Abstract
In the present study, potential of Eisenia fetida to recycle the different types of fresh water weeds (macrophytes) used as substrate in different reactors (Azolla pinnata reactor, Trapa natans reactor, Ceratophyllum demersum reactor, free-floating macrophytes mixture reactor, and submerged macrophytes mixture reactor) during 2 months experiment is investigated. E. fetida showed significant variation in number and weight among the reactors and during the different fortnights (P <0.05) with maximum in A. pinnata reactor (number 343.3 ± 10.23 %; weight 98.62 ± 4.23 % ) and minimum in submerged macrophytes mixture reactor (number 105 ± 5.77 %; weight 41.07 ± 3.97 % ). ANOVA showed significant variation in cocoon production (F4 = 15.67, P <0.05) and mean body weight (F4 = 13.49, P <0.05) among different reactors whereas growth rate (F3 = 23.62, P <0.05) and relative growth rate (F3 = 4.91, P <0.05) exhibited significant variation during different fortnights. Reactors showed significant variation (P <0.05) in pH, Electrical conductivity (EC), Organic carbon (OC), Organic nitrogen (ON), and C/N ratio during different fortnights with increase in pH, EC, N, and K whereas decrease in OC and C/N ratio. Hierarchical cluster analysis grouped five substrates (weeds) into three clusters—poor vermicompost substrates, moderate vermicompost substrate, and excellent vermicompost substrate. Two principal components (PCs) have been identified by factor analysis with a cumulative variance of 90.43 %. PC1 accounts for 47.17 % of the total variance represents “reproduction factor” and PC2 explaining 43.26 % variance representing “growth factor.” Thus, the nature of macrophyte affects the growth and reproduction pattern of E. fetida among the different reactors, further the addition of A. pinnata in other macrophytes reactors can improve their recycling by E. fetida.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Macrophytes (aquatic plant community) comprise a diverse group of flora that include angiosperms, ferns, mosses, liverworts, and some freshwater macroalgae that occur seasonally or permanently in wet environments (Chambers et al. 2008). Commonly, four morphotypes (forms) are used to classify aquatic macrophytes: submerged, floating-leaved, emergent, and free-floating (Sculthorpe 1985). These plants are capable of colonizing several kinds of aquatic environments (lakes, lagoons, wetlands, rivers, reservoirs, waterfalls, and even bromeliad tanks) with a wide range of limnological features, presenting high plasticity and adaptation ability. Aquatic macrophytes are important resource in the maintenance of trophic chains and in biogeochemical processes, but they can also be deleterious if present in excess (Bianchini et al. 2010). However, due to various anthropogenic activities such as discharge of sewage, the accumulation of nutrients in an aquatic ecosystem leads to eutrophication resulting in massive growth of the macrophytes with impaired recreational activities (swimming, fishing, and boating), stunted fish growth, and decrease in localized dissolved oxygen levels, which can cause fish kills. Furthermore, excessive growth especially during summer results in large quantities of organic matter, that when decomposed via bacteria and other microbes, results in high rates of microbial respiration with low oxygen levels causing threat to aquatic fauna (Sinha et al. 2008).
Effective weed control in aquatic habitats is difficult and the effects are commonly short term. The method most commonly applied to manage/control the nuisance of aquatic weeds (macrophytes) includes habitat manipulation, biological, chemical, and mechanical control methods. However, these methods can be highly disruptive causing adverse environmental impacts and are relatively inefficient (Caffrey and Monahan 2006; Najar and Khan 2012). Another option for the lake management authorities is the mechanical harvesting of the weeds during their peak growth season and the technique is most commonly used in management of fresh water weeds in the Kashmir region of India. But after the harvesting and collection of weeds by mechanical weed harvesters, the weeds are kept on lake shores. The harvested weeds does not possess any economic value in its present form and are not transported by local municipal committees to the landfill sites, as the weeds does not fall under the category of municipal solid waste. The harvested weeds remain laid on lake shores and becomes a source of odor problem, thus posing a challenge to the lake management authorities for their proper disposal. However, if these harvested weeds are turned into materials useful in agriculture and horticulture, there would be great savings in primary plant nutrients and metabolic energy (Edwards et al. 2011), in addition a solution to the nuisance of harvested fresh water weeds.
Vermicomposting is efficient ecobiotechnological process to stabilize the organic waste (Sinha 2009; Suthar 2009) into a plant nutrient rich material, i.e., vermicompost which is rich in microbial populations and plant nutrient substances that may acts as suitable plant growth media for sustainable agriculture (Suthar 2012). Vermicomposting is the joint action of earthworms and microorganisms, although microbes are responsible for biochemical degradation of organic matter and earthworms are the important drivers of the process, conditioning the substrate and altering the biological activity (Dominguez 2004). Vermicomposting has been applied in managing a wide range of organic wastes such as crop residues, sewage sludge, oil palm waste, hospital waste (organic), pineapple waste, livestock excreta, human feces, and industrial refuse (Gupta and Garg 2008; Yadav and Garg 2009; Mainoo et al. 2009; Hayawin et al. 2010; Indrajeet and Singh 2010; Pramanik and Chung 2010; Yadav et al. 2010). In the vermicomposting process, earthworms maintain aerobic condition in the organic wastes, convert a portion of it into biological biomass and respiration products whereas expel the remaining stabilized product as vermicompost with disappearance in objectionable odors (Suthar 2012). According to Suthar (2007a), vermicomposting is more appropriate and is an efficient technology to convert organic waste into a valuable community resource at low input basis.
Vermicomposting of fresh water weeds helps not only in their management but at the same time, the recovery of nutrients locked up in fresh weeds from the aquatic system and their availability and use in terrestrial system (agriculture and horticulture). The objective of the present research is to manage the menace of fresh weeds (macrophytes) through vermicomposting with production of valued added product as vermicompost.
