Abstract
Vermicomposting of water hyacinth is a good alternative for the treatment of water hyacinth (Eichhornia crassipes) and subsequentially, beneficial for agriculture purposes. The bioavailability and leachability of heavy metals (Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr) were evaluated during vermicomposting of E. crassipes employing Eisenia fetida earthworm. Five different proportions (trials 1, 2, 3, 4, and 5) of cattle manure, water hyacinth, and sawdust were prepared for the vermicomposting process. Results show that very poor biomass growth of earthworms was observed in the highest proportion of water hyacinth (trial 1). The water soluble, diethylenetriaminepentaacetic acid (DTPA) extractable, and leachable heavy metals concentration (percentage of total heavy metals) were reduced significantly in all trials except trial 1. The total concentration of some metals was low but its water soluble and DTPA extractable fractions were similar or more than other metals which were present in higher concentration. This study revealed that the toxicity of metals depends on bioavailable fraction rather than total metal concentration. Bioavailable fraction of metals may be toxic for plants and soil microorganisms. The vermicomposting of water hyacinth by E. fetida was very effective for reduction of bioavailability and leachability of selected heavy metals. Leachability test confirmed that prepared vermicompost is not hazardous for soil, plants, and human health. The feasibility of earthworms to mitigate the metal toxicity and to enhance the nutrient profile in water hyacinth vermicompost might be useful in sustainable land renovation practices at low-input basis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The water hyacinth (Eichhornia crassipes) is one of the most intransigent weeds of the world (Gajalakshmi et al. 2002). Efforts to control or destroy it by chemical, biological, mechanical, or hybrid means has not achieved total success (Gajalakshmi et al. 2002). The nutrient levels in its water body increase very rapidly due to its decaying body, which eventually generates the problem of eutrophication in aquatic systems (Gupta et al. 2007). The water hyacinth has been used in phytoremediation due to its high affinity and accumulation capacity of several metals from the aqueous medium (Mishra and Tripathi 2009; Agunbiade et al. 2009; Chunkao et al. 2012). The vermicomposting, followed by land application, represents one of the most economical ways for treatment and final disposal of water hyacinth, because it combines material recycling and biomass disposal at the same time. This weed has been successfully used for vermicomposting by many researchers (Gajalakshmi et al. 2001, 2002; Gupta et al. 2007).
During vermicomposting process, earthworms can hasten the composting process to a significant extent with production of a better quality of compost as compared with those prepared through traditional composting methods (Gupta and Garg 2008). Vermicomposting has been considered as a sustainable, potential, and cost-effective alternative for the treatment of this noxious weed. Through vermicomposting, earthworms ingest, grind, and digest organic waste, and finally convert it into a much finer, humified, and microbially active material by the cooperative action of earthworms and microorganisms. Microbes are conscientious for biochemical degradation of organic matter, however; earthworms are the important drivers of the process by conditioning the substrate and altering the biological activity (Gupta and Garg 2008; Khwairakpam and Bhargava 2009; Vig et al. 2011).
The presence of heavy metals in the final vermicompost of water hyacinth hoists serious concern about the undesirable environmental impact, as a result of extreme application to agricultural lands. Heavy metal uptake by plants and consecutive accumulation in human tissues and biomagnification through the food chain causes both human health and environment concern (Wong and Selvam 2006). The mobility of trace metals, their bioavailability, and related eco-toxicity to the plants depend strongly on their specific chemical forms or ways of binding rather than the total metal concentration (Fuentes et al. 2006; Gupta and Sinha 2007). The term “bioavailability” of any element is used to indicate that part of total concentration of the element eagerly soluble in water and considered as easily available to the plants and soil microorganisms. The water soluble fraction of heavy metals has the highest potential for contamination of food chain, surface water, and groundwater (Hait and Tare 2012).
Earthworms may accumulate nonessential toxic heavy metals when they are exposed to heavy metal-contaminated soils (Li et al. 2010). They can reduce possible toxic effects of heavy metals through mitochondrial and cytoplasmic fractions (Jain et al. 2004; Singh and Kalamdhad 2012). The concentration of metals in earthworms is controlled by three factors such as absorption, elimination, and biotransformation (Dominguez-Crespo et al. 2012). The accumulation of heavy metals in earthworms depends on mainly bioavailable fraction of metal rather than the total metal concentration (Li et al. 2010). There is very limited literature available on the bioavailability and leachability of heavy metals during vermicomposting of water hyacinth. Therefore, the aim of study was to assess the bioavailability and leachability of heavy metals (Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr) in different proportion of water hyacinth with cattle manure during vermicomposting process using Eisenia fetida.
