base cations and silica) from the catchments to river and lake water (Skjelkvåle et al. 2005). Higher cation concentrations would affect the direct photolysis of compounds that form complexes with Ca2+ and Mg2+, such as tetracycline (Werner et al. 2006). The increase in temperature would also affect the concentration of dissolved inorganic nitrogen due to higher phytoplankton productivity (Sommaruga-Wögrath et al. 1997; Rogora et al. 2003).
Biological processes could have considerable impact on water alkalinity because of the consumption of sulphate. The latter would be transported to surface waters together with base cations (e.g. Ca2+ and Mg2+), and it would be preferentially removed by biota (Schindler 1997 and 2009). In particular, the formation of organic sulphur species coupled with the permanence of dissolved base cations would lead to an increase of water alkalinity (Schindler 1997), with subsequent increase of pH and of the [CO3 2−]/[HCO3 −] ratio. Such a phenomenon would enhance the generation of CO3 ·− because carbonate reacts faster with·OH compared to bicarbonate. However, the occurrence of CO3 ·− is also largely affected by DOM that has two depressing effects on the steady state [CO3 ·−]. The first effect is the scavenging of·OH and, therefore, the inhibition of CO3 ·− production upon·OH-induced oxidation of carbonate and bicarbonate. The second effect is the direct scavenging of CO3 ·− by DOM itself. Indeed, a parallel increase of carbonate and bicarbonate on the one side and of DOM on the other would often lower the CO3 ·− levels instead of enhancing them (Minella et al. 2013a). Higher leaching from the catchment could actually increase the DOM loading in surface waters, to an extent that largely depends on the environment and climatic zone. A considerable DOM increase could take place as a consequence of warming at elevated latitudes, but the phenomenon could be less important in more temperate environments (Freeman et al. 2001). In contrast, leaching of DOM could be decreased in drought-affected regions and the outcome would be a decline of the DOC levels (Schindler 1997). Another issue is that, where observed, the DOC enhancement would be largely accounted for by organic acids (Skjelkvåle et al. 2005). The latter would both scavenge·OH and lower the [CO3 2−]/[HCO3 −] ratio, the two effects being detrimental to the occurrence of CO3 ·− (Hoigné 1990).
Depth and particularly water depth fluctuations could be highly dependent on climate change as they reflect the dynamic balance between water input (precipitation, catchment run-off) and water loss (outflow and evaporation) (Carere et al. 2011; Adrian 2009; Rosenzweig et al. 2007). Furthermore, if the increment of the average temperature is regionally associated with a decrement of precipitation, it can increase the demand of freshwater from lakes and rivers for agriculture and other human activities. This could exacerbate the fluctuation of the water level during the dry season. Global warming might shift the zone of water scarcity from the tropical ridge to more temperate latitudes, including most notably the Mediterranean region. The onset of water scarcity could mean that several permanent water bodies around the Mediterranean may become highly fluctuating, intermittent or even ephemeral (Ayache et al. 2009; Petrovic et al. 2011; Segui et al. 2010). Therefore, they would show marked depth minima during the dry season. An additional consequence of climate change is the increased likelihood of extreme precipitation events, causing large and sudden modifications of water depth.
A key issue linking water chemistry (which in turn affects photochemistry) and depth is represented by the way water is lost during the dry season. Among the possible mechanisms, three major ones can be listed: (i) water runaway in a river or from a lake via an emissary stream, which is not compensated for by inflow; (ii) water seepage to the underlying aquifer through the sediment and (iii) water evaporation because of the increase in temperature, as the dry season usually coincides with summer (Mason et al. 1994). In the first two cases, one can assume for simplicity that the water depth decreases but that the concentration of solutes in the remaining water does not vary. Cases (i) and (ii) will be considered together under the term of “outflow”. In the third case, water evaporates, but most solutes would not, thereby undergoing evaporative concentration in the remaining water. Evaporative concentration may be offset by processes such as precipitation of poorly soluble salts (e.g. CaCO3 and MgCO3) and microbial consumption of DOM. However, evaporative concentration may also lead to increased eutrophication and, in such a case, DOM might increase even further because of microbial processes. Overall, water evaporation is expected to produce higher concentration values of dissolved species (Schindler 1997; Minella et al. 2013b), and evaporative concentration in freshwater environments can induce the formation of brines where salinity is considerably higher compared to seawater (Zanor et al. 2012).