Materials and methods
Macrophytes
Macrophytes were collected from three lakes of Kashmir Valley (Dal Lake, Khushalsar Lake, and Anchar Lake) and brought to the laboratory were washed thoroughly with tap water followed by ethylenediamine tetra acetic acid solution (0.01 M) and finally rinsed with deionized water (Gupta 1999). After removing the adhered material, they were segregated, identified, and allowed to drain out excess water for 2 days under sunshine and then dried in an oven at 70 °C for 24 h.
Earthworm cultures
Eisenia fetida was picked from several cultures maintained in the laboratory, with cow dung used as feed. Earthworm biomass reported as live weight is taken after rinsing adhered material off the worm and blotting them dry.
Vermireactors
Circular plastic tubs (43 × 30 cm) with a hole at the bottom were used as reactors, into each of which 20 healthy adult earthworms were introduced. 100 g of weed and 20 g of cow dung (w/w dry weight) were added in each reactor. Water was sprinkled on tubs to maintain a moisture level of 55–60 % (Garg et al. 2006). Reactors in triplicates for 15, 30, 45, and 60 days interval were run and terminated at the end of set fortnights for observation in terms of conversion of weeds, cocoon production and changes in biomass of earthworms. Earthworms were picked from the reactors by hand sorting method. The recycling rate (%) was calculated after separating left over weed from vermicompost.
Azolla pinnata reactor (APR), Trapa natans reactor (TNR), Ceratophyllum demersum reactor (CDR) comprised of the macrophytes A. pinnata, T. natans, and Ceratophyllum demersum respectively. However, free-floating macrophytes mixture reactor (FFMMR) had a mixture of A. pinnata, T. natans, Salvinia natans, Nelumbo nucifera, Nymphaea alba, Hydrocharis dubia, and Nymphoides peltata, whereas submerged macrophytes mixture reactor (SMMR) had a mixture of C. demersum, Myriophyllum spicatum, Hydrilla verticillata, Potamogeton pectinatus, and Potamogeton crispus.
Vermicompost
Vermicompost samples were collected at the end of 15, 30, 45, and 60 days (Kaviraj and Sharma 2003) and air dried at room temperature and then kept in well labeled air tight plastic containers for further use. Vermicompost was analyzed for pH, electrical conductivity, organic nitrogen (Jackson 1973), organic carbon (Walkley and Black 1934), phosphorous (Anderson and Ingram 1993), and potassium (Simard 1993).
Multivariate statistical analysis
Cluster analysis
Cluster analysis (CA) is an exploratory data analysis tool for solving classification problems, based on unsupervised learning. CA enables stepwise aggregation of objects according to the similarity of their features. As a result, a hierarchically or non-hierarchically ordered cluster is formed and is typically illustrated by a dendrogram (tree diagram). A single cluster describes a group of objects that are more similar to each other than to objects outside the group. Of all cluster analysis, hierarchical agglomerative cluster is the most common approach. In the present study, hierarchical agglomerative CA was performed based on the normalized data set (mean of observations over the whole period) by means of the Ward’s method using squared Euclidean distances as a measure of similarity (Lattin et al. 2003).
Principal component analysis
Principal component analysis (PCA) is applied to reduce the dimensionality of a data set consisting of a large number of interrelated variables and this reduction is achieved by transforming the data set into a new set of variables—the principal components (PCs) which are orthogonal (non-correlated) and are arranged in decreasing order of importance. An eigenvalue greater than 1 was considered significant and taken as criterion for extraction of principal components required to explain the variance in the data. PCA transforms the original data matrix into a product of two matrices, one of which contains the information about the objects (samples) and the other about the features (analyte concentration). The matrix characterizing objects contains the scores of objects on PCs. The other characterizing features is a square matrix and contains the set of eigenvectors (understood as weights/loadings) of the original features in each PC. Liu et al. (2003) classified the factor loadings as strong, moderate, and weak corresponding to absolute loading values of >0.75, 0.75–0.50, and 0.50–0.30 respectively. Cluster analysis and principal component analysis was carried out on the data set of seven variables (mean body weight, growth rate, relative growth rate, cocoon production, increase in number, increase in biomass, and recycling of macrophytes by E. fetida).
Data analysis
Data sets were subjected to multivariate statistical techniques (Zar 2009): two-way analysis of variance (ANOVA), CA, PCA, and factor analysis. Pearson correlation coefficient (r) was employed to examine the relationship between the vermicomposting time duration with physicochemical characteristics of vermicompost. Tukey’s t test was used as a post hoc analysis to compare the means. All statistical analyses and graphical presentations were performed using SPSS statistical software (Version 16.0), SigmaPlot (Version 11.0), and PAST (Version 1.93).