Materials and methods
Earthworms (Eisenia fetida) culture
Exotic earthworm species E. fetida was collected from the Central Plantation Crops Research Institute (CPCRI), Indian Council of Agricultural Research, Regional Station, Kahikuchi, Guwahati, India. For developing the cultures, perspex bin sizes 450 × 300 × 450 mm were fabricated in the laboratory. For aeration and drainage purpose, 16 holes of 10-mm diameter were drilled along the longer sides and 16 more at the bottom, respectively. The earthworm’s bedding was prepared using chopped hay (about 50 mm), cow dung, banana pulp (chopped about 50 mm), and tree leaves; all were partially degraded. The bedding was watered to keep it moist to enable the worms to breathe. The earthworms were cultured in partially degraded cattle manure.
Feedstock materials
Water hyacinth, cattle (cow) manure, and sawdust were used for the preparation of different waste mixtures. Water hyacinth was collected from the Amingaon industrial area near the Indian Institute of Technology Guwahati campus. Cattle manure was obtained from a dairy farm near the campus. Sawdust was purchased from a nearby saw mill. Prior to composting, the maximum particle size in the mixed waste was restricted to <1 cm in order to provide better aeration and moisture control.
Experimental set-up
The experiments were conducted in duplicate in bamboo containers (reactors) of curved shaped (Radius 120 mm, depth 90 mm, and volume 904.70 cm3). Temperature in the experimentation room was maintained at 25 ± 1 °C, which is the optimum temperature for E. fetida. These reactors were kept in room temperature. Figure 1 shows the pictorial view of bamboo-made vermicompost reactors and vermicompost. The earthworm weight was calculated according to the weight of the feedstock added and the number of days for experimentation, based on the literature suggested; earthworms can consume materials half of their body weight per day under favorable conditions (Haimi and Huhta 1986; Khwairakpam and Bhargava 2009). The moisture level was maintained about 60–70 % throughout the study period by periodic sprinkling of adequate quantity of tap (potable) water. To prevent moisture loss, the reactors were covered with gunny bags. The proper aeration was provided by perforated reactor design and periodic turning of waste mixture. The reactor was designed for a total weight of 1.5 kg for 45 days (based on worm mass added) composting period. Acclimatized 120 earthworms (adult and juvenile, average weight of 50 g) were randomly picked from the perspex bin culture and used for the purpose of this investigation. The composition of waste material in different trials was as follows:
-
Trial 1: water hyacinth (100 %) + cattle manure (0 %) + sawdust (0 %) + earthworms
-
Trial 2: water hyacinth (80 %) + cattle manure (10 %) + sawdust (10 %) + Earthworms
-
Trial 3: water hyacinth (70 %) + cattle manure (20 %) + sawdust (10 %) + earthworms
-
Trial 4: water hyacinth (60 %) + cattle manure (30 %) + sawdust (10 %) + earthworms
-
Trial 5: water hyacinth (50 %) + cattle manure (40 %) + sawdust (10 %) + earthworms
These mixtures were turned manually every 15 days in order to provide proper aeration to earthworms and on the same day, earthworm biomass was counted. Homogenized samples (free from earthworms, hatchlings, and cocoons) of the feed material were drawn at 0, 15, 30, and 45 days from each reactor. The 0 day refers to the time of initial mixing of wastes before inoculation of earthworms. The samples were dried at 105 °C in oven for 24 h; dried samples were ground and passed from 0.2-mm sieves and stored for further analysis.
Physico-chemical analysis
Each sample was analyzed for the following parameters: pH, electrical conductivity (EC) (1:10 w/v waste: water extract), organic matter, and ash content (Kalamdhad et al. 2009). Flame photometer (Systronic 128) was used for analysis of Na, K, and Ca concentration and atomic absorption spectrometer (Varian SpectrAA 55B) was used for analysis of Mg, Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr concentration after digestion of the 0.2-g sample with 10-mL H2SO4 and HClO4 (5:1) mixture in block digestion system (Pelican equipments Chennai-India) for 2 h at 300 °C. Water-soluble heavy metals were determined after extraction of 2.5-g sample with 50 mL of distilled water (sample: solution ratio = 1:20) at room temperature for 2 h in a shaker at 100 rpm (Ciavatta et al. 1993). Diethylenetriaminepentaacetic acid (DTPA) extractable metals were obtained by mechanically shaking 4-g ground sample (screened through 0.22-mm sieve) with 40 mL of 0.005-M DTPA, 0.01-M CaCl2 and 0.1-M (triethanolamine) buffered to pH 7.3 at 100 rpm (Guan et al. 2011). The standard toxicity characteristic leaching procedure (TCLP) method according to environmental protection agency (EPA) Method 1311 (1992) was applied to the solid samples in order to determine the potential leachability of heavy metals. According to this method, 5-g solid sample (size less than 9.5 mm) with 100 mL of acetic acid at pH 4.93 ± 0.05 (pH was adjusted by 1-N NaOH) (sample: solution ratio = 1:20) was taken in 125-mL borosilicate glass bottle and kept at room temperature for 18 h in a shaker at 30 ± 2 rpm. The suspensions were centrifuged for 5 min at 10,000 rpm, and then, it was filtered through Whatman no. 42 filter paper and stored in a plastic reagent bottle at 4 °C for analysis of selected heavy metals.