Climate change and surface-water (photo)chemistry
Even in very sensitive ecosystems such as lakes, climate change is only operational on the long term, and an assessment of climate-connected variations in photochemistry would require long-term series of dedicated measurements. The photochemical reactivity of surface waters has been the object of some point studies in some environments, but no long-term campaigns have been undertaken so far. Accordingly, there is no information about the photochemical behaviour in definite environments in the last 20–30 years, and the data of the next decades are clearly still unavailable. For this reason, the relationship between climate change and surface-water photochemistry is largely unknown and almost non-existent as a research topic. Furthermore, other processes that can alter photochemical reactivity operate on the ecosystems at the same time as climate change, thereby acting as confounding factors. They are for instance the anthropogenic release of micro- and macro-nutrients (Skjelkvåle et al. 2005), which may significantly affect water chemistry, as well as changes in incident irradiance that may be linked with solar cycles or with the slow recovery of stratospheric ozone, the latter mostly affecting the UVB flux (Zepp et al. 2011). As far as water chemistry is concerned, long-term series are often available for some chemical parameters that are monitored to assess the ecological status of water bodies (Evans et al. 2001). Luckily, some of these parameters are also essential to understand water photochemistry, such as nitrate, nitrite, carbonate, bicarbonate and most notably DOC, which is a measure of DOM and from which CDOM can also be obtained through a modelling approach.
We have recently developed a model that predicts the photochemical reactivity of surface waters as a function of water chemistry, absorption spectrum (which can be modelled from DOC if unavailable), depth and the incident irradiance spectrum of sunlight. The model was originally intended to predict the photochemical fate and photochemical persistence of pollutants in surface-water bodies, and it has been validated against the available field data for the photodegradation of several pollutants (see Table 1).
The same model can be used to predict the steady-state concentrations of photogenerated transients such as·OH, CO3 ·−, 1O2 and 3CDOM*. They can be predicted on the basis of data concerning nitrate, nitrite, DOC, carbonate and bicarbonate. The effects of water-level fluctuations can be predicted as well. So far, the model has been used to assess the photochemical impact of changes in water chemistry and depth at constant sunlight intensity, thereby not considering irradiance or spectral modifications caused by solar cycles or by the recovery of stratospheric ozone. Such modifications could be taken into account in the model, by changing the input data concerning the incident spectral photon flux density. The case studies carried out to date (at constant photon flux density) are presented below.
The first lake ecosystem to be investigated was Lake Maggiore, a subalpine lake located in NW Italy at the border between Piedmont, Lombardy and the Swiss Canton of Ticino (Fig. 2) (Minella et al. 2011).
Lake Maggiore is characterised by a fairly elevated anthropic pressure (over 600,000 inhabitants live in its surroundings) and has undergone a process of eutrophication (starting from the 1960s) followed by re-oligotrophication (environmental recovery due to wastewater treatment, starting from the 1990s) that is typical of several lakes in the subalpine region. It is a good case study because of the availability of complete water chemistry data of photochemical significance (also including DOC measurements) from the early 1990s. The relevant trends show a statistically significant decrease of both nitrate and DOC, most likely connected with decreased human impact through treated wastewater discharge. In contrast, the statistically significant increase of alkalinity and bicarbonate could be due to a combination of climate change and decreased acidic depositions (Minella et al. 2011; Rogora et al. 2012). By far, the most important issue from a photochemical point of view is the decrease of DOC, which causes the modelled/predicted [·OH] and [CO3 ·−] to significantly increase over time (see Fig. 3). The reason is that DOM (measured by DOC) is the main·OH scavenger, and it has an even more marked effect on CO3 ·−, through inhibition of formation (·OH consumption) and direct scavenging. In the case of CO3 ·−, some contribution to its increase over time comes from increased bicarbonate and carbonate, but the decrease of DOC plays a much more important role.
