Abstract
In this study, the occurrence and sources of five cataloged antibiotics and metabolites were studied in Jiulongjiang River basin, south China. Nineteen antibiotics and 13 metabolites were detected in water samples from 16 river sampling sites, wastewater from 5 swine-raising facilities, and effluent from 5 wastewater treatment plants (WWTPs). The results showed that 12 antibiotics and 6 metabolites were detected in river water samples. Sulfonamides (SAs) and their metabolites were detected at high concentrations (8.59–158.94 ng/L). Tetracyclines (TCs) and their metabolites were frequently detected in swine wastewater, and the maximum concentration was up to the level in milligram per liter. Macrolides (MLs) and β-lactams (β-Ls) were found in all WWTP effluent samples and some river samples, while they were never found in any of the swine wastewater samples. SAs and quinolones (QNs) were detected in all samples. Hierarchical cluster analysis of 16 surface water samples was applied to achieve the spatial distribution characteristics of antibiotics in the Jiulongjiang River. As a result, two categories were obviously obtained. Principal component analysis and redundancy analysis showed that TCs and SAs as well as their metabolites were the major antibiotics in Jiulongjiang River, and they mainly originated from swine wastewater, while the QNs, MLs, and β-Ls in the Jiulongjiang River came from WWTP effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are commonly (85 % of production) utilized in animal agriculture and medicine to prevent and treat diseases. They are also used as growth promoters in the animal industry and agriculture (Kummerer 2009). However, many antibiotics used in veterinary (Pfeifer et al. 2005) and medical situations are poorly absorbed by organisms, and most of these antibiotics are excreted unaltered or as metabolites in feces or urine (Chee-Sanford et al. 2001; Heuer et al. 2008). In recent years, antibiotics and their metabolites have been detected in municipal sewage (Li et al. 2009; Lindberg et al. 2010), surface water (Roberts and Thomas 2006; Zhang et al. 2011), groundwater (Hu et al. 2010), soil (Christian et al. 2003), sediment (Pei et al. 2006), and sludge (Göbel et al. 2005; Lindberg et al. 2010). The types and concentrations of antibiotics in the environment vary from areas to countries, depending on consumption level and use patterns of the use of antibiotics. In addition, the chemical and physical properties of antibiotics affect their occurrence and distribution in the environment. For example, tetracyclines have a high affinity to soil and sediment (Figueroa et al. 2004; Li and Zhang 2010; Rabolle and Spliid 2000), while sulfonamides show high solubility and chemical stability in water (Thiele-Bruhn et al. 2004; Thiele 2000).
As suspicious environmental contaminants, antibiotics, and their metabolites are biologically toxic. Previous studies have proven the reproduction toxicity of sulfadiazine (SD), tetracycline (TC), and chlorotetracycline (CTC) to Daphnia magna (Wollenberger et al. 2000). N4-Acetyl sulfonamides as metabolites of sulfonamides (SAs) can cause renal toxicity because of their precipitation in the kidneys (Nouws et al. 1985). Another study indicated that the mode of several tetracycline metabolites on tetracycline-resistant bacteria is different from that of their parent compounds (Halling-Sorensen et al. 2002). Moreover, it must be taken into consideration that antibiotics and their metabolites may lead to the development and spread of antibiotics resistance bacteria as well as antibiotics resistance genes in the long term (Boxall et al. 2003; Martinez 2008). Therefore, it is important to assess these metabolites in environmental risk assessment since metabolites can still be active against bacteria and also can be converted back to the parents (Pfeifer et al. 2005).
The Jiulongjiang River is the second largest water system in Fujian Province, southern China and is composed of two major tributaries (Xixi River and Beixi River) and many small tributaries. With the rapid growth of the urban population in the Jiulongjiang River basin, there are numerous inputs of medical antibiotics from municipal sewage systems into the river. Moreover, the upstream city of Longyan is the uppermost livestock farming area of Fujian Province, leading to a lot of veterinary antibiotics entering into the river. Although a few studies have shown that levels of antibiotics were high in Jiulongjiang River (Zhang et al. 2011, 2012), the sources of antibiotics in the Jiulongjiang River remain unclear. Therefore, it is very urgent to develop a comprehensive regional study to characterize the occurrence, distribution, and sources of antibiotics and their metabolites in Jiulongjiang River basin.
