Abstract
The insecticide chlordecone is a contaminant found in most of the banana plantations in the French West Indies. This study aims to search for fungal populations able to grow on it. An Andosol heavily contaminated with chlordecone, perfused for 1 year in a soil–charcoal system, was used to conduct enrichment cultures. A total of 103 fungal strains able to grow on chlordecone-mineral salt medium were isolated, purified, and deposited in the MIAE collection (Microorganismes d'Intérêt Agro-Environnemental, UMR Agroécologie, Institut National de la Recherche Agronomique, Dijon, France). Internal transcribed spacer sequencing revealed that all isolated strains belonged to the Ascomycota phylum and gathered in 11 genera: Metacordyceps, Cordyceps, Pochonia, Acremonium, Fusarium, Paecilomyces, Ophiocordyceps, Purpureocillium, Bionectria, Penicillium, and Aspergillus. Among predominant species, only one isolate, Fusarium oxysporum MIAE01197, was able to grow in a liquid culture medium that contained chlordecone as sole carbon source. Chlordecone increased F. oxysporum MIAE01197 growth rate, attesting for its tolerance to this organochlorine. Moreover, F. oxysporum MIAE01197 exhibited a higher EC50 value than the reference strain F. oxysporum MIAE00047. This further suggests its adaptation to chlordecone tolerance up to 29.2 mg l−1. Gas chromatography–mass spectrometry (GC-MS) analysis revealed that 40 % of chlordecone was dissipated in F. oxysporum MIAE01197 suspension culture. No chlordecone metabolite was detected by GC-MS. However, weak amount of 14CO2 evolved from 14C10-chlordecone and 14C10-metabolites were observed. Sorption of 14C10-chlordecone onto fungal biomass followed a linear relationship (r 2 = 0.99) suggesting that it may also account for chlordecone dissipation in F. oxysporum MIAE01197 culture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cyclodiene insecticide chlordecone (CLD) (1,1a,3,3a,4,5,5,5a,5b,6-decachlorooctahydro-1,3,4-metheno-2H-cyclobuta[cd]pentalen-2-one) was used in the French West Indies (FWI) to control banana weevil, Cosmopolites sordidus, until recently. It was applied in FWI banana plantations for 18 years over two distinct periods. During the first one, from 1972 to 1978, the trademark product Kepone was applied and during the second one, from 1982 to 1993, the trademark product Curlone was applied. Over that 18-year period, approximately 300 tons of chlordecone was applied to banana plantations in the French West Indies. Pollution monitoring in the context of the National Action Plan for chlordecone revealed the presence of chlordecone in soils, rivers, springs, and drinking water as well as in food crop products such as root vegetables (Le Déault and Procaccia 2009). Cabidoche et al. (2009) showed that in soils used for banana production (i.e., representing 20,000 ha in the FWI) chlordecone concentrations ranged between 0.2 and 37.4 mg kg−1. In addition, due to its environmental persistence, sorption, and biomagnification potential, this organochlorine insecticide was classified as a persistent organic pollutant in May 2009 and added to Annex A of the Stockholm Convention. Recently, Multigner et al. (2010) showed a higher prevalence of prostate cancers in FWI populations exposed to chlordecone. In addition, a recent study reports that cognitive, visual, and motor development of young children exposed to chlordecone is impacted (Dallaire et al. 2012). As expected because of its high potential for biomagnification, chlordecone also contaminated aquatic biota, including fish and giant shrimp called ouassou (Coat et al. 2006; Coat et al. 2011), and crops, notably locally produced vegetables (Cabidoche and Lesueur-Jannoyer 2012; Clostre and Lesueur-Jannoyer 2012). Consequently, French authorities regulated fishing and marketing of marine wildlife and forbade the consumption of fish and shrimps produced from rivers. They also forbade the consumption of certain species of game birds potentially contaminated with chlordecone in Guadeloupe and defined recommendations for soil usage to avoid vegetable contamination.
Taking into account the recalcitrance of chlordecone to biodegradation, Cabidoche et al. (2009) suggested that leaching is the major process responsible for chlordecone dissipation from contaminated soils. By applying a simple leaching model based on first-order desorption kinetics, taking into account soil organic carbon content (SOC) and the SOC/water partitioning coefficient (Koc) as input parameters, Cabidoche et al. (2009) suggest that chlordecone contamination will last for several decades in Nitisol, centuries in Ferralsol, and half a millennium in Andosol soils of the French West Indies. Faced with this critical situation, we urgently need to search for possible solutions to treat this widespread pollution. Keeping in mind the large contamination area that rules out any ex situ treatments, any practical strategy for decontaminating chlordecone-polluted soils in the French West Indies will have to employ in situ methods. Among possible cleaning-up strategies, bioremediation strategies based on the use of the purifying capabilities of the soil microflora could be considered. Indeed, recently, Dolfing et al. (2012) studied the Gibbs free energy data of chlordecone and concluded that there are no thermodynamic reasons why chlordecone-respiring or chlordecone-fermenting organisms should not exist. However, despite this thermodynamic consideration, there are only a few numbers of studies about chlordecone biodegradation. Under anaerobic conditions Methanosarcina thermophila, a methanogenic bacterium that produces methane, transformed up to 86 % of 14C10-chlordecone within 10 days, leading to the accumulation of polar and nonpolar 14C-labeled metabolites (Jablonski et al. 1996). Although the mechanisms and metabolites of chlordecone transformation have not been further identified, M. thermophila was suggested to perform a reductive dechlorination of chlordecone. Pseudomonas sp. KO3, a bacterial strain purified from a mixed culture enriched under aerobic conditions from sewage sludge-collected lagoon water, was shown to convert Kepone to monohydro-kepone and to a lesser extent to dihydro-kepone. These findings were criticized by Cabidoche et al. (2009), who considered that the authors did not describe the initial contents in mono-hydro- and di-hydro-chlordecone known to contaminate Kepone due to incomplete chlorination during the production process. This lack of information weakens the findings of that study since both metabolites observed here may derive from an incomplete chlorination of the molecule during the manufacturing process, not from a biodegradation process. A former study of George et al. (1986) reported chlordecone degradation by three Pseudomonas spp. strains, with apparent degradation ranging between 15 and 25 % with an error of 10 % on chlordecone HPLC concentration measurements after 2 weeks of incubation. But details were missing in this study casting doubt on the conclusion that chlordecone is degradable under aerobic conditions. More recently, Pseudonocardia sp. strain KSF27, a soil bacterium isolated from Japanese agricultural soils frequently treated with endosulfan, was tested using a soil–charcoal perfusion method with aldrin trans-diol as a structural analog of dieldrin. Pseudonocardia sp. strain KSF27 degraded not only dieldrin, but also other persistent organochlorine pesticides, such as endosulfan sulfate, heptachlor, and chlordecone (Sakakibara et al. 2011). Interestingly, although organochlorine pesticides are mainly degraded by bacterial populations in soil habitats (Porto et al. 2011), several studies evidence their biodegradation by fungal populations. Indeed, Ortega et al. (2011) showed that Penicillium miczynskii, Aspergillus sydowii, and Trichoderma sp. could grow on the insecticide dichlorodiphenyldichloroethane (DDD). Trichoderma sp. was found able to degrade DDD, and its biodegradation ability was increased with the addition of H2O2. Aspergillus terreus, Aspergillus niger, and Cladosporium oxysporum can biodegrade endosulfan (Bhalerao and Puranik 2007; Mukherjee and Mittal 2005). Fungal enzymatic activities can also degrade several organophosphates. Indeed, Fusarium oxysporum cutinase can be involved in the degradation of malathion (Kim et al. 2005) and A. sydowii, A. flavus, and F. oxysporum phosphatases can hydrolyze several pesticides (Hasan 1999). Li et al. (2006) evidenced that Purpureocillium lilacinum (formerly P eacilomyces lilacinus) could degrade phoxim, an organophosphate insecticide. Although fungal isolates are known to harbor an impressive enzymatic toolbox responsible for pesticide degradation, their involvement in chlordecone degradation is not yet described to our knowledge.