Modelled time trends of [·OH] (smoothed red solid line) and [CO3 ·−] (smoothed black solid line) in the top 1-m layer of Lake Maggiore. For both series, the linear fit line (black solid thin) is reported with the related error band (95 % confidence level, black dashed line (Minella et al. 2011)
The radicals·OH and CO3 ·− are involved in the self-depollution potential of surface waters. Their statistically significant increase over time suggests a parallel increase of such a potential, which is connected with the decrease of anthropic disturbance rather than with climate issues. Therefore, it is suggested that policies aimed at restoring the ecological status of surface-water environments would also improve their ability to get rid of bio-refractory pollutants. Such policies would thus reach an additional and, probably, largely unintended effect.
Due to the DOC trend, 3CDOM* and 1O2 are expected to decrease with time, but the predicted decrease is not statistically significant. The likely explanation is that CDOM absorbs sunlight almost completely, and a decrease of DOC causes a rather limited decrease of the photon flux absorbed by CDOM itself. Indeed, absorption saturation means that the trend of the absorbed photon flux versus DOC is not linear but tends to flatten out, thus providing limited sensitivity to DOC variations.
Similar conclusions were obtained from the modelling of the long-term data series of water chemistry for Lake Peipsi (Minella et al. 2013a). This lake is very different from Lake Maggiore in terms of water depth, trophicity, climatic zone and surrounding environment. Lake Peipsi is located in Southern Nordic Europe, at the border between Estonia and Russia. It consists of Lake Peipsi sensu stricto, the largest and deepest northern part, Lake Lämmijärv, the middle strait-like part, and Lake Pihkva, the southern and most shallow part. It is a polymictic shallow lake with an average depth of 7 m. Figure 2 reports the map of the lake.
Lake Peipsi is subject to a strong human impact, mainly through nutrients discharge that has considerably increased in the last decades. The ongoing eutrophication of the lake has caused considerable environmental damage: perhaps the most noteworthy effect is that the shallow lake waters can become anoxic in windless, still and warm summer nights, with considerable fish kills through lack of oxygen (Kangur et al. 2005; Kangur et al. 2007). Records of chemical parameters of photochemical significance exist since the early 1970s, which allows a long-term assessment of photochemistry. Measures of DOC are unfortunately unavailable, but the COD has been measured instead, which is allowed by the elevated organic loading of the lake. Considering that COD and DOC can be satisfactorily correlated in lake water (Chang et al. 1998) and that the DOC has not been routinely measured in most lakes before the 1990s, the long COD record of Lake Peipsi has a considerable added value for photochemistry assessment.
Lake-water eutrophication has caused a significant increase over time of the concentration values of nitrate, nitrite and of COD, which is an opposite behaviour compared to the re-oligotrophicating Lake Maggiore. Moreover, climate warming is also expected to increase the levels of organic matter in high-latitude environments (Freeman et al. 2001). Carbonate and bicarbonate have increased over time in Lake Peipsi, probably because of decreasing acidic depositions and increasing pH that have been common phenomena in Southern Nordic Europe over the considered time scale (Skjelkvåle et al. 2005). As far as photochemistry is concerned, the model for Lake Peipsi suggests a statistically significant decrease of both [·OH] and [CO3 ·−] and a statistically significant increase of [3CDOM*] and [1O2] (see Fig. 4). A likely explanation of both effects would be the increase of organic matter, measured as COD: in fact, DOM scavenges both·OH and CO3 ·− and inhibits the formation of CO3 ·−. These issues would make DOM the key driver in the decrease over time of both [·OH] and [CO3 ·−], despite the significant increase of bicarbonate. The models of Lake Peipsi photochemistry also suggest a statistically significant increase of both [3CDOM*] and [1O2] because CDOM is expected to increase when organic matter increases.
a, b Time trends of measured COD (a, solid circles) and modelled [3CDOM], [·OH] and [CO3 ·−] (a and b, smoothed solid lines) in the top 1 m of Lake Peipsi. For each data series, the linear fit line is reported in black solid thin lines, with the related error band (95 % confidence level, black dashed line) (Minella et al. 2013a)
As far as pollutant phototransformation is concerned, there would be a partial compensation between the decrease of [·OH] and [CO3 ·−] and the increase of [3CDOM*] and [1O2], which would also depend on the reactivity of each single pollutant toward the different reactive species. However, because·OH is the transient that is less likely to form harmful secondary pollutants from xenobiotics, its decrease would often be more important than the increase of 3CDOM* and 1O2. Therefore, it can be concluded that worsening water quality, as in the case of the eutrophicating Lake Peipsi, would also produce an overall decrease in the photochemical self-depollution potential.