Results and discussion
Increase in number
E. fetida exhibited differing behavior in terms of multiplication in different substrates (macrophytes) over the duration of 2 months. Increase in earthworm number was highest among the APR (343.3 ± 10.23 %) and FFMMR (285 ± 5.40 %). However, SMMR showed a decrease in number of earthworms by 16.7 ± 2.66 % during the first fortnight and subsequently increased to 105 ± 5.77 % during the fourth fortnight (Table 1). Increase in number of E. fetida varied significantly among the treatments (F4 = 4.44, P <0.05) and during different fortnights (F3 = 24.70, P <0.05). The variability in increase in the number of earthworms among different reactors could be due to the difference in physiochemical characteristics of macrophytes and corroborates Suthar (2007b). Organic waste palatability for earthworms is directly related to the chemical nature of the organic waste that consequently affects earthworm growth parameters. In general earthworms exhibited a positive growth over the period of experimental time in all the reactors that shows the potential of E. fetida in the recycling of fresh water weeds. Kaviraj and Sharma (2003) reported doubling in earthworm number in 42 days during the vermicomposting of municipal solid waste and is attributed to decreasing C/N ratio with time duration (Nedgwa and Thompson 2000). Organic wastes which provide earthworms with sufficient amount of easily metabolizable organic matter and non-assimilated carbohydrates, favor growth and reproduction of earthworms (Edwards 1998). Since E. fetida exhibited increase in number (multiplication) in all most all reactors containing different types of weeds over the time period, thus shows the its wide adoptability to vermicompost different types of organic wastes. Domínguez and Edwards (2011) also reported E. fetida as tolerant to the environmental conditions (nature) of organic wastes and has been found to be very competitive during the vermicomposting process (Domínguez et al. 2010).
Increase in weight (%)
Among the different reactors, APR showed the highest increase in weight of 98.62 ± 4.23 during the fourth fortnight, however, SMMR showed a decrease of 3.3 ± 1.67 % during first fortnight and increased to 41.07 ± 3.97 % during fourth fortnight (Table. 2). Increase in weight of E. fetida differed significantly among the reactors (F4 = 29.51, P <0.05) and during different fortnights (F3 = 54.95, P <0.05). Increase in weight over the period of time in all the reactors is due to the adoptability of E. fetida to feed on wide variety of organic wastes with different chemical characteristics. Rathinamala et al. (2008) also reported increase in body weight of E. eugeniae feeding on six different organic substrates and is attributed to palatability of substrate and its nutrient availability. According to Kale and Krishnamoorthy (1981a), earthworm shows enhanced growth and reproduction capabilities when feeding on substrate having more available form of nutrients. Pramanik and Chung (2011) reported the presence of fungi during vermicomposting processes and becomes additional supplement to the earthworms which contributes to the increased weight of the earthworms (Adi and Noor 2009). Earthworms gained weight when reared in cultures of certain fungal species as microorganism are always the favorite food of earthworms and plays important role in their nutrition (Edwards and Fletcher 1988).
Cocoon production
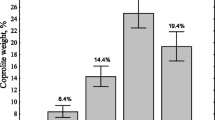
Cocoon production by E. fetida started from the first fortnight in all the reactors as the earthworms used for the study were in clitellates state. Cocoon production by E. fetida varied significantly (F4 = 15.67, P <0.05) among different reactors but with no significant variation (F3 = 2.59, P <0.05) during different fortnights. Cocoon production exhibited maximum value of 176.33 ± 2.02 in APR and minimum of 30 ± 1.15 in SMMR (Table. 3). The difference in the rate of cocoon production is related to the biochemical characteristics of the weeds, the important factor that influences the time taken to reach sexual maturity and onset of reproduction (Flack and Hartenstein 1984). Edwards et al. (1998) concluded that the important difference between the rates of cocoon production in the different wastes is related to the quality of the organic waste material. Suthar (2007b) reported nitrogen content of the substrate as an important factor related to cocoon production. Cocoon production was relatively more in APR and FFMMR, as these reactors contain more nitrogen content (APR: 8.40 ± 0.10 g/kg to 12.30 ± 0.90 g/kg and FFMMR: 6.2 ± 0.10 g/kg to 8.06 ± 0.03 g/kg) during different fortnights as compared to the other reactors, thus, it might be the reason for more cocoon production in these two reactors. The higher nitrogen content in APR and FFMMR is attributed to higher initial value of nitrogen in Azolla pinnata weed as it is the principal substrate in APR and part of substrate in FFMMR. The results are also supported by the findings of Gaur and Singh (1995) that the nitrogen content of vermicompost depend on the initial nitrogen content of the waste.
Mean body weight (g)
Mean body weight observed at the end of the experiment showed a range from 0.39 ± 0.01 in CDR to 0.61 ± 0.03 in FFMMR (Fig. 1a). The mean weight of E. fetida differed significantly (F4 = 13.49, P <0.05) among the reactors and during different fortnights (F3 = 5.12, P <0.05) of recycling. The variation in mean body weight among different reactors could be based on their nutritional status. Kale and Krishnamoorthy (1981b) have reported that the nature of available food influence earthworm activity. Mean body weight showed a decreased trend over the time period in all the reactors and is related with the conversion of the most of the substrate to vermicompost (Suthar 2010), which cannot further support their growth and also decrease in the share of substrate for each individual due the emergence of juveniles. In addition, the cocoon production could be another factor responsible for the decrease in mean body weight. Furthermore, when E. fetida received food below a maintenance level, it lost weight at a rate which depends upon the quality and nature of its ingestible substrate (Neuhauser et al. 1980).
Growth rate (mg/worm/day)
E. fetida showed a highest growth rate of 10.88 ± 2.88 in TNR and lowest of 0.44 ± 0.19 in SMMR (Fig. 1b). Growth rate of E. fetida among different reactors did not vary significantly (F4 = 2.36, P <0.05), but there was significant variation in its growth rate (F3 = 23.62, P <0.05) during the time periods. The growth rate is a good index to compare the growth of earthworms in different organic wastes or feeds, and in the present study, results are similar to the reports of Edwards et al. (1998) as growth rate of 0.6 mg/worm/day to 7.6 mg/worm/day have been documented. The variation in growth rate among the different reactors could be due the palatability of the macrophytes and their nutrient values. Kale and Krishnamoorthy (1981a) reported that variation in the acceptability of organic waste depends on texture as well as chemical nature of diets/feed. Therefore, it is hypothesized that earthworm growth patterns in this study were related to the chemical profile of the macrophytes.