In this context, the purpose of our study was to search for the presence of fungal isolates able to grow on mineral salt medium added with chlordecone as the sole carbon source in heavily contaminated French West Indies soils. To do so, Andosol soil samples contaminated with up to 35 mg kg−1 of chlordecone were continuously perfused with a chlordecone solution for 14 months using a soil–charcoal perfusion system (Takagi and Yoshioka 2000). Enrichment cultures were then initiated using a mineral salt medium added with chlordecone as the sole carbon source. During the time course of the enrichment, aliquots of the enrichment culture were plated onto potato–dextrose–chlordecone solid medium to isolate chlordecone-tolerant fungal species. Fungal isolates were then classified into operational taxonomic units (OTUs) according to their morphological characteristics and further identified using a DNA-based approach. The isolates were then screened for their tolerance to chlordecone and their ability to grow on it. The degrading ability of the fungal isolate able to grow on chlordecone as the sole carbon source was then studied using a radiorespirometry approach and gas chromatography–mass spectrometry (GC-MS) analysis.
Materials and methods
Chemicals
[12C U]-Chlordecone Pestanal® (1,1a,3,3a,4,5,5,5a,5b,6-decachlorooctahydro-1,3,4-metheno-2H-cyclobuta[cd]pentalen-2-one) was purchased from Sigma-Aldrich (Schnelldorf, Germany) (chemical purity 99.7 %). [14C U]-Chlordecone was purchased from Moravek Biochemicals (Brea, CA, USA) at a specific activity of 1,443 MBq mmol−1 (radiochemical purity 99.9 %). The solubility of chlordecone is 2.7 mg l−1 at 25 °C (Kilzer et al. 1979).
Media
The mineral salt (MS) medium used in the present study contained 1.6 g of K2HPO4, 0.4 g of KH2PO4, 0.2 g of MgSO4 7H2O, 0.1 g of NaCl, 0.02 g of CaCl2, and 1 ml of trace element solution per liter. The trace element solution contained ZnSO4·7H2O (0.2 mg), MnSO4·H2O (1.8 mg), H3BO3 (2 mg), CuSO4 (0.1 mg), and Na2MoO4 (0.25 mg) in 1 l. To prepare the agar plates, 15 g of agar (Biokar Diagnostics) per liter was added to the medium. The medium was autoclaved and then supplemented with chlordecone (C10Cl10O) as a carbon source using a Bransonic® ultrasonic water bath 221, 10 ml of filter-sterilized (NH4)2SO4 solution (100 g l−1) as the sole nitrogen source, 1 ml of filter-sterilized FeSO4·6H2O solution (5 g l−1), and 1 ml of filter-sterilized vitamin solution per liter. The vitamin solution contained 40 mg of biotin and 100 mg of thiamin–HCl per liter. For tolerance tests, the MS medium was supplemented with 20 ml of filter-sterilized glucose solution (250 g l−1) after autoclaving, as the main carbon source.
The phosphate buffer contained K2HPO4 (1 g), KH2PO4 (1 g), MgSO4, 7H2O (40 mg), in 1 l adjusted to pH 6.6. The medium was autoclaved and then supplemented with 1 ml of filter-sterilized FeSO4·6H2O solution (5 g l−1).
The potato dextrose agar (PDA) medium used in the present study contained 39 g of PDA (Fluka Analytical, Sigma-Aldrich) and 5 g of agar per liter. The medium was autoclaved and then supplemented with 30 mg of chlordecone per liter using an ultrasonic water bath. Throughout the cultivation period, all the agar plates were maintained in plastic bags in order to prevent desiccation.
F. oxysporum strains and storage conditions
The strains used in this study were F. oxysporum MIAE00047 (Collection de Microorganismes d'Intérêt Agro-Environnemental, UMR AgroEcologie, Institut National de la Recherche Agronomique, Dijon, France) as the reference strain (Alabouvette 1986) and fungal isolates MIAE01197 to 01208 and MIAE01219 to 01309 isolated from a French West Indies Andosol contaminated with chlordecone (this study). To preserve F. oxysporum strains, conidia were collected from the sporulation culture 7 days after inoculation by scraping and using 1 ml of chlordecone–potato–dextrose–broth medium. This suspension was mixed with an equal volume of 50 % (v/v) sterilized glycerol, distributed in 1 ml aliquots, and stored at −80 °C.
Soil sampling
The soil was sampled in June 2010 from a banana plantation located in Guadeloupe (French West Indies, 61°37′15″W; 16°03′57″N) by the team of Yves-Marie Cabidoche (INRA, Guadeloupe, France). This plantation was regularly treated with chlordecone at 6 kg ha−1 year−1 from 1982 to 1993. Soil samples were collected in accordance with ISO 10381-6 recommendations (Soil quality–Sampling–Part 6: Guidance on the collection, handling and storage of soil for the assessment of aerobic microbial processes in the laboratory). A composite sample of the surface horizon (0–15 cm) made of at least five random samplings (approximately 5 kg) was constituted. The soil sample was then shipped to INRA Dijon at 4 °C. It was then sieved to 2 mm and stored at 4 °C until use.
Soil physicochemical properties
The soil physicochemical properties were determined by the Laboratory of Soil Analysis (INRA, Arras, France) following standardized procedures. Granulometry analyses showed that it was composed of 128 g kg−1 of clay (<2 μm), 99 g kg−1 of fine silt (2–20 μm), 60 g kg−1 of coarse silt (20–50 μm), 37 g kg−1 of fine sand (50–200 μm), and 676 g kg−1 of coarse sand (0.2–2 mm). Physicochemical analyses yielded 79.8 g kg−1 of organic carbon, 6.11 g kg−1 of total nitrogen, a C/N ratio of 13, 138 g kg−1 of organic matter, a pH value of 5.21 measured in water, and a pH value of 4.53 measured in KCl. It was classified as an Andosol (FAO-WRB, 1998) and was rich in allophane, an amorphic clay resulting from the transformation of andesitic rock (volcanic origin). The structural properties and the spatial arrangement of allophane aggregates constitute a trap for the chlordecone molecule, which is thus mechanically retained (Fernandes et al. 2010). This soil was shown to be contaminated with 35.4 mg kg−1 of chlordecone and with 0.65 mg kg−1 of β-monohydrochlordecone (Martin-Laurent et al. 2013).
Enrichment culture
The enrichment culture was performed using a soil–charcoal perfusion method as initially described by Takagi et al. (2000) and modified later by Takagi et al. (2009). Briefly, 50 g of soil (dry weight equivalent) was mixed with 2.5 g of autoclaved activated charcoal A100 (2 g, grain size 5–10 mm, BET specific surface area of 100 m2 g−1, pH 7.8; Toyo Denka Kogyo, Kochi, Japan). The soil–charcoal mixture was placed in the perfusion system and continuously perfused with 50 ml of mineral salt solution containing 2 mg l−1 of chlordecone. The soil–charcoal mixture was perfused for 14 months in the dark at 28 °C. Over this period, the chlordecone mineral salt solution was replaced once a month.