Climate change and water depth
Water scarcity in the dry season can cause several effects on water bodies. The most evident one can be classified as water loss by outflow and/or evaporation, not compensated for by inflow (Moreno et al. 2010; Muller and Deil 2005; Larned et al. 2010). The cases of pure outflow and pure evaporation are quite rare, and one would usually observe a combination of the two. The modelling of water loss in the two extreme cases and for a hypothetical lake with an average chemical composition similar to that of lakes located in temperate zones is quite easy. The results are reported in Fig. 5 (Minella et al. 2013b). The figure also includes a mixed scenario (half of the water lost by evaporative concentration and the other half by outflow).
Modelled trends of [·OH] (a), [CO3 ·−] (b), [1O2] (c) and [3CDOM*] (d) with decreasing column depth, due to water loss (outflow, evaporative concentration and mixed case) (Minella et al. 2013b)
The decrease of water depth would in most cases be favourable to photochemistry because shallower water columns are thoroughly illuminated by sunlight. However, outflow would particularly enhance processes mediated by·OH and CO3 ·− because, at equal concentration of solutes, shallower water columns would mostly favour the photochemistry of species that only absorb poorly penetrating UV radiation, such as nitrate and nitrite. In contrast, the depth decrease would affect to a lesser extent reactions induced by 1O2 and 3CDOM*. The latter transients are exclusively generated by CDOM, which also absorbs visible radiation that penetrates more deeply into the water column (Minella et al. 2013b).
As far as evaporative concentration is concerned, the same photon flux would be absorbed by the sensitisers in a smaller volume and the formation rates of transient species would be increased as a consequence. In the case of·OH and CO3 ·−, this phenomenon would be offset by the contemporary increase of the concentration values of radical scavengers, such as DOM. The two opposite effects would cancel out almost exactly, yielding unmodified steady-state [·OH] and [CO3 ·−] (see Fig. 5). In contrast, the inactivation kinetics of 1O2 and 3CDOM* would not be altered by solutes undergoing evaporative concentration (unless very concentrated systems are formed); thus, higher formation rates would produce higher steady-state concentrations (Minella et al. 2013b). From the reported discussion, it can be inferred that the prevailing process of water loss could deeply alter the photochemical reactions taking place in a water body.
It is also important to remind that climate change can deeply affect water circulation in lakes, with important changes in the duration of stratification periods. The lack of circulation in the water column during the hot seasons, as a consequence of higher temperatures and weaker winds, can deplete the concentration of oxygen in the deep layers with marked changes in the lake properties (e.g. changing climate might transform lakes from oligomictic to total meromictic/holomictic). Moreover, the lake epilimnion can be exposed to sunlight for longer periods because of longer summer stratification (Wetzel 2001), which would increase the importance of photochemical processes in the stratified surface waters. Therefore, more extensive photoprocessing of pollutants could be observed in the epilimnion (but little or no phototransformation would take place in the hypolimnion) and the photoinduced formation of photoactive humic-like substances could also be enhanced (De Laurentiis et al. 2013), which could in turn impact the in-water photoprocesses.
An additional issue is that the increased carbonate/bicarbonate ratio produced by increasing alkalinity could be offset by enhanced dissolution of atmospheric CO2 (Adrian 2009). Further effects could be caused by an onset of or a recovery from acidic depositions (Skjelkvåle et al. 2005). The associated variations of pH have a potentially important impact on oxidation processes because, for instance, Fenton and Fenton-like reactions and nitrite photochemistry are favoured under acidic conditions (Vermilyea and Voelker 2009; Vione et al. 2001 and 2004). Moreover, the photoinduced mineralisation of DOM gets faster with decreasing pH (Anesio and Graneli 2004; Vione et al. 2009), possibly because of the enhanced photochemistry of the complexes between Fe and organic ligands.