Relative growth rate (%)
Relative growth rate of the earthworm E. fetida varied from 8.58 ± 1.66 in FFMMR to 50.15 ± 5.80 in APR (Fig. 1c). Relative growth rate exhibited no significant variation (F4 = 1.55, P <0.05) among different reactors, but significant variation (F3 = 4.91, P <0.05) was observed during different fortnights. Relative growth rate was maximum during first fortnight in TNR and CDR. However it was highest during the second fortnight in APR, FFMMR and SMMR reactors, though in subsequent fortnights the growth rate was not equally significant in these three reactors as compared to TNR and CDR reactors. In TNR and in CDR, the easy palatability of these macrophytes has resulted in maximum relative growth gain during the first fortnight. In case of CDR, the leaves are highly dissected, which make them easier to ingest by earthworms. According to Battle and Mihuc (2000), the accelerated decomposition of C. demersum was most likely due to its large surface area of the highly dissected leaves. Overall, the relative growth rate was highest in APR, where the presence of bacteria and cyanobacteria in A.pinnata act as supplementary diet for earthworms and result in biomass increase. The presence of bacteria and cyanobacteria in the specialized leaf cavities of A. pinnata act as protein source for earthworms (Chakrabarty et al. 2009). Microorganisms are the favorite food of earthworms and play an important role in their nutrition (Edwards and Fletcher 1988). The decrease in relative growth rate over the time period observed in all the reactors is related to decreased food availability (Garg et al. 2005).
Recycling of macrophytes
Recycling of macrophytes was 100 % in all the reactors at the end of experiment period except for the SMMR (Table 4). Recycling potential of E. fetida showed significant variation (F4 = 5.20, P <0.05) among different reactors as well as during different fortnights (F3 = 591.70, P <0.05) of recycling. In general, the chemical nature of the organic waste influences the palatability of earthworms directly or indirectly, which consequently affect earthworm efficiency in recycling. Percent recycling was low during the first fortnight in all the reactors by E. fetida and the reason might be that the earthworms, initially cultured with cow dung as principal feed, took some time to acclimatize with the change over to macrophytes as principal feed. Gajalakshmi et al. (2001) also reported an increase in recycling/conversion rate after initial acclimatization. Another reason could be the softening of the weed (macrophytes) with the passage of time in addition to increased earthworm number (Najar and Khan 2010). The percent recycling shows increased trend over the period of time in all the reactors. At the end of the fourth fortnight, entire quantity of macrophytes was recycled in all the reactors except for the SMMR. Throughout the experiment, E. fetida exhibited remarkable difference in recycling among different reactors and this difference is related to the chemical characteristics of the macrophytes. Curry and Schmidt (2007) reported distinct preferences for different kinds of plant materials by earthworm. Difference in C/N ratios, particle size, protein, and crude fiber contents can affect recycling of organic waste by earthworm during vermicomposting (Ganesh et al. 2009). However, in case of SMMR where percent recycling over the period of 2 months was least and could be attributed to presence of polyphenols as SMMR contains M. spicatum species as part of the substrate in which polyphenols has been reported (Bauer et al. 2009). Suthar (2007b) also attributed low performance of Perionyx excavatus in crop residues to the presence of polyphenols and related substances in them.
Cluster analysis
CA was applied to find out similarity among the different reactors (macrophytes) based on the performance of E. fetida. It resulted in a dendrogram (Fig. 2), grouping all the substrate (macrophytes) into three clusters—poor vermicompost substrates (PVS), moderate vermicompost substrate, and excellent vermicompost substrate (EVS).
Cluster 1, which corresponds to PVS, in which E. fetida showed an increase of 105 ± 5.77 % in number, 41.07 ± 3.97 % in weight and a total cocoon production of 30 ± 1.15 over the 60 days duration, where presence of chemical retardant could be the reason for poor growth and subsequently reproduction of E. fetida. Jesikha and Lekeshmanaswamy (2013) also reported retarded growth of Eudrilus eugeniae in Pongamia leaf waste due the presence of polyphenols. Cluster 2 corresponds to moderate vermicompost substrate represented by the TNR and CDR where E. fetida exhibited an increase from 176.67 ± 3.33 to 185 ± 2.88 (number), 76.16 ± 3.06 to 79.15 ± 4.68 (weight) and cocoon production from 24.67 ± 2.60 to 42.33 ± 6.93. Cluster 3 corresponds to EVS, comprising of APR and FFMMR where E. fetida recoding an increase of 285 ± 5.40 to 343.30 ± 10.23 % (number), 69.16 ± 1.49 to 98.62 ± 4.23 % (weight) and total cocoon production from 90.33 ± 3.92 to 162.33 ± 1.45. Maximum growth and reproduction of E. fetida was observed in the reactors were the A. pinnata is principal substrate or part of substrate and corresponds to its nitrogen content. Nitrogen content of the substrate affects the growth and reproduction patterns in composting earthworms (Kale et al. 1982). Thus, the cluster results revealed different characteristics of the substrates corresponding to the chemical nature of the substrate that affects growth and reproduction in E. fetida.
The significance of cluster analysis results in this study indicate that this technique is useful in offering reliable classification of different substrates (macrophytes) on the basis of the performance of E. fetida and the performance itself is affected by the nature of the substrate. Thus cluster analysis enables that the performance of vermicomposting species can be improved by supplementing the main substrate with the material/waste that supports the growth and reproduction of the species, as the performance of the vermicomposting species affects overall process of waste recycling.