The purposes of this study are as follows: (1) to characterize the occurrence and distribution of five cataloged antibiotics and metabolites in Jiulongjiang River surface water, WWTP effluent, and swine wastewater and (2) to ascertain the sources of antibiotics in Jiulongjiang River by cluster analysis, principal component analysis (PCA), and redundancy analysis (RDA). To our knowledge, this is the first systematic regional study on the occurrence, distribution, and sources of selected antibiotics and their metabolites in Jiulongjiang River by the analysis of their physicochemical properties and a multivariate statistical analysis method.
Materials and methods
Chemicals and materials
According to the results of our previous study (Zhang et al. 2011), 19 antibiotics and 13 of their standard metabolites including six sulfonamides and three of their metabolites, three tetracyclines and ten of their metabolites, six quinolones, two macrolides, and two β-lactams were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). More details are shown in Supporting Information SI1. Standard solutions (100.00 mg/L) were prepared by dissolving 0.5000 mg standard antibiotics in 5 mL 10 % methanol solution, individually. They were kept in brown glass vials in a refrigerator (4 °C). Oasis HLB solid phase extraction (500 mg, 6 mL) was obtained from Waters Corporation (Milford, MA, USA). HPLC grade methanol and acetone were purchased from TEDIA Company (Fairfield, OH, USA). Milli-Q water (Millipore, USA) was used throughout the study. Unless otherwise indicated, chemicals used in the analysis were analytical grade.
Sampling sites and sample collection
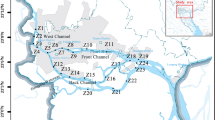
As is shown in Fig. 1, 16 river sampling points were established from putative sources along Jiulongjiang River. These included 12 sites on the tributary of the Beixi (B1–B12) and 4 sites on the Xixi (X1–X4). B3, B8, B9, B11, and X4 are located downstream of major cities along Jiulongjiang River. B1, B2, B3, and B4 are sited at traditional livestock farming areas in the Longyan district. Five effluent samples (S1–S5) were collected from several WWTPs located within Jiulongjiang River basin. S1 is situated in Longyan City, and S5 is located in Zhangzhou City. Five swine wastewater samples were collected in the Longyan district. All samples were collected in the high water season (July 2012) with pre-cleaned brown glass bottles and stored at 4 °C during transportation to the laboratory, where water samples would be stored under 4 °C conditions until pretreatment within 24 h from the sample collection.
Sample pretreatment
Sample pretreatment was carried out as described previously with minor modification (Zhang et al. 2011). Briefly, triplicates of all water samples were extracted using Oasis HLB extraction cartridges (500 mg, 6 mL) and then eluted with 6.0 mL methanol to achieve the target fraction. After drying under a gentle nitrogen stream and then being redissolved in a 10 % methanol solution, the final extracts were transferred tto 2-mL amber vials for liquid chromatography-tanden mass spectrometry (LC-MS/MS); ultra-performance liquid chromatography-tanden mass spectrometry (UPLC-MS/MS) analysis. More details are shown in Supporting Information.
LC–MS/MS and UPLC–MS/MS analysis
SAs, quinolones (QNs), macrolides (MLs), and β-lactams were analyzed by LC–MS/MS system (ABI 3200Q TRAP) as described previously with slight revision (Zhang et al. 2011). Briefly, the Inertsil ODS-SP column (4.6 × 150 mm, 5 μm; GL Science, Inc., Japan) was maintained at 40 °C, with injection volumes of 20 μL. Purified water with 0.1 % formic acid (v/v) (phase A) and methanol (phase B) was used as chromatographic mobile phases at a total flow rate of 1 mL/min. Tetracyclines and their metabolites were analyzed by UPLC–MS/MS system with the same mobile phases at a total flow rate of 0.2 mL/min. The eluent gradients for the LC–MS/MS and UPLC–MS/MS are shown in SI2.