Biodegradability is a parameter that relates initial and final content of vermicompost organic matter during composting and was also calculated for each trial. Biodegradability coefficient (Kb) was calculated using the equation (Yadav and Garg 2009):
where OM f is the organic matter content at the end of process and OM i is the organic matter content at the beginning of the process.
All results reported are the means of three replicates. Repeated measures treated with analysis of variance (ANOVA) and Tukey’s test were made using Statistica software. The objective of the statistical analysis was to determine any significant differences among the parameters analyzed for different trials.
Results and discussion
Earthworm population and biomass in different trials
Earthworm production is an incorporated and important feature of any vermicomposting process (Deka et al. 2011). The survival of earthworms was very less in trial 1 which contains 100 % water hyacinth in comparison to other trials. The feed with higher proportion of water hyacinth in might not have adequate amount of easily metabolizable organic matter and non-assimilated carbohydrates which could be essential for the growth and reproduction of the earthworms (Gupta et al. 2007). The number of earthworms showed statistical difference (F = 16.4, P <0.001) in different feed mixtures. The number of earthworms increased on the 30th day of experiment in all trials but maximum biomass growth was observed in trial 5 followed by trials 4, 3, 2, and 1 (Fig. 2). The earthworms started to decrease after the 30th day due to the exhaustion of food at the end of composting (Yadav and Garg 2009). Furthermore, the higher population of earthworms could increase mortality, reduced cocoon production per earthworm, and reduced growth rate due to conversion of fresh organic matter into earthworm casts (Yadav and Garg 2011). Similar observations have been reported by Vig et al. (2011) during vermicomposting of tannery sludge with cow dung. Initially, cocoon production rate was lower, and with time, it was enhanced; but new hatchlings declined after 30 days. The maximum number of cocoons and hatchlings were produced in trial 5 and minimum in trial 1 (Fig. 2). Higher percentage of water hyacinth in the feed mixture was not favorable for the earthworms’ growth and affected the biomass production during the vermicomposting process. This might be due to the fact that the higher proportion of water hyacinth in the feed mixture made it harder and more tensile, which was not easily used by the earthworms (Gupta et al. 2007). The higher increase in biomass production resulted from the higher percentage of cattle manure addition. The continued existence, biomass production, and reproduction of earthworms are the best indicators to assess the vermicomposting process. The number of earthworms biomass (F = 50.7, P <0.001) number of cocoons (F = 31, P <0.001) and number of hatchlings (F = 104.9, P <0.001) shows statistical differences in different feed mixtures.
Physico-chemical analysis
The water hyacinth and cattle manure-containing feed mixtures had higher moisture and organic contents initially. Sufficient moisture is one of the most important requirements for earthworms during vermicomposting. They require moisture in the range 60–70 %. Excess moisture content may create anaerobic conditions which may be fatal to earthworms (Garg and Gupta 2011). The feed mixtures used in the present study were having moisture within the recommended range. The pH is an important parameter which greatly affects the vermicomposting process. The acceptable pH range, suitable for earthworms and microorganisms activity, is 5.5–8.5 (Yadav and Garg 2011). The pH was increased significantly (F = 90.1, P <0.001) from 6.07, 6.08, 6.50, 6.75, and 6.80 to 7.53, 7.57, 7.64, 7.68, and 7.70 in trials 1, 2, 3, 4, and 5, respectively, during the vermicomposting process (Table 1). An increase in the pH of final vermicompost may be due to excess of organic nitrogen not required by microbes, released as ammonia, which gets dissolved in water and increases the pH of the vermicompost (Vig et al. 2011). Composting and vermicomposting process itself have buffering capacity, as it always stays near neutrality to either initial waste material with alkaline or acidic pH. The neutralization reaction occurring due to presence of carboxylic and phenolic groups in humic acid and ammonium ions formed at the end of the process (Garg and Gupta 2011).