Principal component analysis (PCA)
Principal component analysis was carried out on the data set of seven variables to identify the various factors that influence E. fetida in its recycling potential. Two principal components (PCs) have been identified by factor analysis which explained 90.43 % of total variance. PC1 explaining 47.17 % and PC2 43.26 % of total variance. The eigenvalues, percentage variance counted, cumulative percentage variance and component loadings are given in Table 5 and the biplot which is the graphical representation of factor loadings in different components is given in Fig. 3. Among the two principal components (PCs), the PC1 explaining 47.17 % of total variance, has strong positive loading on cocoon production, mean body weight and increase in earthworm number. Thus PC1 represents reproduction factor. PC2 explaining 43.26 % of total variance, has strong positive loading on biomass increase, growth rate and relative growth rate and there by PC2 represents growth factor. Type of macrophyte had a much more important effect on the growth and reproduction of E. fetida. In the reactors with A. pinnata as the substrate or part of substrate, a higher growth rate and reproduction in E. fetida was observed. The higher nitrogen content and microorganisms acts as nutrient sources (Domínguez et al. 2000). Thus recycling of different types of macrophytes by E. fetida can be enhanced by the addition of A. pinnata which results in increase in number of earthworms in reactors that could be of further use.
Physiochemical characteristics of vermicompost
Physiochemical characteristics of macrophyte vermicompost in different reactors during different fortnights are given in Table 6.
Two PCs has been identified by PCA with a total variance of 79.17 %. The eigenvalues, percentage variance counted, cumulative percentage variance and component loadings are given in Table 7. PC1 accounts for 39.82 % of total variance and is having strong positive loading of ON and P whereas strong negative loading of C/N ratio. Positive loading of ON and P are associated with mineralization of the organic waste, whereas negative loading of C/N ratio is associated with the loss of organic carbon from the substrate in the form of CO2. The PC2 which accounts for 39.35 % of the total variance exhibited strong positive loading of EC, K, and pH. Positive loading of EC, K, and pH is due to the release of different minerals salts in available form during the process of vermicomposting.
The pH showed a range from 7.41 ± 0.02 in APR to 8.2 ± 0.04 in SMMR at the end of fourth fortnight. Among the reactors, pH varied significantly (F4 = 8.79, P <0.05) and during different fortnights (F3 = 31.23). The pH in this study was positively correlated with the time period of vermicomposting (r = 0.998, P <0.01, APR; r = 0.964, P <0.05, CDR; r = 0.996, P <0.01, FFMMR; r = 0.992, P <0.01, SMMR; and r = 0.965, P <0.05, TNR). Variation in pH among the reactors is due to the difference in chemical composition of the macrophytes since recycling of different substrates could result in the production of different intermediate compounds resulting in different pH in reactors. Muthukumaravel et al. (2008) also reported relative increase in pH over the period of vermicomposting and attributed it to the decomposition of nitrogenous substrates.
Electrical conductivity (EC) varied from 0.49 ± 0.01 mS/m in APR to 1.33 ± 0.05 mS/m in SMMR. EC showed significant variation among the reactors (F4 = 44.04, P <0.05) and during different fortnights (F3 = 27.40, P <0.05). The EC in this study was positively correlated with time period of vermicomposting process (r = 0.998, P <0.01, APR; r = 0.967, P <0.05, CDR; r = 0.992, P <0.01, FFMMR; r = 0.990, P <0.05, SMMR; and r = 0.989, P <0.05, TNR). Increase in EC during vermicomposting process is due the increase in the soluble salt level resulting from the mineralization activity of earthworms and microorganisms present in the organic substance and those in the gut of earthworms (Karmegam and Daniel 2009). Similar findings with other earthworm species on vermicomposting of leaf litter, weeds, and agricultural residues are those of Yadav and Garg (2011).
Potassium (K) content ranged in the vermicompost from 25.43 ± 1.4 mg/g in APR to 52.33 ± 1.85 mg/g in CDR. K differed significantly among different reactors (F4 = 13.39, P <0.05) and during different fortnights (F3 = 20.19P <0.05). Potassium content in this study was strongly and positively correlated with vermicomposting duration (r = 0.996, P <0.01, APR; r = 0.970, P <0.01, CDR; r = 0.995, P <0.01, FFMMR; r = 0.999, P <0.01, SMMR; and r = 0.995, P <0.01, TNR). Several studies have revealed that vermicomposting of organic wastes significantly enhanced the concentration of potassium (Suthar 2008; Yadav et al. 2010). Variation in potassium concentration among the reactors has been attributed to differences in the chemical nature of the initial raw material.
Available phosphorous (P) content exhibited a value from 386.10 ± 6.75 μg/g in TNR to 610.66 ± 6.69 μg/g in SMMR vermicompost. Variation in P was significant among the reactors (F4 = 30.18, P <0.05) and for different fortnights (F3 = 42.26, P <0.05). The phosphorous in this study also correlated positively with vermicomposting duration (r = 0.994, P <0.01, APR; r = 0.993, P <0.01, CDR; r = 0.968, P <0.05, FFMMR; r = 0.998, P <0.01, SMMR; and r = 0.963, P <0.05, TNR). The increased phosphorus content in vermicompost clearly indicates earthworm mediated phosphorus mineralization (Suthar 2009). The release of phosphorous in available form is partly by earthworm gut phosphatases and further release may be by phosphorous-solubilizing microorganisms in casts (Ghosh et al. 1999). It is well established that microorganisms play an important role in phosphorous release from the wastes. Vinotha et al. (2000) reported that the microflora play an important role in enhanced phosphatase activity in worm casts. Therefore, it is suggested that earthworm-processed waste material contains high concentration of plant metabolites, due to enhanced microbial activity during the vermicomposting process.