All analyzed compounds were detected by electrospray tandem mass spectrometry in positive-ion mode (ESI+) with multiple reactions monitoring. The optimal conditions for analytes monitoring are summarized in Table SI3.
Analytical method validation
Recoveries of the 32 target compounds were determined for surface water by the standard addition method at two different concentration levels (10.00 and 100.00 ng/L) in triplicate. Limits of detection of the antibiotics were determined as the lowest concentrations resulting in a signal-to-noise (S/N) ratio of 3. Limits of quantification were calculated with a S/N ratio of 10. Relative standard deviations (in percent) were calculated from triplicate injections for each of three samples spiked at the same concentration which ranged from 0.5 to 5 %. Analytical method validation for water samples is described in Table SI3.
Statistical analysis
Cluster analysis and PCA of antibiotics in surface waters were performed using the SPSS 19.0 for Windows (SPSS, Inc.). The canonical correlation analysis (CCA) and RDA among surface water, municipal wastewater, and swine wastewater were achieved using the SAS 9.2 for Windows.
Results and discussion
Occurrence of antibiotics in the surface waters
Figure 2 and Table SI4 show the concentrations of selected antibiotics and metabolites in Jiulongjiang River surface waters. Twelve antibiotics and six metabolites were detected in surface water samples. SAs and their metabolites (MSAs) were detected with high concentrations (8.59–158.94 ng/L). In particular, their concentrations were high at B1–B4 where many swine farms are located. The concentrations of SAs in this study are relatively lower than those in Haihe River, northern China (210–385 ng/L) (Luo et al. 2011). SD, sulfamethazine (SM2), and sulfamethoxazole (SMZ) were frequently detected in our study, whereas sulfadimethoxine (SDM) and sulfamerazine (SM1) were below the limit of detection.
TC, oxytetracycline (OTC), and CTC were detected only in B1–B4 with concentrations of 0.45–61.15 ng/L. This result agreed with previous studies that TCs were seldom detected in natural water (Hirsch et al. 1999; Sarmah et al. 2006). As for the metabolites of TCs, 4-epitetracycline (ETC) and 4-epioxytetracyline (EOTC) were also only found in B1–B4, but isochlortetracycline (ICTC) occurred in almost all water samples with a maximum concentration of 72.65 ng/L in B2. However, the toxicological efficiency of ETC and EOTC has been reported to be in the same range as that of their parent compounds (Halling-Sorensen et al. 2002).
Except for ofloxacin (OF) that was detected in 11 surface water sites with concentrations of 5.88–6.81 ng/L, there were almost no detections in all samples for other QNs. This result may due to the characteristics that QNs are easily photolytic in water (Belden et al. 2007; Prabhakaran et al. 2009) and absorbable in sediments (Li and Zhang 2010; Nowara et al. 1997). The concentrations of OF in our study are consistent with previous studies of Jiulongjiang River estuary (Sun et al. 2009; Zhang et al. 2011) as well as in Yellow River (Zhou et al. 2011). However, the concentrations in our study were lower than those in Haihe River and Liao River, with maximum concentrations of 653 and 50.5 ng/L, respectively (Zhou et al. 2011). Roxithromycin (ROX) was detected at concentrations of 6.72–9.11 ng/L with the high detection frequency of 81.25 %. Erythromycin (ERY) was less frequently detected (37.5 %) with a range concentration of 1.06–1.13 ng/L. However, the concentration of ERY was as high as 423 ng/L in Pearl River in high water season (Xu et al. 2007). Cephalexin and cephradine were only found in B3 with the concentrations of 20.45 and 28.50 ng/L, respectively.
Cluster analysis of antibiotics in surface waters
To ascertain the characteristics of the spatial distribution of antibiotics in the Jiulongjiang River basin, a cluster analysis of 16 surface water samples was implemented. The cluster analysis was achieved by ward connection, and all data were z-score normalized. Figure 3 shows the cluster analysis results. Two categories were obviously obtained from the dendrogram. Category 1 contains samples of B5–B12 and X1–X4, while category 2 includes B1–B4. This result indicates the significant difference of concentration and distribution of antibiotics between B5–12, X1–X4, and B1–B4. In fact, B1–B4 are located in the animal husbandry industry areas, while the others are mainly surrounded by residential areas. So, the concentration and composition of antibiotics in category 1 samples were affected by human activities, whereas category 2 samples were influenced by the discharge of livestock breeding.