EC reflects the salinity of the composting product and its suitability for plant growth. High EC in the final product is undesirable because it will inhibit plant rooting and also reduce the transportation of water and nutrients to the plants (Fang and Wong 1999; Chiang et al. 2007). The EC was decreased significantly (F = 9.8, P <0.001) in the range of 5.7–27.3 % in all trials during the process (Table 1). The decreasing trend of EC during vermicomposting agrees with the finding of another researcher (Vig et al. 2011). Organic matter of the final vermicompost was significantly (F = 131, P <0.001) lower as compared to the initial composted materials. The maximum reduction of organic matter was observed in trial 5 (54.5 %) followed by trial 4 (51.6 %), trial 3 (38.6 %), trial 2 (16.4 %), and trial 1 (5.0 %) during the process. The reduction of organic matter during vermicomposting is consistent with other findings (Gupta et al. 2007; Khwairakpam and Bhargava 2009; Garg and Gupta 2011). The reduction of organic matter might be due to the loss of CO2 as well as consumption of the available carbon as a source of energy by the earthworms and the microorganisms in all the trials (Khwairakpam and Bhargava 2009). The highest values of biodegradability constant (Kb) was observed in trial 5 (0.79) followed by trial 4 (0.78), trial 3 (0.68), trial 2 (0.40) and trial 1 (0.11). The ash content is an important investigative parameter for decomposition and mineralization of the substrates. The ash content was increased in all trials significantly (F = 83.6, P <0.001) in the range of 6.2–54.5 % during the vermicomposting process. Significantly, increase in ash content indicated the higher rate of volatilization and mineralization of the organic matter (Gupta et al. 2007; Khwairakpam and Bhargava 2009).
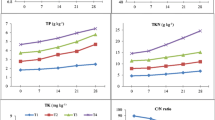
The macro-nutrients (Na, K, Ca, and Mg) are required in very less quantity for the adaptation of earthworms during the vermicomposting process and these nutrients are also required for plant growth. Table 1 illustrates the concentration of macro-nutrients Na (F = 51, P <0.001), K (F = 137, P <0.001), Ca (F = 33.5, P <0.001), and Mg (F = 43.3, P <0.001) were increased in all trials throughout the vermicomposting process. The augmented number of micro flora present in the gut of earthworms in the case of vermicomposting might have played an important role in this process and increased nutrients concentration in the vermicompost (Khwairakpam and Bhargava 2009; Hait and Tare 2012). Furthermore, nutrient increase during process might be due to net loss of dry mass (Amir et al. 2005; Singh and Kalamdhad 2013a).
Heavy metals are important trace elements for the well-being of plants, animals, and humans, but their excess is known to have toxic effects. Heavy metals were increased from initial feed mixtures but their enrichment was within permissible limit. The increase in the content of total metals in vermicompost may be due to reduction in the weight and volume of the final product (Vig et al. 2011). An increase in heavy-metal concentration in the final vermicompost of different wastes was reported by another researcher (Kaushik and Garg 2003). The total concentration of Cu, Fe, and Ni was reduced in trial 4 about 9.6, 17.2, and 8.0 %, respectively, from the initial concentration. Very little amount of Fe was also reduced in trials 3 and 5 about 11.6 and 5.6 %, respectively. The total concentration of Mn and Cd was reduced about 12.1 and 24.6 %, respectively, in trial 3 from the initial concentration (Table 2). The reduction in metal content in trials 3, 4, and 5 showed better decomposition as well as earthworm-growth activities. Therefore, it is suggested that metal loss was related to the earthworm activity in the waste decomposition; furthermore, earthworms can accumulate heavy metals in their tissues if reared in contaminated soils for longer duration (Suthar and Singh 2008). The reduction of heavy metals was also reported by other researchers (Jain et al. 2004). The variation in Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr concentrations in different trials were significant (F = 242, P <0.001 for Zn; F = 9, P <0.001 for Cu; F = 49, P <0.001 for Mn; F = 32.6, P <0.001 for Fe; F = 92.6, P <0.001 for Ni; F = 34.8, P <0.001 for Pb; F = 471, P <0.001 for Cd; F = 6.2, P <0.001 for Cr).
