Abstract
In this study, two carbon materials [chicken manure biochar (CMB) and black carbon (BC)] were investigated for their effects on the reduction of hexavalent chromium [Cr(VI)] in two spiked [600 mg Cr(VI) kg−1] and one tannery waste contaminated [454 mg Cr(VI) kg−1] soils. In spiked soils, both the rate and the maximum extent of reduction of Cr(VI) to trivalent Cr [Cr(III)] were higher in the sandy loam than clay soil, which is attributed to the difference in the extent of Cr(VI) adsorption between the soils. The highest rate of Cr(VI) reduction was observed in BC-amended sandy loam soil, where it reduced 452 mg kg−1 of Cr(VI), followed by clay soil (427 mg kg−1) and tannery soil (345 mg kg−1). X-ray photoelectron microscopy confirmed the presence of both Cr(VI) and Cr(III) species in BC within 24 h of addition of Cr(VI), which proved its high reduction capacity. The resultant Cr(III) species either adsorbs or precipitates in BC and CMB. The addition of carbon materials to the tannery soil was also effective in decreasing the phytotoxicity of Cr(VI) in mustard (Brassica juncea L.) plants. Therefore, it is concluded that the addition of carbon materials enhanced the reduction of Cr(VI) and the subsequent immobilization of Cr(III) in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is introduced into the terrestrial and aquatic environments through indiscriminate disposal of wastes from various industries including electroplating, leather tanning, timber treatment, pulp production, and petroleum refining (Zhitkovich 2011). Chromium is used as trivalent Cr [Cr(III)] [e.g., ([Cr(H2O)6]2(SO4)3)] in the tannery industry and as hexavalent Cr [Cr(VI)] [e.g., (K2Cr2O7)] in the timber treatment industry (Barnhart 1997). Chromium(VI) is highly toxic and carcinogenic even when present in very low concentrations in water (Owlad et al. 2009). The maximum recommended concentration of Cr(total) in ground water and waste water is 2 mg L−1, whereas for Cr(VI), it is only 0.05 mg L−1 (Park et al. 2004).
While cationic Cr(III) species are strongly retained onto the negatively charged soil particles, the anionic Cr(VI) is very weakly adsorbed and, is readily available for plant uptake and leaching to groundwater (Leita et al. 2009). Leaching studies have indicated that Cr(VI) is readily leached compared with Cr(III) and other metallic anions, such as arsenate (As(V); Carey et al. 1996; Bolan and Thiagarajan 2001). Chromium(VI) can be reduced to Cr(III) in the environments where a ready source of electrons such as carbon and reduced iron [Fe(II)] is available, and Cr(VI) reduction is enhanced in acid rather than alkaline conditions (Hsu et al. 2009). Reduction of Cr(VI) to Cr(III) and subsequent immobilization of Cr(III) through adsorption and precipitation reactions in Cr(VI)-contaminated soils and industrial effluents are of primary importance in the remediation process.
Addition of organic amendments such as manures, biosolids, biochar, and black carbon has been shown to act as an electron donor and provide the energy source for the soil microorganisms involved in the reduction of metal(loid)s, such as Cr [i.e., Cr(VI) to Cr(III)] and arsenic [i.e., As(V) to As(III)], and non-metals, such as nitrogen (N) [i.e., nitrate (NO3 −) to gaseous N (NO, N2O and N2) denitrification (Jardine et al. 1999; Park et al. 2011). Recently, there has been an increasing interest in the use of biochar in enhancing carbon sequestration and soil quality (Kookana et al. 2011; Bolan et al. 2012). The potential value of biochars in controlling the bioavailability of metal(loid)s and pesticides in relation to environmental remediation needs to be examined (Park et al. 2011; Kookana 2010).
Reduction of Cr(VI) to Cr(III) in soils is a proton (H+) consumption (or hydroxyl (OH−) release) reaction, resulting in an increase in soil pH (Park et al. 2006). An increase in soil pH is likely to enhance the immobilization of Cr(III) through adsorption resulting from pH-induced increase in surface charge and precipitation as Cr(OH) (Adriano et al. 2004). Although a number of studies have examined the phytotoxicity of Cr(VI) ( Chen et al. 2010; Chiu et al. 2009; Lee et al. 2006), there is a notable dearth of information about the effect of carbon amendments, such as biochar and black carbon, on the reduction of Cr(VI) to Cr(III) and its subsequent immobilization and bioavailability. In this study, the effect of two carbon amendments on the reduction, mobility, and phytoavailability of Cr(VI) in two spiked soils and one tannery sludge contaminated soil was examined. The immobilization of Cr(III) following the reduction of Cr(VI) to Cr(III) is discussed in relation to pH-induced adsorption and precipitation reactions.