Principal component analysis of antibiotics in surface waters
PCA was applied to provide a description of the internal relationships between the antibiotic concentrations and the sampling stations and to find out the main sources of antibiotics in Jiulongjiang River. For this purpose, 15 compounds (those detected in more than 20 % of samples) were included in this statistical evaluation. Four principal components (PC1–4, eigenvalue of >1, total variance of >80 %) were extracted, and they accounted for 55.17, 15.53, 12.82, and 7.51 % of the total variance, respectively. More details are shown in Fig. 4. The most relevant compounds in PC1 are OTC, EOTC, CTC, TC, SD, SM2, sulfameter, ICTC, and SMZ. The results suggest that PC1 can mainly be explained by the composition of SAs, TCs, and TC metabolites (MTCs). PC2 can be explained by MLs because of the correlation with ERY and ROX. PC3 is most related to n-acetyl sulfamethazine and n-acetyl sulfadiazine, the two metabolites of SAs. While PC4 is correlated with OF, it is obvious that PC2 and PC4 have relationships with human medical treatments, for MLs and OF are mainly used in humans. However, PC1 and PC3 came from veterinary drugs, with an aggregate contribution of about 68 %.
Occurrence of antibiotics in WWTP effluent
The occurrence of antibiotics and their metabolites in WWTP effluents is presented in Fig. 5 and Table SI4. The highest total antibiotics concentration was obtained in S1, followed by S5 and S2. Extremely high concentrations of SAs were detected in S1 (194.90 ng/L) and S5 (246.00 ng/L) compared to the other sites (6.52–55.5 ng/L). SMZ (2.96–137.00 ng/L) and SD (1.31–28.40 ng/L) were detected in all samples, while SDM and SM1 were below the limit of detection in all samples. The concentration levels in the present study coincide with those in WWTP effluents in Spain (Garcia-Galan et al. 2011) and Hong Kong (Li et al. 2009). The metabolites of SAs were ubiquitous in all WWTP effluent samples with concentrations ranging from 89.85 to 249.43 ng/L. Similar to SAs, QNs were detected with high concentrations in S1 (332.90 ng/L) and S5 (200.70 ng/L) in comparison with the other samples (25.30–71.65 ng/L). Norfloxacin (NOF) (13.10–172.00 ng/L) and OF (11.95–91.50 ng/L), which have been proven to be widely used in medical treatments (Oliphant and Green 2002), were commonly detected in WWTPs, while ciprofloxacin (CIP) was detected only in S1 and S5. Compared to our results, NOF in WWTP effluents in Sweden, France, Greece, and Italy were lower (30–80 ng/L), but OF levels were higher (120–580 ng/L) (Andreozzi et al. 2003). This phenomenon may be explained by the consumption levels and use patterns of QNs in different cities and regions. Macrolides and β-lactams were detected with high concentrations (14.11–58.85 and 46.42–248.38 ng/L, respectively) in all WWTP effluent samples. However, concentration ranges of ROX and ERY in WWTP effluent samples in Beijing were 54–360 and 51–300 ng/L, respectively (Gao et al. 2012). These results suggest that the main use of macrolides and β-lactams is in human medical treatments by comparison with their occurrence in swine wastewater in our study.
TCs were found only in S1, S2, and S3. Progressive decreases of the total TCs concentration were found from 175.13 to 11.90 ng/L in S1–S3. The concentration of CTC (102.75 ng/L) is relatively higher than that of TC (34.58 ng/L) and OTC (37.80 ng/L) in S1, while only OTC was detected in S3. Similar to TCs, the levels of MTCs decreases progressively from S1 to S5 with the lowest concentration of 11.85 ng/L. The total concentration of MTCs detected in S1 (434.30 ng/L) was much higher than those in the other sewage plants (about 100 ng/L or even lower). On one hand, the diminishing concentrations of TCs and MTCs can be explained by their high affinity to soil and sediment. On the other hand, TCs and MTCs were mainly derived from livestock farms located in the upstream of Jiulongjiang River. Concentrations of ICTC and EOTC in S1 were proven to be the major metabolites in WWTPs and were 309.75 and 37.3 ng/L, respectively. The concentrations of other MTCs were too low to detect in all WWTPs, with the exception of doxycycline (DXC) in S1 (87.25 ng/L).