Bioavailability of heavy metals
Water solubility of heavy metals
It is extremely necessary to determine the water-soluble heavy metals in vermicompost before agronomic application because this metal fraction is positively more biologically dynamic and it has the highest prospective of contaminating to food chain (Hsu and Lo 2001; Iwegbue et al. 2007; Hait and Tare 2012). Figure 3 illustrates the changes in water soluble Zn, Cu, Mn, Fe, and Cr contents during 45 days of vermicomposting period. Water soluble Ni, Pb, and Cd contents were not detectable in initial materials and during the vermicomposting process. The water-soluble concentration of Zn was reduced in all trials in the range of 35.8–65.5 % of the total Zn during the process. The water solubility of Cu reduced in the range of 19.3–64 % of the total Cu in all trials except trial 1 during the process. The water solubility of Cu increased slightly in trial 1 and might be due to poor growth of earthworm biomass, resulting to incomplete degradation of organic matter (Gupta et al. 2007). The decrease in water solubility of Cu and Zn might be due to formation of humic substances during vermicomposting process where humic substances contain carboxyl groups, which can form complex with metals (Singh and Kalamdhad 2013b). The water soluble concentration of Mn was reduced in the range of 46.2–84.6 % of the total Mn in all trials during the process. The water soluble concentration of Fe was reduced in the range of 6.7–58.8 % of the total Fe in all trials except trial 4. The water solubility of Fe increased in trial 4 and can be attributed to a greater rate of degradation in the presence of higher concentrations of different microorganisms within earthworm intestine (Hait and Tare 2012). The water solubility of Cr was reduced in all trials in the range of 35.5–76.7 % of the total Cr during the process.
Hait and Tare (2012) have demonstrated that vermicomposting caused considerable reduction in concentration of water soluble metals like Cu, Zn, and Cr during vermicomposting of sewage sludge. When the organic matter was passing through the gut of the earthworm; some part of its digested, and pH and microbial activity of the gut were increased. As a consequence, the possibilities of binding of metals to ions and carbonates (i.e., more soluble fractions) increased in ingested material. Therefore, the rate of bioaccumulation of water soluble fraction of metals could be increased when it passes through the worm’s gut (Suthar 2009). The order of water soluble metal concentration in the vermicomposted water hyacinth was Mn > Cu > Zn > Cr = Fe. The bioavailability of heavy metals decreased during vermicomposting process, and it might be due to bioaccumulation of metals by E. fetida and organometallic complex formation (Singh and Kalamdhad 2012). The mobility and bioavailability of heavy metals decreased during vermicomposting in the course of two major types of cellular adaptation to toxicity of metals: one involves binding of metals to nuclear proteins and the formation of inclusion nuclear bodies; the second type is a cytoplasmic process involving synthesis of a specific metal binding protein, metallothionein within the chloragogenous tissue (Hait and Tare 2012). The variation in water soluble Zn, Cu, Mn, Fe, and Cr concentrations in different trials were significant (F = 15.1, P <0.001 for Zn; F = 55, P <0.001 for Cu; F = 247.9, P <0.001 for Mn; F = 1,210, P <0.001 for Fe; F = 17.9, P <0.001 for Cr). Water soluble fraction of heavy metals (Zn, Cu, Mn, Fe, and Cr) was significantly correlated with pH, organic matter, electrical conductivity, ash content, and total metal concentration (Table 3).
Plant availability of heavy metals (extraction with DTPA)
The metal toxicity is not caused by the presence of heavy metals, but it depends on metal concentration, toxicity, mobility in free form, the route of uptake mechanism, and bioavailability if it is accumulated in plants (Vig et al. 2011). The immobilization of metals during vermicomposting process appears to be a valuable and easy substitute. Generally, metal fraction extracted with chelating agent DTPA can be considered as potentially available for plant uptake (Garcia et al. 1995; Fang and Wong 1999; Chiang et al. 2007). The DTPA extractable Pb and Cd were not detectable during the vermicomposting process. The DTPA extraction efficiency is the ratio of metal extracted with DTPA solution to the total concentration of metal (Singh and Kalamdhad 2013a). The DTPA extraction efficiency was reduced in the ranges: 4.0–33.6 % for Zn, 9.2–57.7 % for Cu, 36.5–54.6 % for Fe, 63.7–73.7 % for Ni except for trial 1, and 15.2–51.3 % for Cr (Fig. 4) during the vermicomposting process. The percentage reduction of Zn and Cu might be due to immobilization of Cu and Zn atoms in microorganism cells decomposing the organic materials and to the formation of organometallic complex by earthworms (Bhattacharya and Chattopadhyay 2006). The DTPA concentration of Mn was increased in all trials except trial 2 in the range of 3.7–53 % of the total Mn during the process. In trial 2, concentration of Mn was reduced about 34.2 % of total Mn concentration. Introduction of earthworms for vermicomposting tend to increase the DTPA extractability of Mn in all trials except trial 2, this behavior was attributed to a greater rate of degradation in the presence of higher concentrations of different microorganisms within earthworm intestine (Bhattacharya and Chattopadhyay 2006).