Materials and methods
Soil and carbon amendments
Two uncontaminated mineral soils low in organic matter content which varies in their texture were used in this study (Table 1). These soil samples were collected from the Viticultural Research Station in Nuriootpa (Calcic red clay sodosol) and Northern Adelaide Plains (Calcic red sandy loam sodosol), South Australia. A Cr-contaminated soil collected from a long-term tannery waste contaminated site at Mount Barker, South Australia (Avudainayagam et al. 2001) was also used in this study. The pH, cation exchange capacity (CEC), and organic matter content of soils were analyzed using the methods described in Rayment and Higginson (1992).
Two carbon amendments [chicken manure biochar (CMB) and black carbon (BC)] were used to examine their efficiency in reducing Cr(VI) to Cr(III). Black carbon was prepared from an Australian common weed, Solanum elaeagnifolium L. (Choppala et al. 2012; Qiu et al. 2008). Chicken manure biochar was obtained from Pacific Pyrolysis (NSW, Australia).
Reduction of Cr(VI)
Both spiked and field contaminated soils were used to examine the effect of carbon amendments on Cr(VI) reduction. The two uncontaminated soils were mixed with two levels of Cr(VI) (0 and 600 mg Cr(VI) kg−1 soil) using K2Cr2O7 and incubated at field capacity. The spiked and field contaminated soil samples were then mixed with two levels of carbon amendments (0 and 50 g BC/CMB kg−1 soil) and incubated at field capacity for 4 weeks. During the incubation with carbon amendments, soil subsamples were taken at various intervals and extracted with either Milli-Q water or 1 M KH2PO4 at a soil/solution ratio of 1:10 for 16 h and filtered through a 0.45-μm filter. The soil extracts were analyzed for total Cr and Cr(VI).
Spectroscopic characterization of black carbon
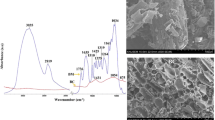
Chromium(VI) reduction by BC was examined by monitoring the surface characteristics of BC before and after reaction with Cr(VI) (600 mg Cr(VI) kg−1 BC). The surface morphology of BC was visualized by environmental scanning electron microscope (ESEM; FEI Quanta 450, Hillsboro, OR, USA), combined with an energy dispersive X-ray spectrometer (EDX; Apollo X silicon drift detector). The ESEM enables to visualize the changes in the structural modifications of BC resulting from the reaction with Cr(VI). The EDX was used to determine the chemical composition and mapping of elements including Cr on the surface of BC.
An X-ray photoelectron spectroscopy (XPS) was used to verify BC-induced Cr(VI) reduction by monitoring the oxidation state of the Cr bound to BC surface. Spectra were obtained for BC samples, before and after reaction with Cr(VI) by using an Al Kα X-ray source in Kratos Axis Ultra spectrometer (Hadano, Kanagawa, Japan) operating at 15 kV and 15 mA. Potassium dichromate (K2Cr2O7; Sigma Aldrich) and chromium (III) chloride (Sigma Aldrich) were used as reference samples for Cr(VI) and Cr(III), respectively. High-resolution Cr 2p core level regions were collected using 40 eV pass energy and 0.05 eV step size. It is known that transition metals can suffer from photoreduction during a prolonged XPS measurement (Skinner et al. 1996; Kagwade et al. 2001). In the present study, the first spectrum acquisition sweep was always compared with the final sweep to ensure that no significant changes in spectral envelope (i.e., relative proportion of Cr (VI) vs. Cr (III)) were observed over the analysis period of <2 h.
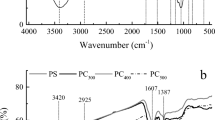
The vibration frequency changes in the functional groups and their role in the reduction of Cr(VI) by BC before and after reaction with Cr(VI) were analyzed using Agilent Cary 600 series Fourier transform infrared spectrometer (FTIR; Santa Clara, USA) scanned between 500 and 4,000 cm−1 and the spectral resolution set to 4.0 cm−1.
Leaching experiment
The spiked and field contaminated soil samples incubated for 4 weeks with carbon amendments were used for the leaching experiment. The soil samples were packed into 60-mL columns (3 cm in diameter and 7.5 cm in height) at a bulk density of 1.4 g cm−3 and leached with 1 mM CaCl2 solution at a flow rate of 20 mL h−1 using a peristaltic pump (Schwab et al. 2007). The columns were leached for six pore volumes, and the leachate samples were collected for every pore volume of the respective soils. The leachate samples were analyzed for total Cr and Cr(VI) immediately after collection, and Cr(III) was calculated by subtracting Cr(VI) from the total Cr.
