Abstract
Phthalates are widely used as plasticizer in various consumer domestic products and are known to disturb the male reproductive function in rodents. This study investigated the involvement of oxidative stress and the atrophy of the testes in pubertal rats exposed to mono-n-butyl phthalate (MBP). Four-week-old pubertal male rats were separated into three groups. In group I, 21 rats were fed rat chow containing 2 % MBP for 3 days. In group II, 21 rats were fed rat chow containing 2 % MBP for 3 days and antioxidant vitamins C (250 mg/kg/day) and E (50 mg/kg/day) were injected daily. In group III, 21 rats were fed standard rat chow and used as controls. After 3 days, each testis was weighed and the germ cell development was evaluated using the Johnsen score. The urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels were measured as a biological marker of oxidative DNA damage. The mean testis weight was significantly lower for group I than groups II or III (p < 0.05). The mean Johnsen score was significantly lower for group I than for groups II or III (p < 0.05). Urinary 8-OHdG concentrations were higher in group I than in groups II or III. Short-time exposure to MBP may therefore induce oxidative DNA damage in rat testes, while antioxidant vitamins administered during exposure may protect against this stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalate esters are known to be endocrine disruptors and are widely used in the plastics industry. They are ubiquitous in the environment and are known to contaminate food and water supplies. Di-n-butyl phthalate induced impairment in both prepubertal and fetal rodent testes by inhibiting testosterone production (Moody et al. 2013; Borch et al. 2006). Our previous studies have shown that mono-n-butyl phthalate (MBP) exposure affects testicular descent and germ cell development in both prenatal and postnatal rats (Imajima et al. 1997; Shono et al. 2000, 2005; Kondo et al. 2006). In prenatal rats, MBP exposure prevented testicular descent by inhibiting the gene expression of insulin-like factor 3 and testosterone production in the fetal testes (Shono et al. 2000, 2005). In postnatal rats, MBP exposure resulted in testicular atrophy and increased germ cell apoptosis in immature rats (Kondo et al. 2006), although no clear mechanism by which the phthalate esters induced testicular damage has yet been elucidated. In these studies, short-term exposure (3 to 10 days) of both prenatal and postnatal rats to MBP induced serious testicular damage. The present study elucidated the possible involvement of oxidative stress in the pathogenesis of testicular atrophy in pubertal rats following very short-term MBP exposure (only 3 days), and whether administration of antioxidant vitamins can protect the testis from the expected harmful effects.
Materials and methods
Chemicals and animals
MBP (purity > 98 %) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Vitamin C was purchased from the Takeda Co., Ltd. (Tokyo, Japan) and vitamin E was obtained from Eizai Co., Ltd. (Tokyo, Japan). Rat chow containing 2 % MBP was produced by Kyudo Co., Ltd. (Fukuoka, Japan). Wistar–King A rats were obtained from a colony maintained at Seac Yoshitomi Int. (Fukuoka, Japan). All rats were provided tap water and food ad libitum in an air-conditioned environment (22 ± 1 °C) with a 12-h light/dark cycle.
Experimental protocols
Pubertal male rats (4 weeks old) were separated into three groups. In group I (n = 21), rats were fed rat chow containing 2 % MBP for 3 days. In group II (n = 21), rats were fed rat chow containing 2 % MBP for 3 days. In addition, antioxidant vitamins C (250 mg/kg/day) and E (50 mg/kg/day) were injected daily for 3 days. The doses of the vitamins were determined by referring to previous reports (Gupta et al. 2004). In group III (n = 21), rats were fed the standard commercial rat chow and were injected with the same volume of saline as group II for 3 days. Group III rats were used as controls. The estimated intake of MBP was calculated for each rat from groups I and II daily. Urine from each rat was collected daily, and levels of urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), a specific biological marker of oxidative DNA damage (Kasai and Nishimura 1984), were measured by high-performance liquid chromatography (SRL Inc, Tokyo, Japan). After 3 days, the rats were sacrificed and the testes were removed. The testes were weighed, fixed in 10 % formalin solution, and embedded in paraffin. Embedded specimens were sectioned at a thickness of 5-μm thickness and were stained with hematoxylin and eosin. Germ cell maturation of each specimen was evaluated using the Johnsen score (JS) (Johnsen 1970). Student's t test was used for the statistical analyses. P values of less than 0.5 were considered to be statistically significant. Our institutional review board approved this study. All animals have received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Results
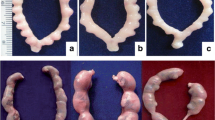
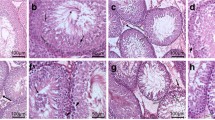
The results are detailed in Table 1. There was no significant difference in the estimated intake volume of MBP between rats in groups I and II. The mean testis weight for group I was significantly lower than groups II or III (p < 0.05). The mean JS for group I was also significantly lower than groups II or III (p < 0.05). The mean level of the urinary 8-OHdG concentration in group I was significantly higher than groups II or III (p < 0.05). Germ cell development was inhibited in the rats exposed to MBP; however, the testes were protected by the simultaneous injection of antioxidant vitamins to some degree (Fig. 1).