Metabolic conversion of highly toxic form Cr (VI) to nontoxic form Cr (III) through mitochondrial and cytoplasmic fractions has also been demonstrated in E. fetida (Jain et al. 2004). However, metal reduction was comparatively higher in trials 2, 3, 4, and 5 due to maximum earthworm activities. On the other hand, trial 1, which exhibited the minimum mineralization rate even in earthworm biomass production, showed the least metal decrease or increase during the vermicomposting process. The reduction of DTPA extractable metals during vermicomposting is also reported by other researchers (Maity et al. 2008; Suthar 2009). Vermicomposting of organic wastes accelerates organic matter stabilization and gives chelating and phytohormonal elements which have a high content of microbial matter and stabilized humic substances (Gupta and Garg 2008; Suthar 2009; Hait and Tare 2012). The interaction of the humic acid with metal is one of the main factors affecting the partitioning of heavy metals during the process. It has a stronger sorption effect on heavy metals, particularly Cu and Zn (Hait and Tare 2012). The variation in DTPA extractable Zn, Cu, Mn, Fe, Ni, and Cr concentrations in different trials were significant (F = 46.2, P <0.001 for Zn; F = 26.8, P <0.001 for Cu; F = 88, P <0.001 for Mn; F = 48, P <0.001 for Fe; F = 292, P <0.001 for Ni; F = 3.7, P = 0.012 for Cr). DTPA extractable fraction of heavy metals (Zn, Cu, Mn, Fe, Ni, and Cr) was significantly correlated to pH, organic matter, electrical conductivity, ash content, and total metal concentration (Table 3).
Leachability of heavy metals
The TCLP is designed to determine the mobility of both organic and inorganic analytes present in liquid, solid, and multiphasic wastes. If an analysis of the liquid fractions of the TCLP extract indicates that regulated heavy metals are present in high concentrations, that even after dilution from the other fractions of the extract, the concentration would be above the regulatory level for those metals, and then the waste is hazardous. The threshold limit for heavy metals contamination in mg/kg is as follows: Cd—20, Cr—100, and Pb—100 (US EPA method 1311, 1992). The leachability of the contaminants from the solid samples to the liquid phase might be influenced by several physico-chemical factors such as type of the leaching medium, particle size, pH, and complexing agents that may be present in the solid sample (Skodras et al. 2009). Table 4 illustrates the changes in leachable Zn, Cu, Mn, Fe, Ni, Cd, Pb, and Cr concentration during 45 days of vermicomposting period. The leachable concentration of Zn, Fe, Pb, and Cr was reduced (% of total metal) in all trials in the ranges of 12.8–49.1, 31–48, 1.1–64.7, and 19.5–56.75 % of total Zn, Fe, Pb, and Cr, respectively, during the vermicomposting period. The leachability of Mn was reduced in trials 4 and 5; however, it was increased in trials 1, 2, and 3 (% of the total Mn) during the vermicomposting process.
The leachability of Cu, Ni, and Cd was reduced in the range of 13.4–62.3, 28.2–71.2, and 30.6–51.9 % of total Cu, Ni, and Cd, respectively, in all trials except trial 1. In trial 1, leachability of these metals increased due to partial degradation and less growth of biomass (Gupta et al. 2007). Reduction of leachable concentration of metals during vermicomposting might be due to accumulation in earthworms. Similar results were also reported by Jain et al. (2004). This study revealed that the leachability of all metals decreases with vermicomposting time; it might be due to increase in pH and complexity of metals humic substances. According to Maity et al. (2008), pH may influence leachability of metals by the following mechanism: an increase in compost pH causes an increase in surface negative charge which can raise cationic adsorption, formation of metal hydroxy ionic species that have a greater affinity for adsorption sites than the metal cations and precipitation of metal as metal hydroxides. In addition, during vermicomposting, metals interact with many chemicals and participate in detoxification processes, as part of the enzymes of the antioxidant systems, such as superoxide dismutase and in metallothioneins (Singh and Kalamdhad 2012). Moreover, the cutaneous absorption of metals was also evidenced in earthworms (Suthar 2009). The reduction of Cu during vermicomposting might be due to high affinity of Cu for functional groups -OH or -COO of humic substances (Kang et al. 2011). The order of leachable heavy metal content in the vermicomposted water hyacinth was Mn > Fe > Zn > Pb > Ni> Cr > Cu > Cd. The variation in leachable Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr concentrations in different trials were significant (F = 50.2, P <0.001 for Zn; F = 8.4, P <0.001 for Cu; F = 12.3, P <0.001 for Mn; F = 30.4, P <0.001 for Fe; F = 20.9, P <0.001 for Ni; F = 4.9, P = 0.003 for Pb; F = 8, P <0.001 for Cd; F = 685, P <0.001 for Cr). TCLP extractable fraction of heavy metals (Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr) was significantly correlated to pH, organic matter, electrical conductivity, ash content, and total metal concentration (Table 5).