Plant growth experiment
A greenhouse plant growth experiment was set up to investigate the effect of carbon amendments on the plant uptake of Cr(VI). The spiked and field contaminated soil samples incubated at field capacity for 4 weeks with carbon amendments were transferred to plastic pots. Eight seeds of mustard plant (Brassica juncea L.) were sown in each pot, and the seedlings were thinned to four plants per pot after 2 weeks. Complete Hoagland nutrient solution, adjusted to pH 5.5, was added regularly (Liu et al. 2008).
The soil pore water samples were collected using Rhizon samplers (Rhizosphere Research Products, Wageningen, The Netherlands) at 1, 2, 3, and 4 weeks after the establishment of the pot experiment and analyzed for Cr(VI). The plants were harvested 8 weeks after sowing and dried at 70 °C using a forced draught oven. The dry weights were recorded, and the plant materials were ground using a Cr-free stainless steel grinder, and analyzed for Cr (Zarcinas et al. 1987).
Chromium measurement
The concentration of Cr in the soil solutions and plant digests was measured using ICP–MS (Agilent, 7500ce, Santa Clara, USA) for total Cr, and Cr(VI) by spectrophotometric method using 1,5-Diphenyl Carbazide (US EPA1995). From these values, the concentration of Cr(III) in the soil solution was calculated.
The amounts of Cr(VI) reduced were estimated from the decrease in the concentration of Cr(VI) in soil solution. The data for the rate of Cr(VI) reduction were described using Eq. 1.
where Y = amount of Cr(VI) reduced (milligrams per kilogram), Y m is the amount of maximum reduction(milligrams per kilogram), r = rate constant (per day), and x = incubation period (days).
Statistical analysis
All measurements, including pH and Cr(VI) concentration, were calculated from the triplicates of each treatment. All calculations and standard deviations of the replicates were determined using Microsoft Excel (Redmond, WA, USA).
The relationships between Cr(VI) reduction in soils and incubation period in the presence of BC and CMB, and between Cr concentration in soil solution and Cr uptake, were derived using regression analysis in Grapher Software (version 9; Golden Software, Golden, CO). Statistical significance of the experimental data was analyzed using a statistical software tool (PASW Statistics, version 18.0.0; SPSS, Inc., Chicago, IL) at a significance level of p < 0.05. Amendment efficiency on decreasing the Cr(VI) phytotoxicity was compared using Duncan’s multiple range test.
Results and discussion
Soil and carbon amendments characteristics
The three soils collected from South Australia varied considerably in their physical and chemical properties (Table 1). The pH varied from slightly acidic (6.14) in the spiked soil to slightly alkaline (7.64) in the field contaminated soil. Organic matter content was in the range of 0.72 to 2.6 %, and CEC ranged from 1.12 to 48.7 cmol kg−1. The dominant clay minerals were kaolinite, chlorite, illite, and feldspar.
In the field contaminated tannery soil, majority of Cr was present as Cr(III) because Cr2(SO4)3 is used in the tanning process (Kilic et al. 2011). There has been some oxidation of Cr(III) to Cr(VI) in this soil. Oxidation of Cr(III) to Cr(VI) has been shown to occur in soils if high-valent manganese (Mn) is present in the soil as an electron acceptor for the reaction to proceed (Dai et al. 2009; Fandeur et al. 2009). Since the field contaminated soil used in this study contained detectable Mn oxide, oxidation of Cr(III) was likely to have occurred (Landrot et al. 2009).
The pH of BC and CMB was 4.61 and 7.35, respectively. The CEC of BC and CMB was 20.47 and 23.75 cmol kg−1, respectively. The pH buffering capacity of CMB was higher (21.27 mmol kg−1 pH−1) than BC (14.67 mmol kg−1 pH−1). The surface areas of BC and CMB were 3.35 and 7.27 m2 g−1, respectively. Scanning electron microscope images demonstrated that these materials had a fine pore structure, which reflected the large surface area compared to non-pyrolysed materials (Fig. 1). The SEM images were similar between these materials and have various fibrous channels.
Reduction of Cr(VI)
In the incubation experiment, the concentration of Cr(VI) in both water and 1 M KH2PO4 extracts decreased with increasing time after Cr(VI) addition. In the case of spiked clay soil, there was a slight difference in Cr(VI) concentration between these two extracts indicating the adsorption of Cr(VI) by this soil. Adsorption of Cr(VI) typically occurs in soils with positive charge components, such as iron and aluminium hydrous oxides, especially when the pH is less than the point of zero net charge (Adriano 2001; Parfitt 1978). Therefore, the amounts of reduced Cr(VI) were estimated from the decrease in 1 M KH2PO4 extractable Cr(VI) concentration in soil (Bolan et al. 2003), which increased with increasing time, and the maximum Cr(VI) reduction occurred within 2 weeks after Cr(VI) addition. Reduction of Cr(VI) to Cr(III) in soils has often been found to be rapid, reaching the maximum within a relatively short time, i.e., in days (Leita et al. 2011).