Organic carbon (OC) content decreased during vermicomposting duration and showed a value between 115 ± 5.70 g/kg in CDR to 290 ± 1.73 g/kg in SMMR at the end of fourth fortnight. OC varied significantly (F4 = 9.85, P <0.05) among the reactors and during different fortnights (F3 = 27.86, P <0.05). The organic carbon content showed negative correlation with the increase in the period of vermicomposting (r = −0.999, P <0.01, APR; r = −0.969, P <0.05, CDR, r = −0.997, P <0.01, FFMMR, r = −0.935, P <0.05, SMMR; and r = −0.966, P <0.05, TNR) and indicates the maturity of the vermicompost. Earthworm activity brings about significant decline in organic carbon level of the waste resources and accelerates waste stabilization process (Singh and Suthar 2012; Tare and Hait 2011). Vermicomposting being a combined operation of earthworm and microorganisms (Singh et al. 2011a), the biological mutuality causes carbon loss in the form of carbon dioxide from the substrates during the decomposition and mineralization of organic waste (Hayawin et al. 2010). The conversion of some part of organic fractions of waste into worm biomass can also reduce the carbon loss from the substrate. However, the difference in the carbon loss patterns among different reactors relates to the variation in chemical nature of the substrates.
Organic nitrogen (ON) showed a value from 6.4 ± 0.52 g/kg in CDR to 9.62 ± 1.7 g/kg in SMMR at the end of experiment. ON exhibited significant variation among different reactors (F4 = 47.69, P <0.05) and during different fortnights (F3 = 23.12, P <0.05). ON content was strongly and positively correlated with vermicomposting duration (r = 0.956, P <0.05, APR; r = 0.991, P <0.01, CDR; r = 0.973, P <0.05, FFMMR; r = 0.971, P <0.05, SMMR; and r = 0.997, P <0.05, TNR) indicating increase in nitrogen content after recycling. Decomposition of organic material by earthworms accelerates the nitrogen mineralization process and subsequently changes the nitrogen profile of the substrate (Cynthia and Rajeshkumar 2012; Suthar 2007b). The organic nitrogen increased linearly in all the reactors over the period of 2 months with a lowest range of 4.80 ± 0.03 to 6.35 ± 0.52 g/kg in CDR and highest range of 8.43 ± 0.10 to 12.30 ± 0.09 g/kg in APR. Earthworm activity enriches the nitrogen profile of vermicompost through microbial mediated nitrogen transformation and the decaying tissues of dead worms in vermicomposting systems result in further enhancement of nitrogen (Suthar 2007b). Satchell (1967) reported that over 70 % of the nitrogen in the tissues of dead earthworm was mineralized in less than 20 days. However, in general, the final nitrogen content of vermicompost is dependent on the initial nitrogen content present in the waste and the extent of decomposition (Chauhan and Joshi 2010).
The C/N ratio exhibited a decreased trend during the vermicompost duration and showed a value between 12.03 ± 1.3 in APR and 30.15 ± 1.98 in SMMR vermicompost. C/N ratio had significant variation among the reactors (F4 = 3.63, P <0.05) and during different fortnights (F3 = 31.89, P <0.05). C/N ratio showed negative correlation with increase in the period of vermicomposting (r = −0.999, P <0.01, APR; r = −0.959, P <0.05, CDR; r = −0.999, P <0.05, FFMMR; r = −0.975, P <0.05, SMMR; and r = −0.991, P <0.01, TNR). The study revealed that the C/N ratio of macrophytes, which is one of the most widely used indices for compost maturation, stabilizes during the vermicomposting process. The C/N ratio of a substrate reflects the organic waste mineralization and stabilization during the process of vermicomposting. The loss of carbon as carbon dioxide through microbial respiration and simultaneously addition of nitrogen by worms in the form of mucus and nitrogenous excretory material, lower the C/N ratio of the substrate Suthar (2008). It is evident from Table 6 that C/Nratios decreased with time in all the vermireactors with a lowest value of 12.03 ± 1.3 in APR. The relevance of the C/N ratio relies on the fact that a decrease in the ratio implies an increase in the degree of humification of organic matter (Singh et al. 2011b). Decline of C/N ratio to less than 20 indicates an advanced degree of organic matter stabilization and reflects a satisfactory degree of maturity of organic wastes Hayawin et al. (2010).
Conclusions
The study demonstrated vermicomposting as an alternate technology for the recycling of different types of macrophytes using E. fetida. PCA revealed that macrophytes affect the growth and reproduction of E. fetida during the process of vermicomposting. Mineralization (C/N ratio) indicated complete recycling of macrophytes into vermicompost in 60 days duration and is rich in basic plant nutrients. Hence, the people having vegetable gardens around the lakes will be benefited by using macrophyte-based vermicompost—a balanced and low-cost organic fertilizer. Large scale utilization of macrophytes helps in controlling these macrophytes considerably and keeps water bodies clean, along with aesthetic improvement.