Soil and charcoal were separated manually. They were then suspended in mineral salt buffer (1/100, w/v) using a Waring Blendor®. Microbial cultures were then initiated starting from these suspensions by inoculating fresh chlordecone mineral salt medium (1/100, v/v). Enrichment cultures were conducted over a 4-month period at 28 °C with shaking at 150 rpm. The cultures were renewed once a month by inoculating fresh chlordecone mineral salt medium (1/100, v/v). For each culture, an aliquot was stored at −20 °C in a glass flask for further chemical analysis.
Fungi were selected on chlordecone–MS agar plates.
Isolation of fungal strains
During the time course of the enrichment cultures, microbes were plated onto MS medium added with chlordecone and incubated at 28 °C. Fungal colonies were retrieved and sub-cultivated on chlordecone–potato dextrose agar (chlordecone–PDA) medium. Fungal isolates were purified by repeated cultures on chlordecone–PDA medium. Pure fungal isolates were deposited in the “Collection de Microorganismes d'Intérêt Agro-Environnemental” under the accession numbers MIAE01197 to 01208 and MIAE01219 to 01309 (UMR AgroEcologie, Institut National de la Recherche Agronomique, Dijon, France). They were classified according to their morphotypes by considering several parameters such as mycelium characteristics, spore color, and shape. On this basis, the fungal isolates with similar morphotypes were grouped into OTUs. A rarefaction curve was obtained by applying the freeware program aRarefactWin.exe (http://www.uga.edu/∼strata/AnRareReadme.html). The diversity of the fungal community isolated from the Andosol was assessed using (a) the Shannon–Wiener index calculated as H′ = −∑pi ln pi, where pi = ni/N and ni is the number of isolates in each morphotype and N the total number of isolates, and (b) the Simpson Diversity index D calculated as D = ∑ (p 2 i).
DNA extraction
DNA was extracted from 500 μl of fungal suspension using the DNeasy Plant Mini Kit according to the manufacturer's instructions (Qiagen, France). DNA was extracted using a rapid minipreparation procedure (Edel et al. 2001) when fungal isolates were recalcitrant to DNA extraction. DNA was purified using the Geneclean® Turbo Kit according to the manufacturer's instructions (MPBio). DNA quality was checked by electrophoresis and stored at −20 °C until use.
ITS amplification and sequencing
The internal transcribed spacer (ITS) located between the 18S rRNA and 28S rRNA of the fungal ribosomal gene was amplified by PCR using pure fungal DNA extracts in a final volume of 25 μl containing 1.5 U of Taq DNA polymerase (Q-biogene, France), 2.5 μl of Taq Polymerase buffer 10× containing 1.5 mM MgCl2 (Q-biogene, France), 1.6 μM dNTPs, 1 μl of 10 μM ITS1F primer (5′-CTTGGTCATTTAGAGGAAGTAA-3′ (Gardes and Bruns 1993), and 1 μl of 10 μM ITS4 primer (5′-TCCTCCGCTTATTGATATGC-3′ (White 1990). PCR amplification was performed with a MasterCycler (Eppendorf, Germany) under the following conditions: 94 °C for 3 min and then 35 cycles each composed of 94 °C for 1 min, 50 °C for 1 min, and an elongation step at 72 °C for 1 min. A final elongation at 72 °C for 30 min was performed. ITS amplicon quality was verified by electrophoresis. Each amplicon was then diluted down to 50 to 100 ng μl−1. ITS amplicons were sequenced by Beckman Coulter Genomics (Takeley, UK) using Sanger technology. ITS sequences will be deposited in GenBank database from KC786979 to KC787040 accession numbers. They were aligned with reference sequences retrieved from GenBank using ClustalX software. A neighbor-joining tree was computed using NJPlot software package (http://pbil.univlyon1.fr/software/njplot.html).
Chlordecone dissipation ability
One-month-old F. oxysporum MIAE01197 cultures grown on chlordecone–MS medium (1.5 mg l−1) were collected. Chlordecone–MS media without fungal inoculums were also analyzed as controls. Three replicates of each treatment (assay and control) were prepared. F. oxysporum MIAE01197 dissipation ability was assessed by GC-MS by modifying the procedure described by Martin-Laurent et al. (2013). Briefly, each culture aliquot was concentrated, and 13C10-chlordecone as an isotopic tracer and anthracene-d10 as an internal standard were added. This mixture was ultrasound treated for 30 min and then filtered. The filtrate was diluted with a solvent and analyzed by GC-MS with an Agilent 7890 A gas chromatograph equipped with a DMPS column coupled to an Agilent 5975 C mass spectrometer. Ionization was performed by electron impact (70 eV). Chlordecone identification was performed by comparing the results with mass spectra in the literature (WILEY257, NIST, Aromalyse databases). 12C10-chlordecone was quantified against an internal calibration with 13C10-chlordecone.
Chlordecone mineralization
The capacity of fungal isolates to mineralize chlordecone was tested by radiorespirometry in liquid cultures. The fungal isolates were grown for 1 week at 25 °C with shaking at 150 rpm, in MS medium with glucose as the main carbon source, supplemented with chlordecone (1.5 mg l−1). They were then collected by centrifugation at 10,000 rpm for 5 min. The pellets were washed twice in ultra-pure H2O and resuspended to 0.1 OD600 unit in MS medium containing 1 mg l−1 of [12C U]-chlordecone and 33.3 Bq ml−1 of [14C U]-chlordecone, with and without glucose. Fungal suspensions were incubated for 3 weeks at 20 °C in a sterile glass jar and shaken (150 rpm). Each jar contained a scintillation vial filled with 5 ml of 0.2 M NaOH to trap CO2. 14CO2 production was monitored all along the incubation period by measuring radioactivity in the NaOH solution by liquid scintillation counting (Wallac 1409), using 10 ml of Ready Safe™ cocktail (Beckman Coulter Inc., USA). Three replicates were performed. Non-inoculated medium was used as control.
At the end of the incubation period, fungal cultures were centrifuged. The collected supernatants were then extracted with dichloromethane (2:1; v/v). The organic phase was then evaporated and resuspended in 500 μl of hexane/acetone (3:1; v/v) and spotted onto glass-backed silica gel thin-layer chromatography (TLC) plates (10 × 20 cm) (Merck). Extracted compounds, including radioactive ones, were separated by migration in hexane/acetone (3:1, v/v) according to Jablonski et al. (1996). Radioactive compounds were detected by autoradiography (Carestream® Kodak® BioMax® MR film 20.3 × 25.4 cm, Sigma-Aldrich, France).
Growth test on chlordecone
The ability of F. oxysporum strains MIAE01197 and MIAE00047 to grow on chlordecone was tested. The strains were grown on glucose–MS medium (7 days, 25 °C, 180 rpm) to prepare spore suspensions. They were then filtered to remove the mycelium. The filtrates were centrifuged (8,000×g, 20 min), the pellets were resuspended in 1 ml of sterilized ultrapure water, and spores were counted using a Malassez counting chamber. The spore suspensions were diluted to obtain a final concentration of 5 × 105 spores ml−1.
The turbidity analysis was performed using a Bioscreen C Microbiological Growth Analyser (Labsystems, Helsinki, Finland). One-hundred-well microplates specifically manufactured for that machine were inoculated with F. oxysporum MIAE01197 or MIAE00047 spore suspensions (50 μl) previously loaded with MS medium (200 μl) containing chlordecone (3 mg l−1) as the main carbon source. Five replicates of each condition were prepared.
The OD600 was recorded every hour over a 7-day period. The experiments were conducted at 25 °C. Data were recorded using Easy Bioscreen Experiment software (EzExperiment) provided by the manufacturer and then exported to a Microsoft Excel Professional 2010 (Microsoft Corporation, Redmond, Washington, USA) sheet for further analysis.