The Y m and r parameters of the equation describing the data (Eq. 1) are presented in Table 2. Both the Y m and the r values increased with the addition of carbon amendments. Black carbon achieved a greater Cr(VI) reduction than CMB. The maximum reduction as predicted using Eq. (1) showed that BC addition to sandy loam sodosol has the highest reduction (75.4 %), followed by clay sodosol (71.2 %) and tannery waste soils (62.9 %), whereas CMB addition achieved a reduction of 55.4, 51.1, and 38.2 %, respectively (Fig. 2). The rates of reduction of Cr(VI) in soils treated with carbon amendments relative to that of the unamended soil were calculated from the r values (Table 2). These values indicate that the addition of BC and CMB caused 1.2–2.3-and 2.5–4.0-fold increases, respectively, in the rate of reduction of Cr(VI) in the soil. This is consistent with the previous findings (Bolan et al. 2003; Choppala et al. 2012; Losi et al. 1994), where a significant increase in the rate of reduction of Cr(VI) in the presence of various carbon amendments such as manures, biosolids, and biochar occurred.
Various reasons could be attributed to the increase in the reduction of Cr(VI) in the presence of the carbon amendments. Black carbon and biochar comprise high surface areas with several surface functional groups. These functional groups serve as electron donors for Cr(VI) reduction. The π electrons from the surface functional groups transfer to reducing compounds (Cr(VI)), and carbon materials get oxidized through abiotic reactions (Joseph et al. 2010). The higher reducing capacity of BC may be due to the protonation of the material during acid treatment to remove silicon. Furthermore, acid treatment increases the acidic functional groups (Chen and Wu 2004), which are critical in the reduction of Cr(VI). The reduction of Cr(VI) to Cr(III), being a H+ consumption (or –OH release) reaction (Eq. 2), was found to increase with a decrease in pH (Lan et al. 2007; Zhong and Yang 2012), which may be one of the reasons for higher Cr(VI) reduction in the presence of protonated BC.
During pyrolysis, as the temperature increases, carbonaceous materials tend to decrease the aliphatic carbon structures and initiate the increase of turbostratic carbon crystallites, where highly dense grapheme sheets are formed. Since the pyrolysis temperatures of BC and CMB are below 550 °C, the carbon structures appear less condensed, and thereby serves as a good source of N and phosphorus (P) which improves the soil fertility (Steinbeiss et al. 2009).
The theoretical amounts of protons required for Cr(VI) reduction in the incubation experiment were calculated using Eq. 2. The amounts of proton consumed during Cr(VI) reduction were also measured from the differences in the pH values between the Cr(VI) treated and the untreated soils, and the pH buffering capacity of the soil. There was a significant relationship between the theoretical and measured amounts of protons required for Cr(VI) reduction, but the theoretical amounts were slightly higher than the calculated values [Fig. S1 of the Electronic supplementary material (ESM)]. The increase in pH due to H+ consumption in Cr(VI) reduction reaction is likely to result in the immobilization of Cr(III) through adsorption (Eq. 3) and precipitation (Eq. 4) reactions which result in the release of H+. Hence, the net effect of Cr(VI) reduction followed by Cr(III) immobilization is a decrease in H+ consumption which may be attributed to the difference in measured and theoretical H+ change.
The increase in Cr(VI) reduction in the presence of carbon materials may also result from an increase in microbial activity. Losi et al. (1994) showed that the addition of a manure compost increased Cr(VI) reduction under both sterile (i.e., abiotic) and non-sterile (i.e., biotic) conditions. However, manure addition caused a larger increase in biotic than abiotic Cr(VI) reduction, indicating its greater contribution to the former process. The results are in agreement with Cifuentes et al. (1996) where Cr(VI) reduced to a greater extent in the presence of organic amendments such as cow manure, yeast extract, and switch grass (Panicum virgatum L.). In another study, Brodie et al. (2011) suggested that addition of electron donor compounds such as lactates increases microbial growth and reduces Cr(VI) at a faster rate. Addition of biochar has been shown to increase the microbial activity of soil, as measured by increased respiration (Choppala et al. 2012; Steinbeiss et al. 2009). Although Cr(VI) reduction can occur through both chemical (abiotic) and biological (biotic) processes, the latter one is considered to be the dominant process, especially in soils that are low in ferrous (Fe2+) ion. The supply of carbon, nutrients, and stimulation of microorganisms are considered to be the important factors enhancing the reduction of Cr(VI) to Cr(III) (Park et al. 2008;Tokunaga et al. 2003).
