Abstract
This study describes the potential application of lipopeptide biosurfactants in removal of petroleum hydrocarbons and heavy metals from the soil samples collected from industrial dumping site. High concentrations of heavy metals (like iron, lead, nickel, cadmium, copper, cobalt and zinc) and petroleum hydrocarbons were present in the contaminated soil samples. Lipopeptide biosurfactant, consisting of surfactin and fengycin was obtained from Bacillus subtilis A21. Soil washing with biosurfactant solution removed significant amount of petroleum hydrocarbon (64.5 %) and metals namely cadmium (44.2 %), cobalt (35.4 %), lead (40.3 %), nickel (32.2 %), copper (26.2 %) and zinc (32.07 %). Parameters like surfactant concentration, temperature, agitation condition and pH of the washing solution influenced the pollutant removing ability of biosurfactant mixture. Biosurfactant exhibited substantial hydrocarbon solubility above its critical micelle concentration. During washing, 50 % of biosurfactant was sorbed to the soil particles decreasing effective concentration during washing process. Biosurfactant washed soil exhibited 100 % mustard seed germination contradictory to water washed soil where no germination was observed. The results indicate that the soil washing with mixture of lipopeptide biosurfactants at concentrations above its critical micelle concentration can be an efficient and environment friendly approach for removing pollutants (petroleum hydrocarbon and heavy metals) from contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrialisation and urbanisation has rendered hydrocarbons and heavy metals as ubiquitous environmental pollutant. Excessive accumulation of petroleum hydrocarbons and heavy metals pollutants in the soil causes serious threat to biota and the environment. Hydrocarbon pollutants are known to have hazardous effect on all forms of life (Guo et al. 2011; Tang et al. 2011). Unlike the hydrocarbon pollutants, metals remain persistent in the environment owing to their non-biodegradability and accumulate throughout the food chain leading to serious ecological and health hazards. Almeida et al. (2013) reported that metal ions hinder the biodegradation of hydrocarbons. Therefore, rapid and simultaneously removal of petroleum hydrocarbons and heavy metal pollutants holds a vital importance in the bioremediation process.
Generally, remediation of heavy metal- and hydrocarbon-contaminated soil is performed with or without excavation by soil washing or soil flushing. Soil washing is a mechanical or chemical or combination of both processes that uses liquids, generally water, to remove pollutants from the soils (Dermont et al. 2008). It is regarded as one of the permanent remediation method available for removal of metals and hydrocarbon contaminants. Ex situ soil washing tries to solubilise the contaminants from the soil with an extracting fluid containing chemical reagents such as surfactants, acids or chelating agents like EDTA. Washing with acid or synthetic surfactant adversely affects chemical and physical structure of soils, thus limiting its reuse. Soil washing with EDTA has health and safety concerns due to slow degradation and inability to recovery EDTA–metal complex (Hong et al. 2002).
The ultimate goal of any remediation process must not only be to remove the contaminant from the polluted soil but also to refurbish soil health so that it restores its potential to support the natural flora and fauna. In this context, biosurfactants (biological counterpart of synthetic surfactants) hold immense potential due to their low toxicity, biodegradability, easy production and possibility of reuse. Biosurfactants do not result in secondary pollution even if they are leaked or discharged into the ecosystem (Kilic et al. 2011).
Most of the biosurfactant (both plant-derived surfactants and microbial surfactant) washing studies reported to date have been performed on the soil artificially contaminated or spiked with pollutants (Pacwa-Plociniczak et al. 2011). To best of our knowledge, combination of lipopeptides biosurfactants has not been applied for the soil washing process. According to Zhu and Feng (2003), mixed surfactant system may improve the performance of surfactant-enhanced remediation of soils, by decreasing the quantity of applied surfactant and, thus, the remediation cost.
The present work describes feasibility of using combination of anionic lipopeptides biosurfactants produced by Bacillus subtilis strain A21 in soil washing process. Study elucidates the best possible condition for soil decontamination of metal and hydrocarbon pollutant by batch type soil washing. Furthermore, washed soil was examined for plant germination feasibility.
Materials and methods
Chemical and physical characterisation of soil
Metal contaminated soil samples were collected from Adityapur Industrial Area’s abandoned dumping site. Soils were air-dried and sieved to remove coarse sand and stone by a 2-mm sieve. Further chemical and physical characteristic of soil samples were analysed as per the method described by Hong et al. (2002).