a Histological development of the germ cells was remarkably inhibited in group I. b The administration of antioxidant vitamins appeared to protect the germ cell development in group II to some degree from the phthalate-induced oxidative stress to the testes. c Normal germ cell development in group III (control) ST spermatid, SC spermatocyte, SG spermatogonia, SE Sertoli cell, JS Johnsen score
Discussion
Oxidative stress is induced by reactive oxygen species or free radicals. Oxidative stress is believed to be involved in the pathophysiology of aging and various age-related diseases, including atherosclerosis, neoplastic diseases, diabetes, chronic inflammatory diseases of the gastrointestinal tract, skin aging, and other disorders (Stohs 1995). Oxidative damage occurs in various levels of tissue, including the cell, nucleus, cytoplasmic proteins, RNA, and DNA (Pryor et al. 2006). 8-OHdG was first reported by Kasai and Nishimura (Kasai and Nishimura 1984) as a specific biological marker of oxidative DNA damage in 1984, and is currently one of the most popular markers for the evaluation of oxidative DNA damage and oxidative stress.
The testis is sensitive to various oxidative stress inducers such as radiation (Hasegawa et al. 1997), high temperature (Shiraishi et al. 2010), and environmental and industrial agents (Koizumi and Li 1992; Ghosh et al. 2002). Oxidative stress has been suggested to cause male reproductive dysfunction associated with male infertility (Kodama et al. 1997). Turner et al. suggested that oxidative stress induced testicular damage caused by various conditions, and that physiological antioxidant defense systems protected the testis from oxidative stress (Turner and Lysiak 2008). Non-enzymatic antioxidant vitamins E and C and other antioxidants also inhibited oxidative damage in testis (Gupta et al. 2004; Agarwal et al. 2007; Uguralp et al. 2005).
Phthalate esters have been reported as testicular toxicants (Moody et al. 2013; Borch et al. 2006; Imajima et al. 1997; Shono et al. 2000) and MBP causes germ cell apoptosis and the atrophy of the testis in rats dependent upon age (Kondo et al. 2006). Exposure to MBP causes increased damage to the testis of immature rats compared to adult rats (Kondo et al. 2006).
In the present study, a 3-day, short-time administration of MBP induced significantly increased the levels of urinary 8-OHdG and severe degeneration of germ cells in immature rats. Furthermore, both the germ cell degeneration and the elevation of urinary 8-OHdG levels were inhibited by the simultaneous injections of antioxidant vitamins E and C. The mechanisms by which MBP induces testicular DNA damage have not yet been fully elucidated. Phthalate have been known to be able to produce free radicals by several pathways in germ cells (Suna et al. 2007). Kim et al. suggested that MBP induced oxidative stress in cultured rat embryonic limb bud cells (Kim et al. 2002). Oxidative stress has been reported to induce testicular DNA damage and germ cell apoptosis in humans and animals (Turner and Lysiak 2008; Kasahara et al. 2002). MBP may induce testicular DNA damage by two pathways; one is the mitochondrial pathway and the other is the Fas system. Oxidative stress alters the association between proapoptotic and antiapoptotic members of the Bcl-2 family on the mitochondrial membrane (Adams and Cory 1998), allowing the release of cytochrome c and induces the fragmentation of a cell's DNA by the activation of a caspase cascade (Green 1998). Certain testicular toxicants, like MBP, induce germ cell apoptosis through a pathway involving Fas and Fas L binding on the cell membrane which initiates the intracellular death domain pathway resulting in DNA fragmentation (Lee et al. 1999). MBP may thus induce and cause testicular DNA damage and germ cell apoptosis in pubertal rats.