Confirmation of BC on the reduction of Cr(VI)
The SEM images of BC samples revealed the porous structure with high surface area. The pore sizes were in the range of 60–200 nm, which are responsible for the high surface area (Fig. 1). There was a notable difference in surface morphology of BC samples before and after treatment with Cr(VI). EDX mapping of elements using SEM confirmed that BC contains 76.5 % C, 11.17 % O, and 5.67 % N. The distribution of Cr in BC observed using backscattered electrons generated from high accelerated voltage (15 kV) in combination with prolonged mapping (90 min) suggested the presence of Cr despite the distribution of Cr being highly heterogeneous. Also, Cr(VI), being a strong oxidizing agent, oxidizes carbon in BC and produces carboxyl functional groups, thereby increasing the retention of Cr(III) species on the surface of BC.
A high-resolution Cr 2p XPS spectrum, collected from BC reacted with Cr(VI), is shown in Fig. 3. Two Cr 2p doublets were observed, corresponding to the Cr 2p 3/2 and Cr 2p 1/2 orbitals of the two species present. The binding energy values for Cr, Cr2O3 (Cr(III)), and K2Cr2O7 (Cr(VI)) were near 574.4, 576.8, and 579.9 eV, respectively (Yang and Chen 2008). Thus, the contributions at 576.9 and 586.8 eV were assigned to Cr(III), and 579.8 and 589 eV to Cr(VI). Hence, it was possible to distinguish the oxidation state and proportion of Cr species present on the BC surface.
Based on the Cr 2p spectrum, BC reduced Cr(VI) by 33 %, and the remaining Cr was present as Cr(III) (Fig. 3). No indication of X-ray-induced photoreduction was observed, and these results were consistent with the phosphate-extractable Cr species that were analyzed independently using ICP-OES (Cr(total) and spectrophotometry (Cr(VI)). Gardea-Torresday et al. (2000) concluded that Cr(VI) bound on the surface of oat (Avena sativa L.) by-products reduced to Cr(III) by positively charged functional groups and subsequently adsorbed by carboxyl groups. Also, Hsu et al. (2009) confirmed that BC was very effective in reducing Cr(VI) to Cr(III) and the resultant Cr(III) species bound to BC surface through surface complexation and precipitation. In a similar study, Park et al. (2004) concluded that most of the Cr bound to the surface of fungal biomass was in the form of Cr(III). The active role of functional groups on the reduction of Cr(VI) was supported by FTIR spectroscopy analysis (Fig. S2 of the ESM). Reduction of Cr(VI) by BC induced some significant modifications in vibrational frequency of functional groups in BC. The FTIR spectrum of BC samples showed that the peak at 3,206.1 cm−1 in the untreated sample has been broadened and shifted to 3,114.9 cm−1 in the Cr(VI)-treated sample, thereby confirming Cr(VI) reduction and consequently, the release of –OH ions. The intensity of the peak at 2,358.6 cm−1 in pure BC decreased in the Cr(VI) treated sample, which may be due to the involvement of amines in the reduction of Cr(VI). However, the suppression of peak at 1,639.9 cm−1 in pure BC and increased peak intensity at 1,603.0 cm−1 indicate stretching of C–O group in amides and the involvement of N–H group of amines in Cr(VI) reduction. Also, the peak suppression at 1,639.9 cm−1, which represents aldehydes/ketones, undergoes hydrolysis and produces carboxyl groups as seen by the increased intensity of peak at 1,384.5 cm−1 (represents hydroxyl groups) in the Cr(VI)-treated BC.
The peak at 1,427.3 cm−1 in pure BC disappeared in the Cr(VI)-treated BC, which was attributed to the C-O-CH3 (methoxyl) deformation and bending of C-OH (hydroxyl) in phenolic structures. The shift of the band from 757.8 cm−1 in pure BC to a new peak at 830.5 cm−1 in Cr(VI)-treated BC was due to the bending of aromatic C-H bonds. Black carbon that contains many functional groups on its surface plays an important role in the reduction of Cr(VI) (Hsu et al. 2009). The resultant Cr(III) species will undergo either adsorption and/or precipitation by BC. Uluozlu et al. (2008) concluded that carboxylic functional groups were responsible for the adsorption of Cr(III) on the lichens biomass. In addition, Banks et al. (2006) postulated that negatively charged functional groups in organic amendments help in retaining Cr(III) ions in water or soils.