Total petroleum hydrocarbon (TPH) pollutants present in the washed and unwashed soils were evaluated on gas chromatography (GC-2010, Shimadzu) installed with flame ionisation detector using Rtx-50 column (25 mm × 1 μm × 30 m). TPH was extracted from soil by mixture of hexane and acetone (1:1). Extract was concentrated and re-dissolved in 1 mL of solvent mixture. For GC, nitrogen flowing at the rate of 1 mL min−1 was used as a carrier gas. The temperature was first set at 40 °C for 5 min and was increased to 80 °C at rate of 2 °C min−1, then to 300 °C at rate of 10 °C min−1 and finally set at 300 °C for 10 min. Flame ionisation detector (FID) and injector temperature were set at 310 °C and 290 °C, respectively. The quantification of TPH was done by the calculating the total peak area of chromatogram.
Lipopeptides biosurfactant production
The biosurfactant producer, strain A21, was isolated from rhizosphere of Parthenium hysterophorus. Bacterium was identified as B. subtilis (Gene bank accession no. JN005770) by morphological, biochemical, physiological and 16S rRNA gene sequencing. The biosurfactant was produced by growing strain A21 on minimal salt medium (4 g L−1 NH4NO3, 4 g L−1 KH2PO4, 5.68 g L−1 Na2HPO4, 0.78 mg L−1 CaCl2, 197.18 mg L−1 MgSO4, 1.112 mg L−1 FeSO4) containing 30 g L−1 of sucrose. For extracting the biosurfactant, cell suspension was centrifuged at 8,000×g for 10 min to prepare the cell-free supernatant (CFS) at 4 °C. The CFS was acidified with 6 N HCl to pH 2 and incubated overnight at 4 °C. The precipitated biosurfactant was collected by centrifugation (15,000×g for 20 min) and dissolved in methanol. After the evaporation of methanol using rotary evaporator, biosurfactant was lyophilised to obtain the off white powder. Stock solution of biosurfactant was made in alkaline water (pH 9) at concentration of 50 mg mL−1.
The biosurfactant was characterised by amino acid and matrix-assisted laser desorption/ionisation (MALDI-TOF) mass spectra analysis. Amino acids were analysed after hydrolysing the peptide bonds in boiling 6 N HCl at 105 °C for 24 h. Further analysis was performed according to Waters Pico Tag method by pre-column derivatisation with Phenylisothiocyanate. MALDI-TOF mass spectra were recorded by using Applied Biosystems Voyager MALDI-TOF instrument containing a 337-nm nitrogen laser for desorption and ionisation.
Surface tension and critical micelle concentration (CMC) determination
The surface and interfacial tension were measured at 25 °C using a duNouy tensiometer (CSC Scientific Company Inc., USA) based on ring detachment method. Interfacial tension measurements were carried out against n-hexane, n-heptane, n-octane and n-dodecane. Critical micelle concentration (CMC) was determined by measuring the surface tension at various dilutions and plotting graph between biosurfactant concentration and surface tension values. Concentration of biosurfactant at which sudden increase in the surface tension was observed was taken as CMC.
Conductivity and zeta potential (ζ) determination
Conductivity of the biosurfactant solution and soil suspension was measured by conductivity meter (CyberScan Con 510, EUTECH). Zeta potential measurement of biosurfactant solution and soil suspension was measured by Zetasizer nano ZS (Malvern Instrument, Malvern, UK).
For soil suspension preparation, 50 mg of soil was added to 25 mL of washing solution or water and agitated at 25 °C for 24 h. All the conductivity and Zeta potential measurements were made at pH 9.
Solubilisation of petroleum hydrocarbon
Total petroleum hydrocarbon extracted from contaminated soil was concentrated and collected by evaporating the mixture of hexane and acetone (1:1) in rotary evaporater under reduced pressure. Nearly 50 mg of collected hydrocarbon was mixed with 50 mL biosurfactant solution in 250 mL seperating funnel. The content in the funnel was shaken in a lateral shaker for 24 h at room temperature. After shaking, the content of the funnel was allowed to settle. This resulted in formation of two separate phases: oil-rich and surfactant-rich phases. Surfactant-rich phase was collected and analysed for hydrocarbon content after solvent (hexane and acetone, 1:1) extraction.