Conclusion
An addition of cattle manure in the feed mixture enhanced the growth and productiveness of the earthworms but reduction of bioavailability and leachability of heavy metals were not co-related with earthworm productiveness during the process. The pH was increased significantly during the process which was the main factor for reducing bioavailability of heavy metals. The total concentration of heavy metals (Cu, Mn, Fe, Ni, and Cd) was decreased in some trials during the vermicomposting process. The vermicomposting of water hyacinth using E. fetida was extremely effective for reduction of water solubility, DTPA extractability, and leachability of heavy metals (Zn, Cu, Fe, Ni, Pb, Cd, and Cr) during the vermicomposting process. Water solubility of Zn, Cu, Mn, and Cr was reduced significantly in all trials except trial 1. DTPA extractability of Cu, Fe, Ni, and Cr was reduced significantly. Leachability of Zn, Cu, Fe, Ni, Pb, Cd, and Cr was reduced significantly during the vermicomposting process. Leachable concentration of heavy metals in all trials was under the threshold limits. Water soluble, DTPA extractable, and leachable heavy metals were positively correlated with pH, organic matter, electrical conductivity, ash content, and total metal concentration. This study suggests that the need for continuous monitoring of bioavailability and leachability of heavy metals is essential rather than total metal concentration in the final vermicompost.
References
Agunbiade FO, Olu-Owolabi BI, Adebowale KO (2009) Phytoremediation potential of Eichhornia crassipes in metal-contaminated coastal water. Bioresour Technol 100:4521–4526
Amir S, Hafidi M, Merlina G, Revel JC (2005) Sequential extraction of heavy metals during composting of sewage sludge. Chemosphere 59:801–810
Bhattacharya SS, Chattopadhyay GN (2006) Effect of vermicomposting on the transformation of some trace elements in fly ash. Nutr Cycl Agroecosyst 75:223–231
Chiang KY, Huang HJ, Chang CN (2007) Enhancement of heavy metal stabilization by different amendments during sewage sludge composting process. J Environ Eng Manage 17(4):249–256
Chunkao K, Nimpee C, Duangmal K (2012) The King’s initiatives using water hyacinth to remove heavy metals and plant nutrients from wastewater through Bueng Makkasan in Bangkok, Thailand. Ecol Eng 39:40–52
Ciavatta C, Govi M, Simoni A, Sequi P (1993) Evaluation of heavy metals during stabilization of organic matter in compost produced with municipal solid wastes. Bioresour Technol 43:147–153
Deka H, Deka S, Baruah CK, Das J, Hoque S, Sarma H, Sarma NS (2011) Vermicomposting potentiality of Perionyx excavatus for recycling of waste biomass of Java citronella—an aromatic oil yielding plant. Bioresour Technol 102:11212–11217
Dominguez-Crespo MA, Sanchez-Hernandez ZE, Torres-Huerta AM, Negrete-Rodríguez MLX, Conde-Barajas E, Flores-Vela A (2012) Effect of the heavy metals Cu, Ni, Cd, and Zn on the growth and reproduction of epigeic earthworms (E. fetida) during the vermistabilization of municipal sewage sludge. Water Air Soil Pollut 22:915–931
Fang M, Wong JWC (1999) Effects of lime amendment on availability of heavy metals and maturation in sewage sludge composting. Environ Pollut 106:83–89
Fuentes A, Llorens M, Saez J, Aguilar MI, Marın ABP, Ortuno JF, Meseguer VF (2006) Ecotoxicity, phytotoxicity and extractability of heavy metals from different stabilised sewage sludges. Environ Pollut 143:355–360
Gajalakshmi S, Ramasamy EV, Abbasi SA (2001) Assessment of sustainable vermiconversion of water hyacinth at different reactor efficiencies employing Eudrilus eugeniae Kingberg. Bioresour Technol 83:131–135
Gajalakshmi S, Ramasamy EV, Abbasi SA (2002) High-rate composting–vermicomposting of water hyacinth (Eichhornia crassipes, Mart Solms). Bioresour Technol 83:235–239
Garcia C, Moreno JL, Hernfindez T, Costa F (1995) Effect of composting on sewage sludges contaminated with heavy metals. Bioresour Technol 53:13–19
Garg VK, Gupta R (2011) Optimization of cow dung spiked pre-consumer processing vegetable waste for vermicomposting using Eisenia fetida. Ecotoxicol Environ Saf 74:19–24
Guan TX, He HB, Zhang XD, Bai Z (2011) Cu fractions, mobility and bioavailability in soil-wheat system after Cu-enriched livestock manure applications. Chemosphere 82:215–222