Leaching of Cr
The cumulative leachate concentration of Cr(VI) in all the three tested soils was higher than Cr(III) concentration. The amount of Cr(VI) leached from calcic red sandy loam and calcic red clay soils was 334.7 and 246.2 mg kg−1, respectively. However, only 123.1 and 19.4 mg kg−1 of Cr(III) were leached from calcic red sandy loam and calcic red clay soils, respectively. The amounts of Cr(VI) and Cr(III) leached from tannery waste contaminated soils were higher among the tested soils (Fig. 4). The leaching experiment confirmed the high mobility and low sorption capacity of Cr(VI), where it leaches rapidly from contaminated soils (Weng et al. 2002). The leaching of total Cr decreased with the addition of carbon amendments which is ascribed to the increase in Cr(VI) reduction and subsequent Cr(III) retention. The amount of leachable Cr(VI) decreased by 87.2 and 80.9 % in calcic red sandy loam and calcic red clay soils, respectively, with the addition of 50 g BC kg−1 soil. In a column experiment, Boni and Sbaffoni (2009) confirmed that Cr(VI) removal efficiency was higher than 99 % in soils that were mixed with organic amendments, which was attributed to the adsorption on organic-based matrix and reduction to Cr(III).
In calcic red sandy loam soil, Cr(III) leaching was decreased by 56.1 and 61.3 % in BC and CMB amended soils, respectively. However, the amount of leached Cr(III) slightly increased in calcic red clay sodosol (15.7 and 7.6 % for BC and CMB, respectively) and tannery waste soil (7.7 % for both amendments), which may be attributed to the reduction of Cr(VI) species. Adsorption of Cr(III) by soils resulting from both non-specific cation exchange and specific adsorption processes at low surface coverage has been reported (Fendorf et al. 1994). The sorption of metal(loid)s such as Pb, Cu, and Cd by BC and biochar occurs through cation exchange reactions (Park et al. 2011).
Plant growth and Cr uptake
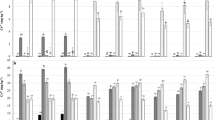
In the absence of carbon addition, the germination was delayed, especially in the field contaminated soil (data not shown). Addition of BC and CMB to control (0 Cr addition) calcic red sandy loam soil increased plant dry matter yield by 9 and 5 %, respectively. In the case of calcic red clay soils, the yield increased by 3 and 2 %, respectively. Plant growth decreased with Cr(VI) addition. In calcic red sandy loam soil that was spiked with 250 mg Cr(VI) kg−1, addition of 50 g BC and CMB kg−1 increased the dry matter yield by 63 and 65 %, respectively. In calcic red clay soil, the growth was increased by 74 and 73 % with the addition of 50 g BC and CMB kg−1 soil, respectively. In tannery waste contaminated soils, the amendments had a significant effect on growth and dry matter yields which increased by 83 and 81 % with the addition of 50 g BC and CMB kg−1 soil, respectively.
The addition of Cr(VI) to the soils retarded plant growth, and the Cr concentration in plant biomass was strongly influenced by the concentration of Cr(VI) in the soil solution. In calcic red sandy loam, calcic red clay, and tannery waste contaminated soils, the Cr levels in plant biomass grown in unamended soil were 24.63, 18.17, and 27.85 mg kg−1, respectively (Fig. S3 of the ESM). The relatively greater concentration of Cr in tannery waste soil compared to other soils may be due to the high levels of Cr from tannery sludge and also from in situ oxidation of Cr(VI). Sorption sites on tannery waste soil that have already been pre-occupied by Cr(III) from tannery waste may lead to low Cr(III) retention by this soil, thereby increasing Cr uptake by plants. The difference in Cr concentration in plant tissue between the spiked soils is attributed to the difference in the adsorption of Cr species between these two soils. However, with the addition of 50 g BC and CMB kg−1 soil, Cr concentration in biomass decreased by 92 and 90 %, respectively, in the sandy loam soil. The same trend followed in other two soils, where tissue Cr concentration was decreased by 86 and 83 % in the calcic red clay soil, and by 79 and 77 % in the tannery waste soil.
Addition of carbon amendments decreased the phytotoxic effect of Cr(VI) on plant growth, and the effect was more pronounced in spiked soils than field contaminated soil which may be attributed to the difference in the bioavailability of Cr between these two soil groups. In spiked soil, Cr(VI) is fresh and hence more readily available for microbial reduction than in field contaminated soils, where the bioavailability of Cr(VI) is considerably low. Chromium(VI) uptake by plant is faster, translocates along with sulphate ions, and damages the plant tissue (Shanker et al. 2005). The increase of Cr(VI) levels in the soil enhanced the uptake of Cr in the plants. The dry matter yield decreased with increasing concentrations of Cr in the plant tissue and Cr(VI) in the soil solution (Fig. 5). Han et al. (2004) observed the decreased number of palisade and spongy parenchyma cells in plant shoots that were grown in Cr(VI)-treated soils, which could be attributed to the decrease in plant biomass. Addition of carbon amendments was very effective in reducing the Cr concentration in plant tissue. The metal(loid) transfer coefficient, as defined by the ratio between plant tissue concentration, and soil concentration decreased with the addition of carbon compounds. This indicates that the addition of carbon compounds decreased the bioavailability of Cr by reducing the readily available Cr(VI) to less available Cr(III) and enhancing the subsequent immobilization of this species.