Tolerance to chlordecone
The tolerance of the fungal isolates to chlordecone was tested by studying their growth in liquid culture. The F. oxysporum MIAE01197 and MIAE00047 strains grown on glucose–MS medium (7 days, 25 °C, 180 rpm) were used to prepare spore suspensions following the protocol described for growth test except that cultures were carried out on MS media (200 μl) with glucose (5 g l−1) as main carbon source and with a gradient of chlordecone content (0, 0.1, 1, and 10 mg l−1). Five replicates of each condition were done.
Toxicity of chlordecone for fungal strains
Chlordecone toxicity was assessed in liquid culture by monitoring mycelium biomass production by MIAE01197 and MIAE00047, starting from spore inoculum. Spore suspensions were prepared from mycelium mats grown in petri dishes with agar medium (10 mM KH2PO4, 4 mM MgSO4, 50 mM NH4Cl, 1 mM CaCl2, 7 mM KCl, 10 g l−1 glucose, 5 g l−1 yeast extract, 10 g l−1 agar) adapted from Abadulla et al. (2000). Briefly, spores were collected with sterile water and counted on a Malassez counting chamber. The spore inoculum was diluted to 3 × 104 spores ml−1. Dose–effect experiments were carried out by inoculating 30 μl of spore suspension (1,000 spores) into 10 ml of liquid medium supplemented with gradual quantities of chlordecone ranging from 0 to 30 mg l−1. Whatever the concentration, chlordecone was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) at 4 % final concentration (v/v). Three replicates were run for each chlordecone concentration tested, plus two controls consisting of DMSO alone or sterile deionized water instead of DMSO. Incubation was statically run for 5 days in the dark at 25 °C. Then, cultures were filtered (30 μm pore size nylon filters) and the mycelium from each flask was collected, dried at 80 °C, and weighed to calculate biomass production as an endpoint. For each condition, EC50 values (effective concentration of chlordecone that decreases biomass production by 50 % compared to the control) were calculated by performing a nonlinear regression on Hill's model using REGTOX software integrating the optimal EC50 value (version EV7.0.5., E. Vindimian, http://eric.vindimian.9online.fr/).
Chlordecone adsorption to fungal biomass
F. oxysporum strain MIAE01197 biomass was produced on MS–glucose and spore suspension prepared as described above. Different spore suspensions were prepared in phosphate buffer to reach 0.1, 0.5, and 1 OD600. All the fungal suspensions were added with 200 Bq of [14C U]-chlordecone. For each fungal suspension, three repeats were done and non-inoculated phosphate buffer was used as control (n tot = 12). All the suspensions were incubated at 20 °C under 150 rpm agitation for 72 h. At different times (i.e., 0, 2, 24, and 72 h of incubation), 1 ml aliquots were taken from the different samples. Aliquots were centrifuged at 10,000 rpm for 5 min. The supernatant was removed and kept to quantify the amount of radioactivity remaining in it. Fungal pellet was resuspended in sterile ultrapure water, transferred in a scintillation flask, and added with 10 ml of Ready Safe™ cocktail (Beckman Coulter Inc., USA). Radioactivity was measured by liquid scintillation counting on both supernatant and fungal pellet.
Statistical analyses
Growth curves of fungal isolates were fitted with the Gompertz model modified by Zwietering et al. (1990) using the SigmaPlot 4.0 software. The equation was y = A ·exp{−exp[1 + μm · exp(1) (λ − t) / A]}, where y is the OD600 value (arbitrary unit), t is time (hour), μm is the maximum specific growth rate (hour−1), A is the maximum OD600 value reached (arbitrary unit), and λ is the lag time (hour). The parameters thus determined were validated by a Student's t test (P < 0.005). Tolerance to chlordecone was estimated by comparing the μmCLD/μmcontrol ratios.
Results
Isolation and morphological characterization of fungal strains isolated from the Andosol
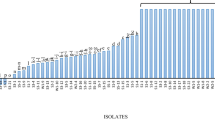
Aliquots of Andosol historically contaminated with 35.5 mg kg−1 of chlordecone were perfused for a year with a chlordecone solution (2 mg l−1) in a soil–charcoal system to exert a selection pressure favorable for the development of chlordecone-tolerant microorganisms. Soil samples were then used to perform enrichment cultures. Four successive 1-month long enrichment cultures were performed. For each enrichment culture, the aliquots were plated on chlordecone–PDA solid medium to isolate fungal isolates from the Andosol. A total of 103 fungal isolates were purified. Using the morphological characteristics observed on chlordecone–PDA medium (mycelium shape: form, elevation, border, surface, and spore pigmentation), these isolates were grouped into 18 different morphotypes (Table 1). Rarefaction analysis, which plots the number of fungal strains isolated from the Andosol as a function of the number of morphotypes detected, showed that the curve did not reach the asymptote. This means that the whole diversity of cultivable fungal species was not fully recovered at that sampling effort (Fig. 1). Morphotypes 1, 2, and 3 were dominant and represented up to 76 % of the fungal isolates. The other 15 morphotypes were represented by a few (1 to 4) isolates. The Shannon–Wiener index (H) was equal to 1.94, suggesting a relatively moderate diversity of cultivable fungal isolates retrieved from the contaminated Andosol, as revealed by rarefaction analysis. The Simpson index (D) was equal to 0.22, indicating codominance of several morphotypes within the cultivable fungal community isolated from the chlordecone-contaminated Andosol.
Rarefaction curve of the observed diversity of the fungal morphotypes as a function of the number of fungal strains isolated from enrichment cultures obtained from the Andosol perfused for 1 year with chlordecone–MS medium in a soil–charcoal device. The rarefaction curve was obtained by applying the freeware program aRarefactWin.exe (http://www.uga.edu/∼strata/AnRareReadme.html). Error bars represent 95 % confidence intervals calculated from the variance of the number of fungal morphotypes
Characterization of the diversity of fungal isolates
In order to estimate the diversity of the fungal isolates, at least one isolate per morphotype was chosen to sequence the 18S–28S ITS of the ribosomal operon. For the three dominant morphotypes, this analysis was performed on several isolates. Altogether 81 ITS sequences were obtained out of which 63 will be deposited in the GenBank database. Comparison of those sequences with known ones available in the database showed similarities ranging from 97 to 100 %. The phylogenetic analysis revealed that all the isolates belonged to the Ascomycota phylum. They were distributed among 11 genera including Metacordyceps, Cordyceps, Pochonia, Acremonium, Fusarium, Paecilomyces, Ophiocordyceps, Purpureocillium, Bionectria, Penicillium, and Aspergillus (Fig. 2). The three dominant morphotypes were identified as F. oxysporum sp. (morphotype 1), Paecilomyces sp./Ophiocordyceps heteropoda/P. lilacinum (morphotype 2), and Aspergillus sp. (morphotype 3). Interestingly, the 33 ITS sequences of morphotype 1 were 99 to 100 % similar to F. oxysporum isolate 281 (accession no. JN232163.1). Conversely, the 13 ITS sequences of morphotype 2 were 100 % similar to Paecilomyces sp. Pa972 (accession no. JQ821350.1), O. heteropoda (accession no. AB084157.1), and P. lilacinum isolate DF12064 (accession no. JQ863231.1) sequences. The last one was similar to Metacordyceps chlamydosporia strain NBAII PC55 (accession no. JX918944.1), Cordyceps chlamydosporia isolate ID05-F0165 (accession no. AB378545.1) and Pochonia chlamydosporia (EU733637.1) sequences. Furthermore, the 15 ITS sequences of morphotype 3 were all similar to Aspergillus ITS sequences, namely A. flavus (100 % similarity with A. flavus accession no. JX157882.1), A. nomius (99 to 100 % similarity with A. nomius isolate A15A, accession no. JQ781730.1), and A. terreus (100 % similarity with A. terreus strain SHPP01, accession no. JF738047.1). This morphotype was dominated by A. flavus, which represented 50 % of the sequenced fungal isolates. It is noteworthy that several other morphotypes represented by a single isolate gathered in the Aspergillus genus (A. flavus morphotype 11; A. nomius morphotypes 10 and 18; and A. terreus morphotypes 13, 14, and 17). Similarly, ITS analysis revealed that identical genera could be found in different morphotypes as Paecilomyces sp./O. heteropoda/P. lilacinum in morphotypes 2, 4, 6, and 7 and M. chlamydosporia/C. chlamydosporia/P. chlamydosporia in morphotypes 2, 4, 6, and 9. At this stage, representative of the morphotype 8 has not been sequenced yet.