Gupta AK, Sinha S (2007) Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour Technol 98:1788–1794
Gupta R, Garg VK (2008) Stabilization of primary sludge during vermicomposting. J Hazard Mater 153:1023–1030
Gupta R, Mutiyar PK, Rawat NK, Saini MS, Garg VK (2007) Development of a water hyacinth based vermireactor using an epigeic earthworm Eisenia fetida. Bioresour Technol 98:2605–2610
Haimi J, Huhta V (1986) Capacity of various organic residues to support adequate earthworm biomass for vermicomposting. Biol Fert Soils 2:23–27
Hait S, Tare V (2012) Transformation and availability of nutrients and heavy metals during integrated composting-vermicomposting of sewage sludges. Ecotoxicol Environ Saf 79:214–224
Hsu JH, Lo SL (2001) Effects of composting on characterization and leaching of copper, manganese, and zinc from swine manure. Environ Pollut 114:119–127
Iwegbue CMA, Emuh FN, Isirimah NO, Egun AC (2007) Fractionation, characterization and speciation of heavy metals in composts and compost-amended soils. Afr J Biotechnol 6(2):067–078
Jain K, Singh J, Chauhan LKS, Murthy RC, Gupta SK (2004) Modulation of flyash-induced genotoxicity in Vicia faba by vermicomposting. Ecotoxicol Environ Saf 59:89–94
Kalamdhad AS, Singh YK, Ali M, Khwairakpam M, Kazmi AA (2009) Rotary drum composting of vegetable waste and tree leaves. Bioresour Technol 100:6442–6450
Kang J, Zhang Z, Wang JJ (2011) Influence of humic substances on bioavailability of Cu and Zn during sewage sludge composting. Bioresour Technol 102:8022–8026
Kaushik P, Garg VK (2003) Vermicomposting of mixed solid textile mill sludge and cow dung with epigeic earthworm Eisenia fetida. Bioresour Technol 90:311–316
Khwairakpam M, Bhargava R (2009) Vermitechnology for sewage sludge recycling. J Hazard Mater 161:948–954
Li L, Xu Z, Wu J, Tian G (2010) Bioaccumulation of heavy metals in the earthworm Eisenia fetida in relation to bioavailable metal concentrations in pig manure. Bioresour Technol 101:3430–3436
Maity S, Padhy PK, Chaudhury S (2008) The role of earthworm Lampito mauritii (Kinberg) in amending lead and zinc treated soil. Bioresour Technol 99:7291–7298
Mishra VK, Tripathi BD (2009) Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes). J Hazard Mater 164:1059–1063
Singh J, Kalamdhad AS (2012) Reduction of heavy metals during composting—a review. Int J Environ Prot 2(9):36–43
Singh J, Kalamdhad AS (2013a) Assessment of bioavailability and leachability of heavy metals during rotary drum composting of green waste (water hyacinth). Ecol Eng 52:59–69
Singh J, Kalamdhad AS (2013b) Bioavailability and leachability of heavy metals during water hyacinth composting. Chem Speciat Bioavailab 25(1):1–14
Skodras G, Grammelis P, Prokopidou M, Kakaras E, Sakellaropoulos G (2009) Chemical, leaching and toxicity characteristics of CFB combustion residues. Fuel 88:1201–1209
Suthar S (2009) Vermistabilization of municipal sewage sludge amended with sugarcane trash using epigeic Eisenia fetida (Oligochaeta). J Hazard Mater 163:199–206
Suthar S, Singh S (2008) Feasibility of vermicomposting in biostabilization of sludge from a distillery industry. Sci Total Environ 394:237–243
US Environmental Protection Agency Method 1311—toxicity characteristic leaching procedure (TCLP), 35 p, July 1992
Vig AP, Singh J, Wani SH, Dhaliwal SS (2011) Vermicomposting of tannery sludge mixed with cattle dung into valuable manure using earthworm Eisenia fetida (Savigny). Bioresour Technol 102:7941–7945
Wong JWC, Selvam A (2006) Speciation of heavy metals during co-composting of sewage sludge with lime. Chemosphere 63:980–986
Yadav A, Garg VK (2009) Feasibility of nutrient recovery from industrial sludge by vermicomposting technology. J Hazard Mater 168:262–268
Yadav A, Garg VK (2011) Industrial wastes and sludges management by vermicomposting. Rev Environ Sci Biotechnol 10:243–276
Acknowledgments
The authors gratefully acknowledge the financial support of the Department of Science and Technology (DST), Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Singh, J., Kalamdhad, A.S. Reduction of bioavailability and leachability of heavy metals during vermicomposting of water hyacinth. Environ Sci Pollut Res 20, 8974–8985 (2013). https://doi.org/10.1007/s11356-013-1848-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1848-x