Soil washing
Soil washing process with biosurfactant mixture was performed in batch experiment. One gram of soil was taken in a series of polycarbonate centrifuge tubes and mixed with 25 mL of solution containing biosurfactants at different concentration (i.e. 0, ½ CMC, CMC, 10CMC, 50CMC) added. Variation in the soil washing efficiency of biosurfactant with change in pH, temperature and agitation condition was also studied. The suspensions were centrifuged at 5,000×g for 12 min, and supernatant was filtered through 0.45 μm nitrocellulose membrane filters. The compositions of metals in the treated soil and supernatants were analysed by atomic absorption spectrophotometer (AA 6800-Shimadzu, Japan).
High-pressure liquid chromatography (HPLC)
Quantitative analysis of the biosurfactant adhering to soil particles was done by high-pressure liquid chromatography (HPLC) using a Phenomax-C18 column (5 μ, 250 mm × 4.6 mm). A mixture of 3.8 mM trifluoroacetic acid (30 vol%) and acetonitrile, flowing at 1 mL min−1, was used as the mobile phase. An aliquot of the 20-μL sample was injected and analysed using an UV detector (UV–VIS detector, Shimadzu, Japan) at 210 nm. The relative biosurfactant concentration in the fresh biosurfactant solution and soil–biosurfactant solution supernatant was determined by comparing the respective peak areas. The area of peaks that eluted between 10 and 40 min were summed to give the total biosurfactant concentration.
Seed germination experiment
Pot experiment was performed by growing the seeds of brown Indian mustard (Brassica juncea) in the soil obtained after soil washing. Soil was partially dried at ambient temperature to remove excess of water before filling it into polypropylene pots. The seeds were pre-soaked in 2 % hypochlorite for 5 min and then washed thoroughly with deionised water, before sowing it into soil. Pots were watered to maintain appropriate moisture in the soil. After 14 days, plants were uprooted and washed to remove the adhering soil from root, air-dried and weighted. Each set of samples consisted of five replicates.
Results and discussions
Chemical and physical characterisation of soil
Adityapur Industrial Area is one the largest industrial areas in India and once held the record of being the largest industrial belt in Asia (http://en.wikipedia.org/wiki/Adityapur). Sampling site was industrial dumping area abandoned after complete filling of site. This site has sparse vegetation indicating its hostility towards normal plant growth, probably due to toxicity of various pollutants. Table 1 shows chemical and physical features of soil samples used in the present study. Soil samples from industrial site indicated alarming level of metals (namely iron, lead, nickel, cadmium, cobalt, copper and zinc) and hydrocarbon pollutants. Presence of pollutants like hydrocarbon and metal ions is known to inhibit/hinder the plant growth or germination (Tang et al. 2011; Adam and Duncan 2002). However, in the garden soil, hydrocarbon and metals contents were very low as compared with the industrial site soil samples.
Characterization of biosurfactant produced by B. subtilis strain A21
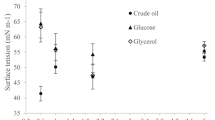
Bacillus species produces structurally diverse lipopeptides with varying applications (Raaijmakers et al. 2010). In present study, B. subtilis strain A21 produced lipopeptide biosurfactants while growing on sucrose supplemented MSM (Fig. 1a). The biosurfactants produced by strain A21 was able to reduce the surface tension of water from 72 to 29 mN m−1 with CMC of 30 mg L−1 and an interfacial tension of less than 2 mN m−1 with n-hexane, n-heptanes, n-octane and n-dodecane. Surface tension, interfacial tension reducing ability and CMC observed in this study suggested it to be an efficient biosurfactant (de Faria et al. 2011; Kim et al. 2010; Mulligan 2005).