Phylogenetic neighbor-joining tree of the ITS sequences of the fungal strains isolated from enrichment cultures obtained from the Andosol perfused for 1 year in a soil–charcoal device. The tree was built from the maximum likelihood criteria, using ClustalX software and NJplot program. Branch lengths are proportional to the evolutionary distances between sequences. Dots mean that bootstrap values are above or equal to 900 out of 1,000 iterations. Isolates are noted in bold and were registered in the MIAE (Microorganismes d'Intérêt Agro-Environnementaux) collection from INRA Dijon (France). In brackets, morphotype number and corresponding abundance are indicated separated by a dash. Several ITS sequences were deposited in the GenBank database under the following accession numbers: KC787016 (MIAE01197); KC786984 (MIAE01198); KC603897 (MIAE01199); KC786992 (MIAE01200); KC787004 (MIAE01204); KC787021 (MIAE01207), KC787023 (MIAE01208); KC787001 (MIAE01242); and KC787036 (MIAE01299). One representative of each fungal species is shown. Reference sequences and corresponding accession numbers retrieved from the GenBank database are separated by a hyphen
Screening of the fungal isolates for growth on chlordecone–MS medium
We focused our attention on the three dominant morphotypes, for which isolates able to grow on chlordecone were searched using a test in Bioscreen. Out of all the isolates tested, only F. oxysporum MIAE01197 was able to grow on liquid mineral salt medium with 3 mg l−1 of chlordecone added as the sole carbon source (Fig. 3a). As a control, F. oxysporum MIAE01197 was unable to grow on liquid mineral salt medium. In addition, the reference strain not previously exposed to chlordecone F. oxysporum MIAE00047 grew neither on MS–chlordecone nor on MS medium (Fig. 3b). Further characterization of growth of MIAE01197 and of MIAE00047 addressed by biomass production showed that both of them were able to weakly grow on liquid mineral salt medium (i.e., approximately 2 mg of biomass within 2 weeks of incubation). However, F. oxysporum MIAE01197 developed a significantly higher biomass (i.e., 7 ± 1 mg) than F. oxysporum MIAE00047 (i.e., 3 ± 1 mg) when grown on liquid mineral salt medium with 10 mg l−1 of chlordecone added as the sole carbon source.
a Growth curves of F. oxysporum MIAE01197 recorded from cultures on chlordecone–MS medium (solid lines). As a control, the growth of F. oxysporum MIAE01197 inoculated MS medium was monitored (dashed lines). b Growth curves of F. oxysporum MIAE00047 grown on MS medium (solid lines). As a control, the growth of F. oxysporum MIAE00047 inoculated MS medium was monitored (dashed lines). For each strain and for each condition tested, five replicates were performed (n tot = 20)
Characterization of F. oxysporum MIAE01197 tolerance to chlordecone
The tolerance of F. oxysporum MIAE01197 and MIAE00047 to chlordecone was addressed by assessing the impact of increasing concentrations of chlordecone (0, 0.1, 1, and 10 mg l−1 of chlordecone) on growth parameters. Both strains grew without a lag phase on glucose–MS medium in the presence of chlordecone. However, the growth rate (micrometer) of F. oxysporum MIAE01197 was significantly increased in response to chlordecone exposure (p < 0.01; n = 5). On the contrary, the growth rate of MIAE00047, the reference strain unexposed to chlordecone, was not affected by chlordecone (Fig. 4).
Tolerance to chlordecone of F. oxysporum MIAE01197 isolated from a chlordecone-contaminated Andosol as compared to that of the reference strain F. oxysporum MIAE00047 not previously exposed to the insecticide. The growth of the two isolates on glucose–MS medium added with 0.1, 1, and 10 mg l−1 of chlordecone or not (control) was monitored over 7 days incubation at 25 °C in the Bioscreen C Microbiological Growth Analyser. For each condition tested, five replicates were performed (n tot = 40). Growth rates (micrometer) were estimated by fitting growth curves with the Gompertz model. Tolerance to chlordecone was estimated by calculating the μm[CLD]/μm[control] ratio. Gray bars represent μm[CLD]/μm[control] values obtained for F. oxysporum MIAE01197 and white bars those obtained for the F. oxysporum MIAE00047 reference strain. Values are the means and standard errors of five replicates. Values labeled with the same letter are not significantly different according to the Student's t test at P < 0.005, n = 5
The tolerance of the two strains to chlordecone was also estimated by calculating the EC50, by quantifying fungal biomass after growth in a liquid culture medium. F. oxysporum MIAE01197 isolated from the chlordecone-contaminated Andosol showed a significantly higher EC50 than the reference MIAE00047 strain (i.e., 29.2 and 18.6 mg of chlordecone per liter, respectively, p < 0.01).
Characterization of F. oxysporum MIAE01197 ability to dissipate chlordecone
In order to address F. oxysporum MIAE01197 dissipation ability, fungal cultures in liquid MS medium added with chlordecone (1.5 mg l−1) were performed. A control consisting in incubating the chlordecone–MS medium without fungal inoculation was added. GC-MS quantification of the chlordecone that remained in the culture medium at the end of the incubation period showed that 1.06 ± 0.26 mg l−1 of chlordecone remained in the control, while 0.60 ± 0.09 mg l−1 remained in the MIAE01197 culture. As compared to the control, approximately 40 % of chlordecone was dissipated by F. oxysporum MIAE01197. Unfortunately, although our GC-MS method allowed us to detect β-monohydro- and dihydro-chlordecone, no chlordecone metabolites could have been observed on MS spectrum.
To further address F. oxysporum MIAE01197 dissipation ability, mineralization experiments using radiorespirometry were carried out with 14C10-chlordecone on cultures grown on chlordecone–MS or glucose–chlordecone–MS liquid media. A rapid but low emission of 14CO2 (i.e., 1.2 % of the initial amount of 14C10-chlordecone added) was noted in F. oxysporum MIAE01197 cultures carried out in glucose-added MS medium, while hardly any emission of 14CO2 was recorded in F. oxysporum MIAE 01197 cultures carried out in chlordecone–MS medium (Fig. 5). At the end of the incubation period, chlordecone residues were extracted using dichloromethane and separated by thin-layer chromatography (Fig. 6). The autoradiographic analysis of the chromatogram showed that 14C10-chlordecone was present in the two F. oxysporum MIAE01197 cultures. S1 spot was observed close to the deposit line for both cultures and control. S2 and S3/S4 spots were observed below and above the chlordecone spot, respectively. These three spots were detected in both cultures, but not in the control culture medium. In addition, 14C-compounds were weakly present in the migration fronts of the two cultures.