Occurrence of antibiotics in swine wastewater
Five swine farms were investigated, and the results are illustrated in Fig. 6 and Table SI4. Extremely high concentrations of antibiotics were detected in all samples in the level of milligram per liter. Consistent with expectation, TCs and MTCs were the major components of our detected targets in swine wastewater; 1.45–10.59 μg/L of TCs were detected in swine wastewater which were much higher than those in surface water and municipal wastewater. The same total concentration levels of TCs were detected in swine wastewater in Hubei Province (0.01–21.69 μg/L) (Tong et al. 2009). However, higher concentrations occurred in breeding wastewater in Jiangsu Province (not detected (ND)–84.30 μg/L) (Wei et al. 2011). ICTC was detected with extremely high concentrations of 29.83–111.25 μg/L, which were much higher than those of its parent (0.55–5.38 μg/L) as well as the other MTCs. This result coincided with the anticipation that CTC forms iso-CTC easily by an irreversible process. In addition, the chemical and structural properties of ICTC are quite different from other metabolites that result in higher solubility in water (Halling-Sorensen et al. 2002). EOTC (0.35–5.68 μg/L) and ETC (0.48–3.94 μg/L) were found in swine wastewater, whereas 4-epi-anhydrotetracycline, anhydrotetracycline, and 4-epi-anhydrochlortetracycline were never detected in any of the samples. This indicates that epi-TCs are primarily converted products, while dehydration products are infrequent and unstable. Besides, demeclocycline and DXC were found up to levels in microgram per liter.
SAs and MSAs were frequently detected in swine wastewater with the total concentrations ranging from 0.18 to 78.10 μg/L. The highest concentration of 51.25 μg/L was detected in H5 for SM2. This is consistent with the results from a pig farm wastewater study in Mekong Delta, where SM2 had concentrations ranging from 18.50 to 19.20 μg/L (Heuer et al. 2008). Taking the concentration distribution of SM2 in surface water into consideration, we can draw a conclusion that SM2 has a potential utility as a molecular marker for livestock-source contamination. The concentration of SMZ in our study (ND–0.04 μg/L) is much lower than the other study of livestock wastewater in Jiangsu (ND–63.6 μg/L) (Wei et al. 2011). ENR was detected only in swine wastewater and S1, which are located at breeding area. That indicated that the main use of NER is veterinary in this area. OF and CIP were also detected at concentrations ranging from 122.75 to 268.00 ng/L and ND to 325.00 ng/L, respectively, even higher than those in WWTP effluent. Compared with the concentration and distribution of QNs in WWTP effluent, we can draw a conclusion that QNs were used not only for humans but also in animals in Jiulongjiang River basin. No macrolides or β-lactams were detected in any of the swine wastewater samples, which they were only used for humans.
Redundancy analysis of antibiotics in surface water, WWTP effluent, and swine wastewater
CCA and RDA were performed to get a comprehensive overview and to investigate possible relationships among the surface waters, WWTP effluents, and swine wastewater. Sixteen surface water samples were grouped into two categories according to the clustering analysis results. Eigenvalues and canonical correlation coefficients of the canonical correlation analysis are shown in Table 1. The results show strong correlations between the canonical variables of river surface water with swine wastewater and WWTP effluents, with the canonical correlation coefficients ranging from 0.7978 to 0.9999(P < 0.0001).