Biosurfactant production by B. subtilis strain A21. a Time courses of growth, surface tension reduction and total carbohydrate utilisation by strain A21. Growth was carried out at 30 °C and 200 rpm agitation. Values given are mean ± SD. of three independent experiments. b MALDI-TOF mass spectrum of biosurfactant obtained from B. subtilis A21
Amino acid analysis of biosurfactant exhibited presence of glutamic acid (Glu), isoleucine, leucine, tyrosine, glutamine, proline, alanine (Ala), valine (Val), threonine and asparitic acid (Asp). Non-protein amino acid ornithine was also detected in the biosurfactant. These amino acids suggest that lipopeptide biosurfactant produced by strain A21 may be mixture of surfactin and fengycin (Kim et al. 2010). Presence of Asp and Glu in the biosurfactant is of special interest as they are negatively charged amino acids and can assist in removing cationic metal contamination during soil washing (Mulligan et al. 1999).
MALDI-TOF mass spectra of biosurfactant gave two separate clusters of peaks, one in the range of 1,030–1,076 m/z (corresponding to surfactin isoforms) and other in range of 1,454–1,542 m/z (corresponding to fengycin isoforms) (Fig. 1b). Like other cyclic lipopeptides produced by B. subtilis, fengycin and surfactin also occur as a mixture of isoforms that vary in both length and branching of the β-hydroxy fatty acid moiety, as well as in the amino-acid composition of the peptide ring (Kim et al. 2010; Coutte et al. 2010). The mass spectra of lipopeptides have peaks which can be attributed to the sodium and potassium adducts. The spectra analysis suggested that surfactin isoforms ranged from carbon chain length of C-14 to C-16 while fengycin isoforms ranged from C-15 to C-17. Both forms of fengycin, i.e. fengycin A (Ala at position 6) and B (Val at position 6), were present in the biosurfactant obtained from strain A21 (Kim et al. 2010; Wei et al. 2010). The mass spectra of surfactin showed the major intensity peak of C-14 surfactin (m/z 1,044) which was present as Na+ adduct form. The mass spectra of fengycin exhibited major intensity peak of C-16 fengycin (m/z 1,512) which was also present as Na+ adduct.
Surfactin has been reported to remove metal ions and hydrocarbon pollutant from soil and sediments (Mulligan et al. 1999; Lai et al. 2009). However, combination of surfactin and fengycin has not been tested for the soil washing process. Anionic nature of surfactin and fengycin would support removal of metal ion pollutant while inter-phase tension reducing ability would support removal of petroleum hydrocarbon pollutants during soil washing process.
Zeta potential and equivalent conductivity measurement
Zeta potential studies were carried to determine the effect of concentration on the net charge of biosurfactant solution. Aqueous solution of biosurfactant exhibited negative potential of −30 mV suggesting it to be an anionic surfactant. Zeta potential of biosurfactant increased with concentration and reached the stable value (−30 to −33 mV) after reaching CMC. Isa et al. (2007) had reported similar change in the zeta potential while studying surfactin solution.
Equivalent conductivity of biosurfactant solution varied with concentration. Equivalent conductivity of biosurfactant solution initially decreased with increase in concentration till it reached CMC. After 50 mg L−1 of biosurfactant, there was a negligible change in the equivalent conductivity. Initial decrease in equivalent conductivity may be due to hiding of charged sites in the micelles as compared to the monomers (Mulligan et al. 1999).
The zeta potential of soil samples collected from industrial site and garden were found to be −36.6 and −28.3 mV, respectively. Negative potential of soil is in accordance with previous reports (Mulligan et al. 1999; Kaya and Yukselen 2005). The zeta potentials of soil samples were also measured in the presence of biosurfactant to determine the interaction between the surfactant and the soil inter-phase. All the measurements were made with 50 times the CMC solution of biosurfactants. Presence of biosurfactant in the soil suspension decreased the zeta potential of soil samples to −56.1 mV. This decrease in zeta potential value indicates sorption of biosurfactant onto the soil particles (Mulligan et al. 1999). Mulligan et al. (1999) considered hydrophobic sorption as a probable reason for adherence of surfactant molecule to the soil particles. Hydrophobic tail of lipopeptides biosurfactant interacts with the hydrophobic surface of soil particle to induce the sorption.
Biosurfactant sorption to soil
Determining the extent of surfactant sorption, on soil during washing process is important for efficient washing. Sorption of biosurfactant on the soil results in loss and reduction in effective concentration, which may render them less efficient and ineffective during soil treatment. Zeta potential studies indicated sorption of biosurfactant to the soil particles.