Kinetics of 14CO2 evolution from 14C10-chlordecone over a 3-week incubation period in cultures of F. oxysporum MIAE01197 grown on chlordecone–MS medium (triangle, dashed lines) or on glucose–chlordecone–MS medium (cross, solid lines). The amount of accumulated 14CO2 is expressed as a percentage of the initial amount of 14C10-chlordecone added to the medium. Three replicates were performed per treatment
Search for 14C10-chlordecone metabolites in F. oxysporum MIAE01197 cultures conducted on glucose–chlordecone–MS medium (lanes 1 and 2) or on chlordecone–MS medium (lanes 3 to 5) using thin-layer chromatography. Non-inoculated chlordecone–MS medium was used as a control (lane 6). 14C-metabolites extracted from the cultures were separated in a glass-backed silica gel layer. Radioactive compounds were detected by autoradiography
In order to check for the possible adsorption of chlordecone to the fungal biomass, an experiment was carried out by incubating different amounts F. oxysporum MIAE01197 in a phosphate buffer presence of 14C-chlordecone. The amount of radioactivity contained in the phosphate buffer and in the fungal biomass was regularly monitored over a 72-h incubation period. A linear relationship was found between the amount of 14C10-chlordecone adsorption and fungal biomass (14C10-chlordecone adsorbed = 32,595 × [fungal biomass] + 0,069, r 2 = 0.99) suggesting that biosorption could occur with F. oxysporum sp. MIAE01197 (Fig. S1).
Discussion
One hundred and three fungal strains were isolated from Andosol contaminated with 35 mg kg−1 of chlordecone. These strains were gathered into 18 morphotypes. Rarefaction analysis showed that the whole diversity of cultivable fungal species was not fully recovered. Shannon–Wiener index suggests a relatively moderate diversity of the isolated cultivable fungal strains. However, this observation should be interpreted keeping in mind that most fungal species are not cultivable (Anderson and Cairney 2004; Bridge and Spooner 2001; Manter and Vivanco 2007; Thorn 1997; Zak and Visser 1996). Consequently, the Andosol may have harbored higher fungal diversity. In addition, incubating the Andosol in a soil–charcoal system and isolating fungal strains under chlordecone selection pressure may also have yielded a moderate diversity of fungal species. As revealed by the Simpson index, several morphotypes (1, 2, and 3) codominated the cultivable fungal community and represented up to 76 % of the total effective. Molecular analysis based on 18S–28S ITS sequencing revealed that morphological classification led to a slight overestimation of the diversity of the fungal strains. Indeed, although the 103 isolates gathered in 18 morphotypes, they were distributed into only 11 genera (Fusarium, Paecilomyces, Ophiocordyceps, Aspergillus, Purpureocillium, Metacordyceps, Pochonia, Cordyceps, Penicillium, Bionectria, and Acremonium). This discrepancy was mainly due to whitish color isolates clustered in morphotype 4 and morphotypes 6 to 14 which did not display any spore pigmentation. Being classified as a separate morphotype according to mycelium shape, ITS sequencing revealed that some of them belonged to the Aspergillus genus explaining why diversity was overestimated. All the fungal strains isolated from the Andosol belonged to the Ascomycota phylum. This observation is in agreement with Rohilla and Salar (2012) who reported that Ascomycota was the dominant phylum (represented by seven genera) among fungal strains isolated from 23 agricultural soils contaminated with different pesticides. In addition, two of our three dominant morphotypes, namely Fusarium sp. (morphotype 1) and Aspergillus sp. (morphotype 3), were also found as predominant genera in pesticide-contaminated agricultural soils (Rohilla and Salar 2012).
Strains gathered in morphotype 1 belonged to Fusarium species which are ubiquitous organisms widely distributed in the environment (Nelson et al. 1994). They are known as plant (Nelson et al. 1994) and occasionally as animal pathogens (Evans et al. 2004) including humans causing broad spectrum of infections (Gorman et al. 2006; Nucci and Anaissie 2002, 2007).
Strains gathered in morphotype 2 belonged to the Hypocreales order including Paecilomyces, Ophiocordyceps, and Purpureocillium genera. Unfortunately, ITS amplification with ITS1F/ITS4 primers failed to distinguish between these genera. To give these strains a clearer affiliation, other molecular markers such as elongation factor or beta-tubuline will have to be investigated. Strains belonging to these genera are known as entomopathogenic fungi infecting emerald ash borers (Johny et al. 2012), whitefly nymphs (Wraight et al. 1998), and cicada larvae (Kobayasi 1938).
Strains gathered in morphotype 3 belonged to the Aspergillus genus which is ubiquitous, including saprophytes living in diverse habitats. They are known as a mycotoxin producer (Caira et al. 2012; Hedayati et al. 2007; Lass-Flörl et al. 2005) such as aflatoxin that impacts human health (Kurtzman et al. 1987). According to a biogeographical study, A. flavus and A. terreus strains occur at expected frequencies in soil and litter in the tropical latitudes (Guadeloupe latitude is 16°09′00) (Klich 2002).
In order to identify a chlordecone-tolerant fungal strain, the strains gathered in the three dominant morphotypes were screened in liquid cultures by monitoring their growth on chlordecone–MS medium using the Bioscreen. Among all the strains we tested, only F. oxysporum MIAE01197 grew on MS medium supplemented with chlordecone as the sole carbon source. F. oxysporum MIAE00047, which had never been exposed to chlordecone, did not grow on chlordecone–MS medium at least using a Bioscreen. The measurement of biomass production showed that both reference and MIAE01197 strains were able to weakly grow on MS medium. This discrepancy between the Bioscreen and biomass production experiments is most likely due to technical limitations of Bioscreen not allowing the detection of weak biomass production. However, only MIAE01197 produced a significantly higher biomass in chlordecone MS medium than MIAE00047. In order to further assess the adaptation of F. oxysporum MIAE01197 to chlordecone, its tolerance to chlordecone was studied in vitro following a method typically used to assess the impact of different pesticides on organism growth (Bhalerao and Puranik 2007; Dritsa et al. 2007; Ortega et al. 2011). F. oxysporum MIAE01197 and MIAE00047 were exposed to a chlordecone gradient in liquid glucose–MS medium. F. oxysporum MIAE01197 growth rate (micrometer) was significantly increased in response to exposure to increasing concentrations of chlordecone (p < 0.01; n = 5), whereas the reference strain growth rate was not affected. F. oxysporum MIAE01197 and MIAE00047 tolerance was also assessed by calculating their EC50 values following the procedure used by different authors for different pesticides (Dorigo et al. 2007; Pesce et al. 2010). F. oxysporum MIAE01197 showed a significantly higher EC50 value than the reference strain MIAE00047 (i.e., 29.2 mg l−1 for MIAE01197 vs 18.6 mg l−1 for MIAE00047). As compared to the reference strain MIAE00047, it even seems to be stimulated by chlordecone exposure up to 10 mg l−1, suggesting its adaptation in response to chlordecone exposure.