The RDA results are stated in Table 2. The RDA of swine wastewater (H1–H5) between breeding area river surface waters (B1–B4) indicated that the breeding area river surface water indicator can be explained by the 82.50 % proportion of shared variance by the first two canonical variables of their own (CV1 RH1 and CV1 RH2) and 68.51 % by the first two CVs of the opposite (CV1 H1 and CV1 H2). The result indicates that the antibiotic composition of breeding area river surface water can be well predicted by swine wastewater with a strong correlation between them. However, the RDA results of WWTP effluents (S1–S5) with B1–B4 show that the breeding area river surface water indicator can only be explained by the 34.89 % proportion of shared variance by their own (CV3 RH1 and CV3 RH2) and 25.63 % by the first two CVs of the opposite (CV3 S1 and CV3 S2). Therefore, we can draw a conclusion that livestock industry antibiotic discharges were the major sources of antibiotics contamination in the river in breeding areas, while domestic pollution sources impacted much less. Analogously, 60.84 % of the total variance for the town area river surface water (B5–B12 and X1–X4) index was explained by CV4 S1–3, and 43.72 % was explained by CV2 H1–3. These results demonstrate that the antibiotics pollution of Jiulongjiang River surface water in mid- and downstream was mainly due to the discharge of domestic sewage into the river. Simultaneously, livestock antibiotics pollution sources have a subordinate influence on it.
Conclusion
In this study, the obvious characteristics of the antibiotics’ spatial distribution in Jiulongjiang River were obtained. SAs, TCs, and their metabolites were detected at high concentrations in all swine wastewater and B1–B4. These results suggest that they originated from veterinary applications in swine farms. However, macrolides and β-lactams were mainly used as medical antibiotics in this area, for their only detection was in WWTP effluent samples and some of the river samples. The sources of antibiotics in the Jiulongjiang River were also demonstrated by principal component analysis and redundancy analysis. The results suggest that the major source was veterinary use in livestock breeding. The above results indicate that the pollution control for livestock breeding activities in Jiulongjiang River basin should be strengthened in the future, and more attention should be given to the Longyan region.
References
Andreozzi R, Marotta R, Paxeus N (2003) Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 50:1319–1330
Belden JB, Maul JD, Lydy MJ (2007) Partitioning and photodegradation of ciprofloxacin in aqueous systems in the presence of organic matter. Chemosphere 66:1390–1395
Boxall ABA, Kolpin DW, Halling-Sorensen B, Tolls J (2003) Are veterinary medicines causing environmental risks? Environ Sci Technol 37:286a–294a
Chee-Sanford JC, Aminov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI (2001) Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl Environ Microbiol 67:1494–1502
Christian T, Schneider RJ, Farber HA, Skutlarek D, Meyer MT, Goldbach HE (2003) Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim Hydrobiol 31:36–44
Figueroa RA, Leonard A, Mackay AA (2004) Modeling tetracycline antibiotic sorption to clays. Environ Sci Technol 38:476–483
Göbel A, Thomsen A, McArdell CS, Alder AC, Giger W, Theiß N, Löffler D, Ternes TA (2005) Extraction and determination of sulfonamides, macrolides, and trimethoprim in sewage sludge. J Chromatogr A 1085:179–189
Gao L, Shi Y, Li W, Niu H, Liu J, Cai Y (2012) Occurrence of antibiotics in eight sewage treatment plants in Beijing, China. Chemosphere 86:665–671
Garcia-Galan MJ, Diaz-Cruz MS, Barcelo D (2011) Occurrence of sulfonamide residues along the Ebro River basin: removal in wastewater treatment plants and environmental impact assessment. Environ Int 37:462–473
Halling-Sorensen B, Sengelov G, Tjornelund J (2002) Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch Environ Contam Toxicol 42:263–271
Heuer H, Focks A, Lamshöft M, Smalla K, Matthies M, Spiteller M (2008) Fate of sulfadiazine administered to pigs and its quantitative effect on the dynamics of bacterial resistance genes in manure and manured soil. Soil Biol Biochem 40:1892–1900
Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225:109–118