Quantitative analysis by HPLC was performed to determine the extent of biosurfactants sorption to the soil. Comparison of HPLC chromatogram (fresh surfactant solution and soil–biosurfactants solutions supernatant) supported above finding. As compared with fresh surfactant solution, lipopeptides detected in soil–biosurfactants solutions supernatant was 50.12 ± 4.02 % less due to its sorption on the soil. Thus, suggesting that nearly 50 % of the biosurfactant molecules get adhered to the soil particles and aid in portioning of pollutant from soil while rest of the biosurfactant molecules remains in the solution helping in stabilising surfactant–pollutant system and preventing re-adherence of pollutant to the soil particles.
Urum et al. (2006) has reported sorption of biosurfactant like rhamnolipids, aescin, lecithin, saponin and tannin on the soil particles during soil washing process. Surfactants that get adsorbed to the soil water inter-phase are considered as a better detergent for soil washing. According to Mulligan (2005), sorption of biosurfactant is essential for removal of soil contaminates. Biosurfactants with small CMC and high degree of sorption to soil have better ability to remove hydrocarbon pollutant, only if washing solution has surfactant concentration much higher than CMC (Urum et al. 2006).
Petroleum hydrocarbon solubilisation
Figure 2a represents solubilisation of petroleum hydrocarbon in the presence of surfactant solution. In distilled water, petroleum hydrocarbon solubilisation was 3.8 mg L−1 while in presence of surfactant it reached to 57.2 mg L−1. Low aqueous solubility of hydrocarbon is primarily due to its hydrophobic nature. Below CMC, extent of petroleum hydrocarbon solubilisation was very low. However, above CMC there was drastic increase in hydrocarbon solubilisation. Thus, suggesting that micelles mediated solubilisation is occurring. In aqueous system, hydrophobic end of the surfactant molecules comes together inside the micelle structure with the hydrophilic end exposed outside. Hydrophobic environment present in the interior of a micelle is suitable for hydrophobic molecules resulting in solubilisation (Mulligan 2005).
TPH is a complex mixture of hundreds of hydrocarbon compounds, ranging from light, volatile, short-chained organic compounds to heavy, long-chained, branched compounds. Biosurfactants exhibits different degree of solubility for different component of TPH and is primarily influenced by chemical nature of the compound (Isa et al. 2007; Wei et al. 2010). The solubilisation ability is one of the important parameter determining the effectiveness of surfactant in removing hydrocarbon pollutant from soil or water. Surfactants with greater solubilisation efficiency have better ability to recovery petroleum hydrocarbon from either soil or water (Urum et al. 2006).
Soil washing and factor influencing its efficiency
Usually, soil washing with water alone is not as efficient as surfactant-water solution. In present study, soil washing with aqueous solution of biosurfactant was also found to be more efficient in removing metal and hydrocarbon pollutants. As evident from Tables 2, 3, 4 and 5, pollutant removing ability of biosurfactant solution was influenced by several factors like concentration, pH, duration and rate of agitation.
Table 2 shows effect of agitation time on pollutant removing ability of biosurfactant solution. For deciding optimum agitation time, agitation was set at maximum possible value of 200 rpm. Amount of pollutant removed by biosurfactant solution gradual increased with time. However, saturation was reached after 24 h of agitation. Furthermore, agitation has no significant influence on amount of metal ion and hydrocarbon removed by biosurfactant solution. Hence, for further studies, agitation time was set at 24 h. Furthermore, soil washing was carried out for 24 h with different agitation rate so as to have optimum agitation condition. Increase in agitation rate increased the amount of metal ions and hydrocarbon removed by biosurfactant solution (Table 3). Most optimum agitation rate for soil washing was obtained at 150 rpm. Higher agitation rate of 150 and 200 rpm caused excessive foaming. Higher agitation rate increased the frequency of interaction between biosurfactant solution and soil particle harboring pollutants.
Biosurfactant from strain A21 exhibited best washing efficiency at pH 9, while lower pH (pH 5 and pH 7) and higher pH 11 reduced the washing efficiency of the solution (Table 4). Lower pH (less than 5) caused precipitation of biosurfactant which was visible as a white precipitate on the soil after centrifugation following soil washing experiments. Earlier, Mulligan et al. (2001) has reported that significant amount of Zn and Cu can be removed from soil at pH 8 and 10 by biosurfactants, respectively. At pH 9, surfactin shows a higher affinity for divalent cations than for monovalent cations (Thimon et al. 1993).