Conclusions
The scarce information that is currently available about the link between surface-water photochemistry and climate change is accounted for by the relatively few studies that have been carried out so far over this issue. At the present state of knowledge, the following conclusions can be drawn:
-
1.
Enhanced catchment run-off of biologically labile sulphate together with more stable base cations could lead to increased water alkalinity and to higher [CO3 2−]/[HCO3 −] ratios. The latter issue could favour CO3 ·− formation because carbonate is considerably more reactive than bicarbonate towards·OH. However, this effect could be largely offset by DOC fluctuations because DOM is strongly anticorrelated with [CO3 ·−]. Therefore, a key issue into the link between photochemistry and climate is represented by the understanding of DOM variations upon temperature increase. A major confounding factor in this context is the fact that DOM variations are also connected to changes in human impact.
-
2.
A decrease in the column depth of water bodies during the dry season would generally enhance photochemical reactions. In particular, water outflow without important changes in the concentration of solutes would favour reactions induced by·OH and CO3 ·−. In contrast, evaporative concentration phenomena would enhance processes that involve 3CDOM* and 1O2.
-
3.
An increased duration of the hot season would prolong the summer stratification period in lakes, thereby enhancing the importance of photochemical reactions in the epilimnion but, at the same time, keeping the deep hypolimnion waters in the dark for a longer time.
It is clear that many additional research efforts will be required to better understand the influence that climate change may have on photochemical processes in surface waters.
References
Adrian R (2009) Lakes as sentinels of climate change. Limnol Oceanogr 54(6):2283–2297
Anesio AM, Graneli W (2004) Photochemical mineralization of dissolved organic carbon in lakes of differing pH and humic content. Arch Hydrobiol 160:105–116
Ayache F, Thompson JR, Flower RJ, Boujarra A, Rouatbi F, Makina H (2009) Environmental characteristics landscape history and pressures on three coastal lagoons in the Southern Mediterranean Region: Merja Zerga (Morocco), Ghar El Melh (Tunisia) and Lake Manzala (Egypt). Hydrobiologia 622:15–43
Boreen AL, Arnold WA, McNeill K (2003) Photodegradation of pharmaceuticals in the aquatic environment: a review. Aquat Sci 65:320–341
Boule P, Bahnemann D.W and Robertson P.K.J (eds) (2005) Environmental Photochemistry Part II, In: The handbook of environmental chemistry, vol. 2, part M, Springer, Berlin
Brinkmann T, Hoörsch P, Sartorius D, Frimmel FH (2003) Photoformation of low-molecular-weight organic acids from brown water dissolved organic matter. Environ Sci Technol 37:4190–4198
Bucheli-Witschel M, Egli T (2001) Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol Rev 25:69–106
Canonica S (2007) Oxidation of aquatic organic contaminants induced by excited triplet states. Chimia 61:641–644
Canonica S, Jans U, Stemmler K, Hoigné J (1995) Transformation kinetics of phenol in water-photosensitization by dissolved natural organic material and aromatic ketones. Environ Sci Technol 29:1822–1831
Carere M, Miniero R, Cicero MR (2011) Potential effects of climate change on the chemical quality of aquatic biota. Trac - Trends Anal Chem 30:1214–1221
Carpenter SR, Benson BJ, Biggs R, Chipman JW, Foley JA, Golding SA, Hammer RB, Hanson PC, Johnson PTJ, Kamarainen AM, Kratz TK, Lathrop RC, McMahon KD, Provencher B, Rusak JA, Solomon CT, Stanley EH, Turner MG, Jake Vander Zanden M, Wu C, Yuan H (2007) Understanding regional change: a comparison of two lake districts. Bioscience 57:323–335
Chang E, Chiang PC, Lin TF (1998) Development of surrogate organic contaminant parameters for source water quality standards in Taiwan, ROC. Chemosphere 37:593–606
Comoretto L, Arfib B, Chiron S (2007) Pesticides in the Rhone river delta (France): basic data for a field-based exposure assessment. Sci Total Environ 380:124–132
Dattilo AM, Decembrini F, Bracchini L, Focardi S, Mazzuoli S, Rossi C (2005) Penetration of solar radiation into the waters of Messina Strait (Italy). Annal Chim 95:177–184
De Laurentiis E, Chiron S, Kouras-Hadef S, Richard C, Minella M, Maurino V, Minero C, Vione D (2012a) Photochemical fate of carbamazepine in surface freshwaters: laboratory measures and modeling. Environ Sci Technol 46:8164–8173