The present results showed that short-term exposure to MBP may induce oxidative DNA damage and the atrophy of the testis in immature rats, with non-enzymatic antioxidant vitamins C and E, can prevent this occurrence.
References
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Agarwal A, Prabhakaran SA, Sikka SC (2007) Clinical relevance of oxidative stress in patients with male factor infertility: evidence-based analysis. AUA Update Ser 26:1–12
Borch J, Axelstad M, Vinggaard AM, Dalgaard M (2006) Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis. Toxicol Lett 163(3):183–190
Ghosh D, Das Sarkar S, Maiti R, Jana D, Das UB (2002) Testicular toxicity in sodium fluoride treated rats: association with oxidative stress. Reprod Toxicol 16:385–390
Green DR (1998) Apoptotic pathways: the roads to ruin. Cell 94:695–698
Gupta RS, Gupta ES, Dhakal BK, Thakur AR, Ahnn J (2004) Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells 17(1):132–139
Hasegawa M, Wilson G, Russell L, Meistrich ML (1997) Radiation induced cell death in the mouse testis: relationship to apoptosis. Radiat Res 147:457–467
Imajima T, Shono T, Zakaria O, Suita S (1997) Prenatal phthalate causes cryptorchidism postnatally by inducing transabdominal ascent of the testis in fetal rats. J Pediatr Surg 32:18–21
Johnsen SG (1970) Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1:2–25
Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, Tokuda M, Nakano Y, Inoue M (2002) Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J 365:849–856
Kasai H, Nishimura S (1984) Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agent. Nucleic Acid Res 12:2137–2145
Kim SH, Kim SS, Kwon O, Sohn KH, Kwack SJ (2002) Effects of dibutyl phthalate and monobutyl phthalate on cytotoxicity and differentiation in cultured rat embryonic bud cells; protection by antioxidants. J Toxicol Environ Health Part A 65(5–6):461–472
Kodama H, Kasai H, Yamaguchi R, Tanaka T, Fukuda J (1997) Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril 68:519–524
Koizumi T, Li ZG (1992) Role of oxidative stress in single-dose, cadmium-induced testicular cancer. J Toxicocol Environ Health 37:25–36
Kondo T, Shono T, Suita S (2006) Age-specific effect of phthalate ester on testicular development in rats. J Pediatr Surg 41:1290–1293
Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K (1999) The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology 140:582–588
Moody S, Goh H, Bielanowicz A, Rippon P, Loveland KL, Itman C (2013) Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinology 154(9):3460–3475
Pryor WA, Houk KN, Foote CS (2006) Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol 291:R491–R511
Shiraishi K, Takihara H, Matsuyama H (2010) Elevated scrotal temperature, but not varicocele grade, reflects testicular oxidative stress-mediated apoptosis. World J Urol 28:359–364
Shono T, Kai H, Suita S, Nawata H (2000) Time-specific effects of mono-n-butyl phthalate on the transabdominal descent of the testis in rat fetuses. BJU Int 86:121–125
Shono T, Shima Y, Kondo T, Suita S (2005) In utero exposure to mono-n-butyl phthalate impairs insulin-like factor 3 gene expression and the transabdominal phase of testicular descent in fetal rats. J Pediatr Surg 40:1861–1865
Stohs SJ (1995) The role of free radicals in toxicity and disease. J Basic Clin Physiol Pharmacol 6:205–228
Suna S, Yamaguchi F, Kimura S, Tokuda M, Jitsunari F (2007) Preventive effect of D-psicose, one of rare ketohexoses, on di-(2-ethylhexyl) phthalate (DEHP)-induced testicular injury in rat. Toxicol Lett 173:107–117
Turner T, Lysiak JJ (2008) Oxidative stress: a common factor in testicular dysfunction. J Androl 29:488–498
Uguralp S, Usta U, Mizrak B (2005) Resveratrol may reduce apoptosis of rat testicular germ cells after experimental testicular torsion. Eur J Pediar Surg 15:333–336
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Shono, T., Taguchi, T. Short-time exposure to mono-n-butyl phthalate (MBP)-induced oxidative stress associated with DNA damage and the atrophy of the testis in pubertal rats. Environ Sci Pollut Res 21, 3187–3190 (2014). https://doi.org/10.1007/s11356-013-2332-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2332-3