Metal ions and hydrocarbon pollutant removing ability is also influenced by biosurfactant concentration. In present study, high biosurfactant concentration increased pollutant removing ability of washing solution, indicating that each molecule may be assisting in removal of positively charged metal ions and hydrophobic pollutants (Table 5). Soil washing with 50 CMC biosurfactant solution removed copper (26.2 %), cadmium (44.2 %), cobalt (35.4 %), lead (40.3 %), zinc (32.0 %) and TPH (64.5 %). Soil washing by rhamnolipids was also found to increase metal removal with increase in concentration (Dahrazma and Mulligan 2007). Pollutant removing efficiency observed in the present study was better than previous reports where biosurfactant solution has been applied as washing agent (Mulligan et al. 1999; Hong et al. 2002; Urum et al. 2006; Dahrazma and Mulligan 2007; Lai et al. 2009; Wang and Mulligan 2009). Some researchers applied series of soil washing to remove significant amount of pollutants (Mulligan et al. 1999). However, in present study, single soil washing by mixture of surfactin and fengycin was found to remove considerable amount of pollutants. As evident from Fig. 2b, biosurfactant from strain A21 was able to remove all fraction of TPH with equal efficiency. Zhu and Feng (2003) have demonstrated that the mixture of synthetic surfactants can increase the solubility of polycyclic aromatic hydrocarbon and thus, improve the performance of surfactant-enhanced remediation of soils. Residual petroleum hydrocarbon left in the soil after single washing process may be attributed to the low efficiency of biosurfactant to partition the soil adhering hydrocarbon into the aqueous system (Lai et al. 2009).
Earlier reports suggest that surfactin from B. subtilis can remove heavy metal like copper, zinc and cadmium (Mulligan et al. 2001). Presence of iron in the combination with organic matter may have inhibited removal of iron during soil washing process (Dahrazma and Mulligan 2007). Anionic surfactants like surfactin are better in sequestration and removal of divalent metal from soil (Mulligan 2005; Thimon et al. 1993). In natural condition, iron remains in trivalent state, thus making removal of iron from contaminated soil by biosurfactant difficult. According Mulligan (2005) removal of pollutant by the biosurfactant occurs through sorption of the surfactant onto the soil surface followed by complexation with the pollutant and subsequent detachment of the metal from the soil into solution. Further metals get sorbed within the surfactant micelles for stabilisation (Mulligan et al. 2001; Wang and Mulligan 2009).
Pot experiment for plant growth
As mentioned earlier, due to presence of various toxic pollutants in the industrial soil, plant growth was sparse at sampling site. To check if soil washing by biosurfactant solution did help in retrieving soil to wellbeing level for plant growth, brown Indian mustard (B. juncea) were grown in pots containing biosurfactant washed soil and water washed soil. Biosurfactant washed soil supported the germination of mustard very much similar to garden soil (positive control). However, the total biomass was less in biosurfactant washed soil by 20 % as compared with normal garden soil (Fig. 3). This may be due to lack of some essential nutrients in biosurfactant washed soil. Mustard failed to germinate in water washed soil indicating its continual toxicity for plant.
Conclusion
The present study exhibits feasibility of using combination of lipopeptide biosurfactant solution for soil washing process. Washing with lipopeptides solution at concentration above CMC removed significant amounts of hydrocarbon and metal pollutants from the soil. The germination experiment indicated that washing of soil with biosurfactant solution made the soil conducive for plant growth. This approach could be very helpful at sites where phytoremediation cannot be practiced due to high level of metal/hydrocarbon contaminates, which inhibits the plant growth. However, after soil washing with lipopeptides biosurfactants, plants can be grown for phytoremediation of polluted sites. Therefore, use of a combination of biosurfactants holds greater potential in bioremediation of contaminated soils.
The application of anionic biosurfactant in extraction of metal from ores can be greener and environment friendly approach. Moreover, influence of biosurfactant/synthetic surfactant present in the soil, on mobility of metal ions within soil, from soil to ground water or nearby water bodies can be an interesting aspect to be explored.