De Laurentiis E, Maurino V, Minero C, Vione D, Mailhot G, Brigante M (2013) Could triplet-sensitised transformation of phenolic compounds represent a source of fulvic-like substances in natural waters? Chemosphere 90:881–884
Delpla I, Jung A-V, Baures E, Clement M, Thomas O (2009) Impacts of climate change on surface water quality in relation to drinking water production. Environ Int 35:1225–1233
Dong MM, Rosario-Ortiz FL (2012) Photochemical formation of hydroxyl radical from effluent organic matter. Environ Sci Technol 46:3788–3794
Donner E, Kosjek T, Qualmann S, Kusk KO, Heath E, Revitt DM, Ledin A, Andersen HR (2013) Ecotoxicity of carbamazepine and its UV photolysis transformation products. Sci Total Environ 443:870–876
Evans CD, Cullen JM, Alewell C, Kopacek J, Marchetto A, Moldan F, Prechtel A, Rogora M, Vesely J, Wright R (2001) Recovery from acidification in European surface waters. Hydrol Earth Syst Sci 5:283–297
Freeman C, Evans CD, Monteith DT, Reynolds B, Fenner N (2001) Export of organic carbon from peat soils. Nature 412:785
Halladja S, Ter Halle A, Aguer JP, Boulkamh A, Richard C (2007) Inhibition of humic substances mediated photooxygenation of furfuryl alcohol by 2,4,6-trimethylphenol. Evidence for reactivity of the phenol with humic triplet excited states. Environ Sci Technol 41:6066–6073
Hobbie JE, Carpenter SR, Grimm NB, Gosz JR, Seastedt TR (2003) The US long term ecological research program. Bioscience 53:21–32
Hoigné J (1990) Formulation and calibration of environmental reaction kinetics: oxidations by aqueous photooxidants as an example. In: Stumm W (ed) Aquatic chemical kinetics. Reaction rates of processes in natural waters. Wiley, New York, pp 43–70
Kangur K, Kangur A, Kangur P, Laugaste R (2005) Fish kill in Lake Peipsi in summer 2002 as a synergistic effect of a cyanobacterial bloom, high temperature, and low water level. Proceedings of Estonian Academy of Sciences Biology, Ecology 54:67–80
Kangur M, Kangur K, Laugaste R, Punning J-M, Möls T (2007) Combining limnological and palaeolimnological approaches in assessing degradation of Lake Pskov. Hydrobiologia 584:121–132
Karst-Riddoch TL, Pisaric MFJ, Smol JP (2005) Diatom responses to 20th century climate-related environmental changes in high-elevation mountain lakes of the northern Canadian Cordillera. J Paleolimn 33(3):265–282
Kuivila KM, Jennings BE (2007) Input, flux, and persistence of six select pesticides in San Francisco Bay. Int J Environ Anal Chem 87:897–911
Larned ST, Datry T, Arscott DB, Tockner K (2010) Emerging concepts in temporary river ecology. Freshw Biol 55:717–738
Latch DE, Packer JL, Arnold WA, McNeill K (2003) Photochemical conversion of triclosan to 2,8-dichlorodibenzo-p-dioxin in aqueous solution. J Photochem Photobiol A Chem 158:63–66
Leavitt PR, Cumming BF, Smol JP, Reasoner M, Pienitz R, Hodgson D (2003) Climatic control of ultraviolet radiation effects on lakes. Limnol Oceanogr 48:2062–2069
Leenher JA, Croue JP (2003) Characterizing aquatic dissolved organic matter. Environ Sci Technol 37:18A–26A
Maddigapu PR, Minella M, Vione D, Maurino V, Minero C (2011) Modeling phototransformation reactions in surface water bodies: 2,4-Dichloro-6-nitrophenol as a case study. Environ Sci Technol 45:209–214
Mason IA, Guzkowska MAJ, Rapley CG, Street-Perrott FA (1994) The response of lake levels and areas to climatic change. Clim Chang 27(2):161–197
Minella M, Rogora M, Vione D, Maurino V, Minero C (2011) A model approach to assess the long-term trends of indirect photochemistry in lake water. The case of Lake Maggiore (NW Italy). Sci Total Environ 409:3463–3471
Minella M, De Laurentiis E, Buhvestova O, Haldna M, Kangur K, Maurino V, Minero C, Vione D (2013a) Modelling lake-water photochemistry: three-decade assessment of the steady-state concentration of photoreactive transients (OH, CO3 − and 3CDOM*) in the surface water of polymictic Lake Peipsi (Estonia/Russia). Chemosphere 90:2589–2596
Minella M, Maurino V, Minero C and Vione D (2013b) Modelling photochemical transformation of emerging organic pollutants in surface waters: effect of water level fluctuations following outflow or evaporation, relevant to arid and semi-arid environments. Int. J. Environ. Anal. Chem., doi: http://dx.doi.org/10.1080/ 03067319.2013.803284 (in press).