References
Adi AJ, Noor ZM (2009) Waste recycling, utilization of coffee grounds, and kitchen waste in vermicomposting. Bioresour Technol 100:1027–1030
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility. A handbook of methods, 2nd edn. CAB International, Wallingford
Battle JM, Mihuc TB (2000) Decomposition dynamics of aquatic macrophytes in the lower Atchafalaya, a large floodplain river. Hydrobiologia 418:123–136
Bauer N, Blaschke U, Beutler E, Gross EM, Jenett-Siems K, Siems K, Hilt S (2009) Seasonal and interannual dynamics of polyphenols in Myriophyllum verticillatum and their allelopathic activity on anabaena variabilis. Aquat Bot 91:110–116
Bianchini JI, Cunha-Santino MB, Milan JAM, Rodrigues CJ, Dias JHP (2010) Growth of Hydrilla verticillata (L.f.) Royle under controlled conditions. Hydrobiologia 644:301–312
Caffrey JM, Monahan C (2006) Control of Myriophyllum verticillatum L in Irish canals by turion removal. Hydrobiologia 570:211–215
Chakrabarty D, Das SK, Das MK (2009) Earthworm (Eudrillus euginae) multiplication through variable substrates. Aquacult Nutr 15:513–516
Chambers PA, Lacoul P, Murphy KJ, Thomaz SM (2008) Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595(1):9–26
Chauhan A, Joshi PC (2010) Composting of some dangerous and toxic weeds using Eisenia fetida. J Am Sci 6(3):1–6
Curry P, Schmidt O (2007) The feeding ecology of earthworms. A review. Pedobiologia 50:463–477
Cynthia JM, Rajeshkumar KT (2012) A study on sustainable utility of sugar mill effluent to vermicompost. Adv Appl Sci Res 3(2):1092–1097
Dominguez J (2004) State of the art and new perspectives on vermicomposting research. In: Edwards CA (ed) Earthworm Ecology, 2nd edn. CRC Press, Boca Raton, pp 401–424
Domínguez J, Aira M, Gómez-Brandón M (2010) Vermicomposting: earthworms enhance the work of microbes. In: Insam H, Franke-Whittle I, Goberna M (eds) Microbes at Work: from wastes to resources. Springer-Verlag, Berlin Heildelberg, pp 93–114
Domínguez J, Edwards CA (2011) Biology and ecology of earthworm species used for vermicomposting. In: Edwards CA, Arancon NQ, Sherman RL (eds) Vermiculture technology: earthworms organic waste and environmental management. CRC Press, Boca Raton, pp 25–37
Domínguez J, Edwards CA, Webster M (2000) Vermicomposting of sewage sludge, Effect of bulking materials on the growth and reproduction of the earthworm Eisenia andrei. Pedobiologia 44:24–32
Edwards CA, Arancon NQ, Sherman R (2011) Vermiculture technology: earthworms, organic wastes and environmental management. CRC press, Boca Raton
Edwards CA (1988) Breakdown of animal, vegetable, and industrial organic wastes by earthworms. In: Edwards CA, Neuhauser EF (eds) Earthworms in waste and environmental management. SPB Academic Publishing, Hague, pp 21–31
Edwards CA, Domínguez J, Neuhauser EF (1998) Growth and reproduction of Perionyx excavatus (Per.) (Megascolecidae) as factors in organic waste management. Biol Fertil Soils 27:155–161
Edwards CA, Fletcher KE (1998) Interaction between earthworms and microorganisms in organic-matter breakdown. Agr Ecosyst Environ 24:235–247
Flack FM, Hartenstein R (1984) Growth of the earthworm Eisenia foetida on microorganisms and cellulose. Soil Biol Biochem 16:126–130
Gajalakshmi SA, Ramasamy EV, Abbasi SA (2001) Potential of two epigeic and two anecic earthworm species in vermicomposting of water hyacinth. Bioresour Technol 76:177–181
Ganesh PS, Gajalakshmi S, Abbasi SA (2009) Vermicomposting of the leaf litter of acacia (Acacia auriculiformis): possible roles of reactor geometry, polyphenols and lignin. Bioresour Technol 100(5):1819–1827
Garg P, Gupta A, Satya S (2006) Vermicomposting of different types of wastes using Eisenia fetida: a comparative study. Bioresour Technol 97:391–395
Garg VK, Chand S, Chhillar A, Yadav A (2005) Growth and reproduction of Eisenia fetida in various animal wastes during vermicomposting. Appl Ecol Environ Res 3(2):51–59
Gaur AC, Singh G (1995) Recycling of rural and urban waste through conventional and vermicomposting. In: Tondon HLS (ed) Recycling of crop, animal, human and industrial waste in agriculture. Fertilizer Development and Consultation Organization, New Delhi, pp 31–49
Ghosh M, Chattopadhyay GN, Baral K (1999) Transformation of phosphorus during vermicomposting. Bioresour Technol 69:149–154
Gupta PK (1999) Soil, plant, water, and fertilizer analysis. Agro Botanica, Bikaner
Gupta R, Garg VK (2008) Stabilization of primary sewage sludge during vermicomposting. J Hazard Mater 153:123–130
Hayawin ZN, Khalil HPSA, Jawaid M, Ibrahim MH, Astimar AA (2010) Exploring chemical analysis of vermicompost of various oil palm fibre wastes. Environmentalist 30:273–278
Indrajeet RSN, Singh J (2010) Vermicomposting of farm garbage in different combination. J Recent Adv Appl Sci 25:15–18
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Pvt. Ltd, New Delhi
Jesikha M, Lekeshmanaswamy M (2013) Effect of Pongamia Leaf Medium on Growth of Earthworm (Eudrilus eugeniae). Int J Sci Res Publ 3(1):1–4
Kale RD, Bano K, Krishnamoorthy RV (1982) Potential of Perionyx excavatus for utilizing organic wastes. Pedobiologia 23:419–425
Kale RD, Krishnamoorthy RV (1981a) Litter preferences in the earthworm Lampito mauritii. P Indian As-Anim Sci 90:123–128
Kale RD, Krishnamoorthy RV (1981b) What affects the abundance and diversity of earthworms in soils? P. Indian As-Anim Sci 90:117–121