References
Adam G, Duncan H (2002) Influence of diesel fuel on seed germination. Environ Pollut 120:363–370
Almeida R, Mucha AP, Teixeira C, Bordalo AA, Almeida CM (2013) Biodegradation of petroleum hydrocarbons in estuarine sediments: metal influence. Biodegradation 24:111–123
Coutte F, Lecouturier D, Yahia SA, Leclere V, Bechet M, Jacques P, Dhulster P (2010) Production of surfactin and fengycin by Bacillus subtilis in a bubbleless membrane bioreactor. Appl Microbiol Biotechnol 87:499–507
Dahrazma B, Mulligan CN (2007) Investigation of the removal of heavy metals from sediments using rhamnolipid in a continuous flow configuration. Chemosphere 69:705–711
de Faria AF, Teodoro-Martinez DS, de Oliveira Barbosa GN et al (2011) Production and structural characterization of surfactin (C14/Leu7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochem 46:1951–1957
Dermont G, Bergeron M, Mercier G, Richer-Lafleche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152:1–31
Guo Y, Wu K, Huo X, Xu X (2011) Sources, distribution, and toxicity of polycyclic aromatic hydrocarbons. J Environ Health 73:22–25
Hong KJ, Tokunaga S, Kajiuchi T (2002) Evaluation of remediation process with plant-derived biosurfactant for recovery of heavy metals from contaminated soils. Chemosphere 49:379–387
Isa MHM, Coraglia DE, Frazier RA, Jauregi P (2007) Recovery and purification of surfactin from fermentation broth by a two-step ultrafiltration process. J Membr Sci 296:51–57
Kaya A, Yukselen Y (2005) Zeta potential of soils with surfactants and its relevance to electrokinetic remediation. J Hazard Mater 120:119–126
Kilic E, Font J, Puig R, Colak S, Celik D (2011) Chromium recovery from tannery sludge with saponin and oxidative remediation. J Hazard Mater 185:456–462
Kim PI, Ryu J, Kim YH, Chi YT (2010) Production of biosurfactant lipopeptides iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol 20:138–145
Lai CC, Huang YC, Wei YH, Chang JS (2009) Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater 167:609–614
Mulligan CN (2005) Environmental applications for biosurfactants. Environ Pollut 133:183–198
Mulligan CN, Yong RN, Gibbs BF, James S, Bennett HPJ (1999) Metal removal from contaminated soil and sediments by the biosurfactant surfactin. Environ Sci Technol 33:3812–3820
Mulligan CN, Yong RN, Gibbs BF (2001) Heavy metal removal from sediments by biosurfactants. J Hazard Mater 85:111–125
Pacwa-Plociniczak M, Plaza GA, Piotrowska-Seget Z, Cameotra SS (2011) Environmental applications of biosurfactants: recent advances. Int J Mol Sci 12:633–654
Raaijmakers JM, de Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062
Tang J, Wang M, Wang F, Sun Q, Zhou Q (2011) Eco-toxicity of petroleum hydrocarbon contaminated soil. J Environ Sci (China) 23:845–851
Thimon L, Peypoux F, Wallach J, Michel G (1993) Ionophorous and sequestering properties of surfactin, a biosurfactant from Bacillus subtilis. Colloids Surf B Biointerfaces 1:57–62
Urum K, Grigson S, Pekdemir T, McMenamy S (2006) A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere 62:1403–1410
Wang S, Mulligan CN (2009) Rhamnolipid biosurfactant-enhanced soil flushing for the removal of arsenic and heavy metals from mine tailings. Process Biochem 44:296–301
Wei YH, Wang LC, Chen WC, Chen SY (2010) Production and characterization of fengycin by indigenous Bacillus subtilis F29-3 originating from a potato farm. Int J Mol Sci 11:4526–4538
Zhu L, Feng S (2003) Synergistic solubilization of polycyclic aromatic hydrocarbons by mixed anionic–nonionic surfactants. Chemosphere 53:459–467
Acknowledgements
The authors thank the Director, IMTECH, for providing the facilities for this work. AKS is thankful to UGC for his fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Singh, A.K., Cameotra, S.S. Efficiency of lipopeptide biosurfactants in removal of petroleum hydrocarbons and heavy metals from contaminated soil. Environ Sci Pollut Res 20, 7367–7376 (2013). https://doi.org/10.1007/s11356-013-1752-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1752-4