Moreno IM, Avila A, Losada MA (2010) Morphodynamics of intermittent coastal lagoons in Southern Spain: Zahara de los Atunes. Geomorphology 121:305–316
Muller JV, Deil U (2005) The ephemeral vegetation of seasonal and semi-permanent ponds in tropical West Africa. Phytocoenologia 35:327–388
Oliveira JL, Boroski M, Azevedo JCR, Nozaki J (2006) Spectroscopic investigation of humic substances in a tropical lake during a complete hydrological cycle. Acta Hydrochim Hydrobiol 34:608–617
Page SE, Arnold WA, McNeill K (2011) Assessing the contribution of free hydroxyl radical in organic matter-sensitized photohydroxylation reactions. Environ Sci Technol 45:2818–2825
Petrovic M, Ginebreda A, Acuna V, Batalla RJ, Elosegi A, Guasch H, de Alda ML, Marce R, Munoz I, Navarro-Ortega A, Navarro E, Vericat D, Sabater S, Barceló D (2011) Combined scenarios of chemical and ecological quality under water scarcity in Mediterranean rivers. Trac - Trends Anal Chem 30:1269–1278
Pham SV, Leavitt PR, McGowan S, Peres-Nato P (2008) Spatial variability of climate and land-use effects on lakes of the northern Great Plains. Limnol Oceanogr 53:728–742
Rogora M, Mosello R, Arisci S (2003) The effect of climate warming on the hydrochemistry of alpine lakes. Water, Air, Soil Poll 148:347–361
Rogora M, Arisci S, Marchetto A (2012) The role of nitrogen deposition in the recent nitrate decline in lakes and rivers in Northern Italy. Sci Total Environ 417–418:214–223
Rosenzweig C, Casassa G, Karoly D.J, Imeson A, Liu C, Menzel A, Rawlins S, Root T.L, Seguin B and Tryjanowski P (2007) Assessment of observed changes and responses in natural and managed systems. Climate change. Impacts, adaptation and vulnerability. Contribution of working group II to the fourth Assessment report of the intergovernmental panel on climate Change, Parry M.L., Canziani O.F., Palutikof J.P., van der Linden P.J. and Hanson C.E., Eds. Cambridge University Press, Cambridge, UK, 79–131.