To characterize F. oxysporum MIAE01197 degrading ability, chlordecone dissipation was monitored by GC-MS. Approximately 40 % of chlordecone was dissipated by F. oxysporum MIAE01197. Unfortunately, no known chlordecone metabolites (β-monohydro- and dihydro-chlordecone) could have been observed on the MS spectrum. In addition, no trace of intermediary metabolites recently suggested by Dolfing et al. (2012) was detected either. Therefore, at this stage, chlordecone dissipation cannot be clearly related to a degradation process. In order to investigate this possibility, radiorespirometry analyses were conducted. F. oxysporum MIAE01197 did not mineralize 14C10-chlordecone when chlordecone was added as the sole carbon source. However, in the presence of glucose a low amount of 14C10-chlordecone evolved to 14CO2 (i.e., 1.2 % of the initial amount of 14C10-chlordecone added). Although several radioactive spots were detected in the F. oxysporum MIAE01197 culture by TLC analyses, most of the radioactivity remained in the 14C10-chlordecone spot, suggesting poor transformation. These last two observations contradict the fact that F. oxysporum MIAE01197 was able to grow on chlordecone as the sole carbon source in liquid medium. Indeed, although chlordecone dissipation was observed by GC-MS, hardly any evolution to 14CO2 was recorded by radiorespirometry, except in the presence of glucose. One could not exclude that MIAE01197 could grow on some contaminants of chlordecone, but this hypothesis seems very unlikely because chlordecone pure at 99.7 % was used at low concentration (i.e., not more than 3 mg l−1) not furnishing enough C to fuel its growth. Keeping in mind the poor ability of F. oxysporum MIAE01197 to transform 14C10-chlordecone, we could explain chlordecone dissipation by biosorption. Indeed, adsorption onto fungal mycelium and/or absorption into the fungal cells has been observed by several authors for different xenobiotics (Aksu 2005; Benoit et al. 1998; Dritsa et al. 2007; Juhasz et al. 2002; Lievremont et al. 1996; Shin et al. 1970; Wu and Yu 2006). Fungal biosorption was even recommended as a water-cleaning strategy to remove heavy metals or organic pollutants by environmental bioremediation (Aksu 2005; Kapoor and Viraraghavan 1995; Yan and Viraraghavan 2003). We evidenced for a linear relationship between the amount of 14C10-chlordecone adsorption and fungal biomass (r 2 = 0.99) suggesting that biosorption could occur with F. oxysporum sp. MIAE01197. However, in our dissipation experiment, fungal biomass was too low to account for 40 % chlordecone removal by biosorption alone.
Conclusion
This study led to the isolation and characterization of 103 fungal strains from a historically contaminated Andosol. All these isolates were affiliated to the Ascomycota phylum known to be present in soils frequently exposed to pesticides. Among them, F. oxysporum MIAE01197 was the only one able to grow on chlordecone while the reference strain, F. oxysporum MIAE00047, not previously exposed to chlordecone did not grow on it. F. oxysporum MIAE01197 was shown to not only tolerate high concentration of chlordecone but also growth to be stimulated by chlordecone. This suggests that in response to long-term exposure to chlordecone in contaminated French West Indies soils, fungal species adapted processes involved in their adaptation. Among possible processes, F. oxysporum MIAE01197 biosorption and degradation abilities were shown to be involved in the dissipation of chlordecone. Chlordecone degradation ability was low and further work is needed to improve it. Biosorption may give insight in cleaning water from chlordecone contamination by developing fungal filter.
References
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gubitz GM (2000) Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol 66(8):3357–3362
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40(3–4):997–1026
Alabouvette C (1986) Fusarium -wilt suppressive soils from the Châteaurenard region: review of a 10-year study. Agronomie 6(3):273–284
Anderson IC, Cairney JWG (2004) Diversity and ecology of soil fungal communities: increased understanding through the application of molecular techniques. Environ Microbiol 6(8):769–779
Benoit P, Barriuso E, Calvet R (1998) Biosorption characterization of herbicides, 2,4-D and atrazine, and two chlorophenols on fungal mycelium. Chemosphere 37(7):1271–1282
Bhalerao TS, Puranik PR (2007) Biodegradation of organochlorine pesticide, endosulfan, by a fungal soil isolate, Aspergillus niger. Int Biodeterior Biodegrad 59(4):315–321
Bridge P, Spooner B (2001) Soil fungi: diversity and detection. Plant Soil 232:147–154
Cabidoche YM, Achard R, Cattan P, Clermont-Dauphin C, Massat F, Sansoulet J (2009) Long-term pollution by chlordecone of tropical volcanic soils in the French West Indies: a simple leaching model accounts for current residue. Environ Pollut 157(5):1697–1705
Cabidoche YM, Lesueur-Jannoyer M (2012) Contamination of harvested organs in root crops grown on chlordecone-polluted soils. Pedosphere 22(4):562–571
Caira M, Posteraro B, Sanguinetti M, de Carolis E, Leone G, Pagano L (2012) First case of breakthrough pneumonia due to Aspergillus nomius in a patient with acute myeloid leukemia. Med Mycol 50(7):746–750
Clostre F, Lesueur-Jannoyer M (2012) Transfert de la chlordécone du sol vers les produits cultivés. Document de synthèse Cirad (in French).
Coat S, Bocquene G, Godard E (2006) Contamination of some aquatic species with the organochlorine pesticide chlordecone in Martinique. Aquat Living Resour 19(2):181–187
Coat S, Monti D, Legendre P, Bouchon C, Massat F, Lepoint G (2011) Organochlorine pollution in tropical rivers (Guadeloupe): role of ecological factors in food web bioaccumulation. Environ Pollut 159(6):1692–1701
Dallaire R, Muckle G, Rouget F, Kadhel P, Bataille H, Guldner L, Seurin S, Chajes V, Monfort C, Boucher O, Thome JP, Jacobson SW, Multigner L, Cordier S (2012) Cognitive, visual, and motor development of 7-month-old Guadeloupean infants exposed to chlordecone. Environ Res 118:79–85
Dolfing J, Novak I, Archelas A, Macarie H (2012) Gibbs free energy of formation of chlordecone and potential degradation products: implications for remediation strategies and environmental fate. Environ Sci Technol 46(15):8131–8139
Dorigo U, Leboulanger C, Berard A, Bouchez A, Humbert JF, Montuelle B (2007) Lotic biofilm community structure and pesticide tolerance along a contamination gradient in a vineyard area. Aquat Microb Ecol 50(1):91–102
Dritsa V, Rigas F, Natsis K, Marchant R (2007) Characterization of a fungal strain isolated from a polyphenol polluted site. Bioresour Technol 98(9):1741–1747
Edel V, Steinberg C, Gautheron N, Recorbet G, Alabouvette C (2001) Genetic diversity of Fusarium oxysporum populations isolated from different soils in France. FEMS Microbiol Ecol 36(1):61–71
Evans J, Levesque D, De Lahunta A, Jensen HE (2004) Intracranial fusariosis: a novel cause of fungal meningoencephalitis in a dog. Vet Pathol 41(5):510–514
FAO/ISRIC/ISSS (1998) World Reference Base for Soil Resources. World Soil Resources Report 84, FAO, Rome
Fernandes P, Lesueur-Jannoyer M, Soler A, Achard R, Woignier T Effects of clay microstructure and compost quality on chlordecone retention in volcanic tropical soils: consequences on pesticide lability and plant contamination. In: 19th World Congress of Soil Science, Soil Solutions for Changing World, Brisbane, Australia., 1–6 August 2010. pp 50–53
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118
George SE, King LC, Claxton LD (1986) High-performance liquid-chromatography separation of chlordecone and its metabolites. Chromatographia 22(1–6):165–167
Gorman SR, Magiorakos AP, Zimmerman SK, Craven DE (2006) Fusarium oxysporum pneumonia in an immunocompetent host. S Med J 99(6):613–616
Hasan HAH (1999) Fungal utilization of organophosphate pesticides and their degradation by Aspergillus flavus and A. sydowii in soil. Folia Microbiol 44(1):77–84
Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW (2007) Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153(6):1677–1692
Jablonski PE, Pheasant DJ, Ferry JG (1996) Conversion of Kepone by Methanosarcina thermophila. FEMS Microbiol Lett 139(2–3):169–173
Johny S, Kyei-Poku G, Gauthier D, van Frankenhuyzen K (2012) Isolation and characterisation of Isaria farinosa and Purpureocillium lilacinum associated with emerald ash borer, Agrilus planipennis in Canada. Biocontrol Sci Technol 22(6):723–732
Juhasz AL, Smith E, Smith J, Naidu R (2002) Biosorption of organochlorine pesticides using fungal biomass. J Ind Microbiol Biotechnol 29(4):163–169
Kapoor A, Viraraghavan T (1995) Fungal biosorption—an alternative treatment option for heavy metal bearing wastewaters: a review. Bioresour Technol 53(3):195–206
Kilzer L, Scheunert I, Geyer H, Klien W, Korte F (1979) Laboratory screening of the volatilization rates of organic chemicals from water and soil. Chemosphere 10:751–761
Kim YH, Ahn JY, Moon SH, Lee J (2005) Biodegradation and detoxification of organophosphate insecticide, malathion by Fusarium oxysporum f. sp pisi cutinase. Chemosphere 60(10):1349–1355
Klich MA (2002) Biogeography of Aspergillus species in soil and litter. Mycologia 94(1):21–27
Kobayasi Y (1938) The genus Cordyceps and its allies. Science reports of the Tokyo Bunrika Daigaku 5
Kurtzman CP, Horn BW, Hesseltine CW (1987) Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Van Leeuwenhoek 53(3):147–158
Lass-Flörl C, Griff K, Mayr A, Petzer A, Gastl G, Bonatti H, Freund M, Kropshofer G, Dierich MP, Nachbaur D (2005) Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br J Haematol 131(2):201–207
Le Déault JY, Procaccia C (2009) Les impacts de l'utilisation de la chlordécone et des pesticides aux Antilles: bilan et perspectives d'évolution. Report no. 1778 of French National Assembly (in French).