Addition of nutrient amendments such as organic matter and nitrate has been shown to increase the biological reduction of Cr(VI) (Oliver et al. 2003). It has often been noticed that the transfer coefficient of various contaminants including metal(loid)s and organic compounds was decreased by minimising their bioavailability through increased retention and degradation (Beesley et al. 2010; Park et al. 2011). For example, Kumpiene et al. (2007) observed that the bioavailability of metal(loid)s such as Pb and Cu was decreased through immobilization using a range of inorganic and organic soil amendments. Similarly, the bioavailability of organic contaminants such as atrazine has been found to be decreased by enhancing their degradation using microbial inoculants (Huang et al. 2009).
Conclusion
Overall, this study indicated that the addition of carbon amendments to Cr(VI)-contaminated mineral soil enhanced the reduction of Cr(VI) to Cr(III) and the subsequent immobilization of Cr(III), thereby reducing the bioavailability of Cr for plant uptake (Fig. 5). These carbon amendments provide a source of electron donor, thereby facilitating the reduction of Cr(VI) to Cr(III) in soils. The Cr(VI) reduction reaction results in an increase in soil pH, thereby increasing the pH-induced negative charge on variable-charge surfaces in both soil and carbon amendments. The increase in surface negative charge facilitates the adsorption of Cr(III), thereby reducing its mobility and bioavailability. Since bioavailability is the key factor for remediation technologies, reduction of Cr(VI) to Cr(III) followed by chemical or biological immobilization of Cr(III) may be a preferred option (Frankenberger and Losi 1995; James 1996). A major inherent problem associated with immobilization techniques in general is that, although the metal(loid)s become less bioavailable, the total contaminant concentration in soils remains unchanged. The immobilized metal(loid) may become bioavailable with time through natural weathering process or through advanced decomposition of soil carbon matter.
References
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer, New York
Adriano DC, Wenzel WW, Vangronsveld J, Bolan NS (2004) Role of assisted natural remediation in environmental cleanup. Geoderma 122:121–142
Avudainayagam S, Naidu R, Kookana RS, Alston AM, McClure S, Smith LH (2001) Effects of electrolyte composition on chromium desorption in soils contaminated by tannery waste. Aust J Soil Res 39:1077–1090
Banks M, Schwab A, Henderson C (2006) Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere 62:255–264
Barnhart J (1997) Occurrences, uses, and properties of chromium. Regul Toxicol Pharmacol 26:S3–S7
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158:2282–2287
Bolan NS, Kunhikrishnan A, Choppala GK, Thangarajan R, Chung JW (2012) Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci Total Environ 424:264–270
Bolan NS, Adriano DC, Natesan R, Koo BJ (2003) Effects of organic amendments on the reduction and phytoavailability of chromate in mineral soil. J Environ Qual 32:120–128
Bolan NS, Thiagarajan S (2001) Retention and plant availability of chromium in soils as affected by lime and organic matter amendments. Aust J Soil Res 39:1091–1104
Boni MR, Sbaffoni S (2009) The potential of compost-based biobarriers for Cr (VI) removal from contaminated groundwater: column test. J Hazard Mater 166:1087–1095
Brodie EL, Joyner DC, Faybishenko B, Conrad ME, Rios-Velazquez C, Malave J, Martinez R, Mork B, Willett A, Koenigsberg S (2011) Microbial community response to addition of polylactate compounds to stimulate hexavalent chromium reduction in groundwater. Chemosphere 85:660–665
Carey PL, McLaren RG, Cameron KC, Sedcole JR (1996) Leaching of copper, chromium, and arsenic through some free-draining New Zealand soils. Aust J Soil Res 34:583–597
Chen CP, Juang KW, Lin TH, Lee DY (2010) Assessing the phytotoxicity of chromium in Cr(VI)-spiked soils by Cr speciation using XANES and resin extractable Cr(III) and Cr(VI). Plant Soil 334:299–309
Chen JP, Wu S (2004) Acid/base-treated activated carbons: characterization of functional groups and metal adsorptive properties. Langmuir 20:2233–2242