Schindler DW (1997) Widespread effects of climatic warming on freshwater ecosystems in North America. Hydrol Proc 11:1043–1067
Schindler DW (2009) Lakes as sentinels and integrators for the effects of climate change on watersheds, airsheds, and landscapes. Limnol Oceanogr 54:2349–2358
Segui PQ, Ribes A, Martin E, Habets F, Boe J (2010) Comparison of three downscaling methods in simulating the impact of climate change on the hydrology of Mediterranean basins. J Hydrol 383:111–124
Skjelkvåle BL, Stoddard JL, Jeffries DS, Tørseth K, Høgåsen T, Bowma J, Mannio J, Monteith DT, Mosello R, Rogora M, Rzychon D, Vesely J, Wieting J, Wilander A, Worsztynowicz A (2005) Regional scale evidence for improvements in surface water chemistry 1990–2001. Environ Pollut 137:165–176
Sommaruga-Wögrath S, Koinig KA, Schmidt R, Sommaruga R, Tessadri R, Psenner R (1997) Temperature effects on the acidity of remote alpine lakes. Nature 387:64–67
Sur B, Rolle M, Maurino V, Minero C, Vione D, Brigante M, Mailhot G (2011) Formation of hydroxyl radicals by irradiated 1-nitronaphthalene (1NN): oxidation of hydroxyl ions and water by the 1NN triplet state. Photochem Photobiol Sci 10:1817–1824
Sur B, De Laurentiis E, Minella M, Maurino V, Minero C, Vione D (2012) Photochemical transformation of anionic 2-nitro-4-chlorophenol in surface waters: laboratory and model assessment of the degradation kinetics, and comparison with field data. Sci Total Environ 426:296–303
Vermilyea AW, Voelker BM (2009) Photo-Fenton reaction at near neutral pH. Environ Sci Technol 43:6927–6933
Vione D, Maurino V, Minero C, Pelizzetti E (2001) Phenol photonitration upon UV irradiation of nitrite in aqueous solution. II. Effects of pH and TiO2. Chemosphere 45:903–910
Vione D, Maurino V, Minero C, Pelizzetti E (2004) Phenol nitration upon oxidation of nitrite by Mn(III, IV) (hydr)oxides. Chemosphere 55:941–949
Vione D, Maurino V, Minero C, Pelizzetti E, Harrison MAJ, Olariu RI, Arsene C (2006) Photochemical reactions in the tropospheric aqueous phase and on particulate matter. Chem Soc Rev 35:441–453
Vione D, Lauri V, Minero C, Maurino V, Malandrino M, Carlotti ME, Olariu RI, Arsene C (2009) Photostability and photolability of dissolved organic matter upon irradiation of natural water samples under simulated sunlight. Aquat Sci 71:34–45
Vione D, Khanra S, Das R, Minero C, Maurino V, Brigante M, Mailhot G (2010) Effect of dissolved organic compounds on the photodegradation of the herbicide MCPA in aqueous solution. Wat Res 44:6053–6062
Vione D, Maddigapu PR, De Laurentiis E, Minella M, Pazzi M, Maurino V, Minero C, Kouras S, Richard C (2011) Modelling the photochemical fate of ibuprofen in surface waters. Wat Res 45:6725–6736
Walker CH, Hopkin SP, Sibly RM, Peakall DB (2006) Principles of ecotoxicology, 3rd edn. Taylor & Fancis, New York
Werner JJ, Arnold WA, McNeill K (2006) Water hardness as a photochemical parameter: tetracycline photolysis as a function of calcium concentration, magnesium concentration, and pH. Environ Sci Technol 40:7236–7241
Wetzel RG (2001) Limnology Lake and River Ecosystems, 3rd edn. Academic Press, New York
Williamson CE, Dodds W, Kratz TK, Palmer M (2008) Lakes and streams as sentinels of environmental change in terrestrial and atmospheric processes. Front Ecol Environ 6:247–254
Williamson CE, Saros JE, Schindler DW (2009) Sentinels of change. Science 323:887–888
Zanor GA, Piovano EL, Ariztegui D, Vallet-Coulomb C (2012) A modern subtropical playa complex: Salina de Ambargasta, central Argentina. J South Am Earth Sci 35:10–26
Zepp RG, Erickson DJ III, Paulk ND, Sulzberger B (2011) Effects of solar UV radiation and climate change on biogeochemical cycling: interactions and feedbacks. Photochem Photobiol Sci 10:261–279
Acknowledgments
The PhD grant of EDL was financially supported by Progetto Lagrange–Fondazione CRT. DV also acknowledges financial support by Università di Torino - EU Accelerating Grants, project TO_Call2_2012_0047 (Impact of radiation on the dynamics of dissolved organic matter in aquatic ecosystems - DOMNAMICS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
De Laurentiis, E., Minella, M., Maurino, V. et al. Effects of climate change on surface-water photochemistry: a review. Environ Sci Pollut Res 21, 11770–11780 (2014). https://doi.org/10.1007/s11356-013-2343-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2343-0