Li F, Huang SF, Liu B (2006) Degradation of phoxim by Paecilomyces lilacinus. Chin J Appl Environ Biol 12:104–107 (in Chinese with English abstract)
Lievremont D, SeigleMurandi F, BenoitGuyod JL, Steiman R (1996) Biotransformation and biosorption of pentachloronitrobenzene by fungal mycelia. Mycol Res 100:948–954
Manter DK, Vivanco JM (2007) Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J Microbiol Methods 71(1):7–14
Martin-Laurent F, Sahnoun M, Merlin C, Vollmer G, Lübke M (2013) Detection and quantification of chlordecone in contaminated soils from the French West Indies by GC-MS using 13C10-chlordecone stable isotope as a tracer. Env Sci Pollut Res. doi:10.1007/s11356-013-1839-y
Mukherjee I, Mittal A (2005) Bioremediation of endosulfan using Aspergillus terreus and Cladosporium oxysporum. Bull Environ Contam Toxicol 75(5):1034–1040
Multigner L, Ndong JR, Giusti A, Romana M, Delacroix-Maillard H, Cordier S, Jegou B, Thome JP, Blanchet P (2010) Chlordecone exposure and risk of prostate cancer. J Clin Oncol 28(21):3457–3462
Nelson PE, Dignani MC, Anaissie EJ (1994) Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev 7(4):479–504
Nucci M, Anaissie E (2002) Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis 35(8):909–920
Nucci M, Anaissie E (2007) Fusarium infections in immunocompromised patients. Clin Microbiol Rev 20(4):695–704
Ortega SN, Nitschke M, Mouad AM, Landgraf MD, Rezende MOO, Seleghim MHR, Sette LD, Porto ALM (2011) Isolation of brazilian marine fungi capable of growing on DDD pesticide. Biodegradation 22(1):43–50
Pesce S, Margoum C, Montuelle B (2010) In situ relationships between spatio-temporal variations in diuron concentrations and phototrophic biofilm tolerance in a contaminated river. Water Res 44(6):1941–1949
Porto ALM, Melgar GZ, Kasemodel MC, Nitschke M (2011) Biodegradation of pesticides. In: Stoytcheva DM (ed) Pesticides in the modern world—pesticides use and management. InTech, pp 407–438
Rohilla SK, Salar RK (2012) Isolation and characterization of various fungal strains from agricultural soil contaminated with pesticides. Res J Rec Sci 1:297–303
Sakakibara F, Takagi K, Kataoka R, Kiyota H, Sato Y, Okada S (2011) Isolation and identification of dieldrin-degrading Pseudonocardia sp strain KSF27 using a soil-charcoal perfusion method with aldrin trans-diol as a structural analog of dieldrin. Biochem Biophys Res Commun 411(1):76–81
Shin YO, Chodan JJ, Wolcott AR (1970) Adsorption of DDT by soils, soil fractions, and biological materials. J Agric Food Chem 18(6):1129–1133
Takagi K, Iwasaki A, Kamei I, Satsuma K, Yoshioka Y, Harada N (2009) Aerobic mineralization of hexachlorobenzene by newly isolated pentachloronitrobenzene-degrading Nocardioides sp strain PD653. Appl Environ Microbiol 75(13):4452–4458
Takagi K, Yoshioka Y (2000) Development of a method for the rapid accumulation and isolation of recalcitrant-pesticide decomposing bacteria in soil using charcoal. In: Pesticides, soil microbiology and sustainable agriculture. Abstracts and Final Program of the 3rd International Symposium on Environmental Aspects of Pesticide Microbiology. Leverkusen, Germany, pp 69–70
Thorn G (1997) The fungi in soil. In: van Elsas JD, Trevors JT, Wellington EMH (eds) Modern soil microbiology. CRC, Boca Raton, pp 63–197
White TJ (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic, San Diego, pp 315–322
Wraight SP, Carruthers RI, Bradley CA, Jaronski ST, Lacey LA, Wood P, Galaini-Wraight S (1998) Pathogenicity of the entomopathogenic fungi Paecilomyces spp. and Beauveria bassiana against the silverleaf whitefly, Bemisia argentifolii. J Invertebr Pathol 71(3):217–226
Wu J, Yu HQ (2006) Biosorption of 2,4-dichlorophenol from aqueous solution by Phanerochaete chrysosporium biomass: isotherms, kinetics and thermodynamics. J Hazard Mater 137(1):498–508
Yan GY, Viraraghavan T (2003) Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res 37(18):4486–4496
Zak JC, Visser S (1996) An appraisal of soil fungal biodiversity: the crossroads between taxonomic and functional biodiversity. Biodivers Conserv 5:169–183
Zwietering MH, Jongenburger I, Rombouts FM, Vantriet K (1990) Modeling of the bacterial-growth curve. Appl Environ Microbiol 56(6):1875–1881
Acknowledgments
Chloé Merlin's PhD work was funded by an ADEME/Région Bourgogne. The work was done within the framework of the Biodechlord project funded by the INRA AIP Demichlord part of PNAC 1. The authors would like to thank Yves-Marie Cabidoche for having offered the possibility to work on this subject. This paper pays homage to Yves-Marie Cabidoche, a brilliant INRA researcher who carried out precursor work on chlordecone fate in the French West Indies. We would like to thank Annie Buchwalter for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary data S 1
Amount of 14C10-chlordecone adsorbed to fungal biomass measured in 0.1, 0.5 and 1 OD600 F. oxysporum MIAE01197 suspension cultures. Linear relationship calculated between the amount of 14C10-chlordecone adsorption and fungal biomass. (DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Merlin, C., Devers, M., Crouzet, O. et al. Characterization of chlordecone-tolerant fungal populations isolated from long-term polluted tropical volcanic soil in the French West Indies. Environ Sci Pollut Res 21, 4914–4927 (2014). https://doi.org/10.1007/s11356-013-1971-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1971-8