Karmegam N, Daniel T (2009) Investigating efficiency of Lampito mauritii (Kinberg) and Perionyx ceylanensis Michaelsen for vermicomposting of different types of organic substrates. Environmentalist 29:287–300
Kaviraj SS, Sharma S (2003) Municipal solid waste management through vermicomposting employing exotic and local species of earthworms. Bioresour Technol 90:169–173
Lattin J, Carroll D, Green P (2003) Analyzing multivariate data. Duxbury, New York
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a Blackfoot disease area in Taiwan. Sci Total Environ 313(1–3):77–89
Mainoo NOK, Barrington S, Whalen JK, Sampedro L (2009) Pilot-scale vermicomposting of pineapple wastes with earthworms native to Accra, Ghana. Bioresource Technol 100:5872–5875
Muthukumaravel K, Amsath A, Sukumaran M (2008) Vermicomposting of vegetable wastes using cow dung. E-J Chem 5(4):810–813
Najar IA, Khan AB (2010) Vermicomposting of Azolla pinnata by using earthworm Eisenia fetida. Bioscan 5(2):239–241
Najar IA, Khan AB (2012) Vermicomposting of fresh water weeds (macrophytes) by Eisenia fetida (Savigny, 1826), Aporrectodea caliginosa trapezoides (Duges, 1828) and Aporrectodea rosea rosea (Savigny, 1826). Dyn Soil Dyn plant 6(S1):73–77
Nedgwa PM, Thompson SA (2000) Effect of C to N ratio on vermicomposting in the treatment and bioconversion of biosolids. Bioresour Technol 76:7–12
Neuhauser EF, Hartenstein R, Kaplan DL (1980) Growth of the earthworm Eisenia foetida in relation to population density and food rationing. Oikos 35:93–98
Pramanik P, Chung YR (2010) Efficacy of vermicomposting for recycling organic portion of hospital wastes using Eisenia fetida: standardization of cow manure proportion to increase enzymatic activities and fungal biomass. Environmentalist 30:267–272
Pramanik P, Chung YR (2011) Changes in fungal population of fly ash and vinasse mixture during vermicomposting by Eudrilus eugeniae and Eisenia fetida, Documentation of cellulase isozymes in vermicompost. Waste Manage 31(6):1169–1175
Rathinamala J, Jayashree S, Lakshmanaperumalsamy P (2008) Potential utilization of domestic wastes as a suitable experimental diet to enhance the biomass of Eudrilus eugeniae in various seasons. Eco Env Cons 14(1):43–50
Satchell JE (1967) Lumbricidae. In: Burger A, Raw F (eds) Soil Biology. Academic Press, London, pp 259–322
Sculthorpe CD (1985) The biology of aquatic vascular plants. Koeltz Scientific Books, Königstein-West Germany, 97
Simard RR (1993) Ammonium acetate extractable elements. In: Martin R, Carter S (eds) Soil sampling and method of analysis. Lewis Publishers, Florida, pp 39–43
Singh D, Suthar S (2012) Vermicomposting of herbal pharmaceutical industry solid wastes. Ecol Eng 39:1–6
Singh RP, Embrandiri A, Ibrahim MH, Esa N (2011a) Management of biomass residues generated from palm oil mill, vermicomposting a sustainable option. Resour Conserv Recy 55:423–434
Singh RP, Singh P, Araujo ASF, Ibrahim MH, Sulaiman O (2011b) Management of urban solid waste, vermicomposting a sustainable option. Resour Conserv Recy 55:719–729
Sinha R (2009) Earthworms, the miracle of nature (Charles Darwin’s ‘unheralded soldiers of mankind and farmer’s friends’). Environmentalist 29:339–340
Sinha RK, Bharambe G, Chaudhari U (2008) Sewage treatment by vermifiltration with synchronous treatment of sludge by earthworms, a low-cost sustainable technology over conventional systems with potential for decentralization. Environmentalist 28:409–420
Suthar S (2007a) Production of vermifertilizer from guar gum industrial wastes by using composting earthworm Perionyx sansibaricus (Perrier). Environmentalist 27:329–335
Suthar S (2007b) Vermicomposting potential of Perionyx sansibaricus (Perrier) in different waste materials. Bioresour Technol 98:1231–1237
Suthar S (2008) Bioconversion of post-harvest residues and cattle shed manure into value added products using earthworm Eudrilus eugeniae. Ecol Eng 32:206–214
Suthar S (2009) Growth and fecundity of earthworms, Perionyx excavatus and Perionyx sansibaricus in cattle waste solids. Environmentalist 29:78–84
Suthar S (2010) Recycling of agro-industrial sludge through vermitechnology. Ecol Eng 3:1028–1036
Suthar S (2012) Earthworm production in cattle dung vermicomposting system under different stocking density loads. Environ Sci Pollut Res 19:748–755
Tare V, Hait S (2011) Optimizing vermistabilization of waste activated sludge using vermicompost as bulking material. Waste Manage 31(3):502–5011
Vinotha SP, Parthasarathi K, Ranganathan LS (2000) Enhanced phosphatase activity in earthworm casts is more of microbial origin. Curr Sci 79(9):1158–1159
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 34:29–38
Yadav A, Garg VK (2009) Feasibility of nutrient recovery from industrial sludge by vermicomposting technology. J Hazard Mater 168:262–268
Yadav A, Garg VK (2011) Vermicomposting—an effective tool for the management of invasive weed Parthenium hysterophorus. Bioresour Technol 102:5891–5895
Yadav KD, Tare V, Ahammed MM (2010) Vermicomposting of source separated human faeces for nutrient recycling. Waste Manage 30:50–56
Zar JH (2009) Biostatistical analysis, 5th edn. Prentice Hall, Englewood Cliffs
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Najar, I.A., Khan, A.B. Management of fresh water weeds (macrophytes) by vermicomposting using Eisenia fetida . Environ Sci Pollut Res 20, 6406–6417 (2013). https://doi.org/10.1007/s11356-013-1687-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1687-9