Hu X, Zhou Q, Luo Y (2010) Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ Pollut 158:2992–2998
Kummerer K (2009) Antibiotics in the aquatic environment—a review—part I. Chemosphere 75:417–434
Li B, Zhang T, Xu Z, Fang HH (2009) Rapid analysis of 21 antibiotics of multiple classes in municipal wastewater using ultra performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta 645:64–72
Li B, Zhang T (2010) Biodegradation and adsorption of antibiotics in the activated sludge process. Environ Sci Technol 44:3468–3473
Lindberg RH, Fick J, Tysklind M (2010) Screening of antimycotics in Swedish sewage treatment plants—waters and sludge. Water Res 44:649–657
Luo Y, Xu L, Rysz M, Wang Y, Zhang H, Alvarez PJ (2011) Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River basin, China. Environ Sci Technol 45:1827–1833
Martinez JL (2008) Antibiotics and antibiotic resistance genes in natural environments. Science 321:365–367
Nouws JF, Vree TB, Hekster YA (1985) In vitro antimicrobial activity of hydroxy and N4-acetyl sulphonamide metabolites. Vet Q 7:70–72
Nowara A, Burhenne J, Spiteller M (1997) Binding of fluoroquinolone carboxylic acid derivatives to clay minerals. J Agric Food Chem 45:1459–1463
Oliphant CM, Green GM (2002) Quinolones: a comprehensive review. Am Fam Physician 65:455–464
Pei R, Kim SC, Carlson KH, Pruden A (2006) Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res 40:2427–2435
Pfeifer T, Tuerk J, Fuchs R (2005) Structural characterization of sulfadiazine metabolites using H/D exchange combined with various MS/MS experiments. J Am Soc Mass Spectrom 16:1687–1694
Prabhakaran D, Sukul P, Lamshoft M, Maheswari MA, Zuhlke S, Spiteller M (2009) Photolysis of difloxacin and sarafloxacin in aqueous systems. Chemosphere 77:739–746
Rabolle M, Spliid NH (2000) Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere 40:715–722
Roberts PH, Thomas KV (2006) The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci Total Environ 356:143–153
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Sun G, Su Z, Chen M, Yuan D (2009) Simultaneous determination of tetracycline and quinolone antibiotics in environmental water samples using solid phase extraction-ultra pressure liquid chromatography coupled with tandem mass spectrometry. Chin J Chromatogr 27:54–58
Thiele-Bruhn S, Seibicke T, Schulten HR, Leinweber P (2004) Sorption of sulfonamide pharmaceutical antibiotics on whole soils and particle-size fractions. J Environ Qual 33:1331–1342
Thiele S (2000) Adsorption of the antibiotic pharmaceutical compound sulfapyridine by a long-term differently fertilized loess Chernozem. J Plant Nutr Soil Sci 163:589–594
Tong L, Li P, Wang Y, Zhu K (2009) Analysis of veterinary antibiotic residues in swine wastewater and environmental water samples using optimized SPE-LC/MS/MS. Chemosphere 74:1090–1097
Wei R, Ge F, Huang S, Chen M, Wang R (2011) Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 82:1408–1414
Wollenberger L, Halling-Sorensen B, Kusk KO (2000) Acute and chronic toxicity of veterinary antibiotics to Daphnia magna. Chemosphere 40:723–730
Xu WH, Zhang G, Zou SC, Li XD, Liu YC (2007) Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ Pollut 145:672–679
Zhang D, Lin L, Luo Z, Yan C, Zhang X (2011) Occurrence of selected antibiotics in Jiulongjiang River in various seasons, South China. J Environ Monit: JEM 13:1953–1960
Zhang X, Zhang D, Zhang H, Luo Z, Yan C (2012) Occurrence, distribution, and seasonal variation of estrogenic compounds and antibiotic residues in Jiulongjiang River, South China. Environ Sci Pollut Res Int 19:1392–1404
Zhou LJ, Ying GG, Zhao JL, Yang JF, Wang L, Yang B, Liu S (2011) Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ Pollut 159:1877–1885
Acknowledgments
This work is financially supported by the International Cooperation of Ministry Science and Technology of China (2011DFB91710).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 186 kb)
Rights and permissions
About this article
Cite this article
Jiang, H., Zhang, D., Xiao, S. et al. Occurrence and sources of antibiotics and their metabolites in river water, WWTPs, and swine wastewater in Jiulongjiang River basin, south China. Environ Sci Pollut Res 20, 9075–9083 (2013). https://doi.org/10.1007/s11356-013-1924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1924-2