Chiu CC, Cheng CJ, Lin TH, Juang KW, Lee DY (2009) The effectiveness of four organic matter amendments for decreasing resin-extractable Cr(VI) in Cr(VI)-contaminated soils. J Hazard Mater 161:1239–1244
Choppala GK, Bolan NS, Chen Z, Megaharaj M, Naidu R (2012) The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. J Environ Qual 41:1175–1184
Cifuentes FR, Lindemann WC, Barton LL (1996) Chromium sorption and reduction in soil with implications to bioremediation. Soil Sci 161:233–241
Dai R, Liu J, Yu C, Sun R, Lan Y, Mao JD (2009) A comparative study of oxidation of Cr (III) in aqueous ions, complex ions and insoluble compounds by manganese-bearing mineral (birnessite). Chemosphere 76:536–541
Fandeur D, Juillot F, Morin G, Olivi L, Cognigni A, Webb SM, Ambrosi JP, Fritsch E, Guyot F, Brown GE Jr (2009) XANES evidence for oxidation of Cr (III) to Cr (VI) by Mn-oxides in a lateritic regolith developed on serpentinized ultramafic rocks of New Caledonia. Environ Sci Technol 43:7384–7390
Fendorf SE, Lamble GM, Stapleton MG, Kelley MJ, Sparks DL (1994) Mechanisms of chromium (III) sorption on silica. 1. Chromium(III) surface structure derived by extended x-ray absorption fine structure spectroscopy. Environ Sci Technol 28:284–289
Frankenberger Jr W, Losi M (1995) Applications of bioremediation in the cleanup of heavy metals and metalloids. In H.D. Skipper and R.F. Turco (eds.) Bioremediation: science and applications. SSSA Special Publ. 43. SSSA, Madison, WI, pp 173–210
Gardea-Torresdey J, Tiemann K, Armendariz V, Bess-Oberto L, Chianelli R, Rios J, Parsons J, Gamez G (2000) Characterization of Cr(VI) binding and reduction to Cr (III) by the agricultural byproducts of Avena monida (oat) biomass. J Hazard Mater 80:175–188
Han FX, Sridhar BB, Monts DL, Su Y (2004) Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica juncea. New Phytol 162:489–499
Hsu NH, Wang SL, Lin YC, Sheng GD, Lee JF (2009) Reduction of Cr(VI) by crop-residue-derived black carbon. Environ Sci Technol 43:8801–8806
Huang H, Zhang S, Wu N, Luo L, Christie P (2009) Influence of Glomus etunicatum/Zea mays mycorrhiza on atrazine degradation, soil phosphatase and dehydrogenase activities, and soil microbial community structure. Soil Biol Biochem 41:726–734
James BR (1996) The challenge of remediating chromium-contaminated soil. Environ Sci Technol 30:248–251
Jardine PM, Fendorf SE, Mayes MA, Larsen IL, Brooks SC, Bailey WB (1999) Fate and transport of hexavalent chromium in undisturbed heterogeneous soil. Environ Sci Technol 3:2939–2944
Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia CH, Hook J, Van Zwieten L, Kimber S, Cowie A, Singh BP (2010) An investigation into the reactions of biochar in soil. Soil Res 48:501–515
Kagwade SV, Clayton CR, Halada GP (2001) Causes and prevention of photochemical reduction of hexavalent chromium during x-ray photoelectron spectroscopy. Surf Interface Anal 31:442–447
Kilic E, Puig R, Baquero G, Font J, Colak S, Guerler D (2011) Environmental optimization of chromium recovery from tannery sludge using a life cycle assessment approach. J Hazard Mater 192:393–401
Kookana RS (2010) The role of biochar in modifying the environmental fate, bioavailability, and efficacy of pesticides in soils: a review. Soil Res 48:627–637
Kookana RS, Sarmah AK, Van Zwieten L, Krull E, Singh B (2011) Biochar application to soil: agronomic and environmental benefits and unintended consequences. Adv Agron 112:103–143
Kumpiene J, Lagerkvist A, Maurice C (2007) Stabilization of Pb-and Cu-contaminated soil using coal fly ash and peat. Environ Pollut 145:365–373
Lan Y, Deng B, Kim C, Thornton EC (2007) Influence of soil minerals on chromium(VI) reduction by sulfide under anoxic conditions. Geochem Trans 8:4
Landrot G, Ginder-Vogel M, Sparks DL (2009) Kinetics of chromium (III) oxidation by manganese (IV) oxides using quick scanning X-ray absorption fine structure spectroscopy (Q-XAFS). Environ Sci Technol 44:143–149
Lee DY, Shih YN, Zheng HC, Chen CP, Juang KW, Lee JF, Tsui L (2006) Using the selective ion exchange resin extraction and XANES methods to evaluate the effect of compost amendments on soil chromium (VI) phytotoxicity. Plant Soil 281:87–96
Leita L, Margon A, Pastrello A, Arcon I, Contin M, Mosetti D (2009) Soil humic acids may favour the persistence of hexavalent chromium in soil. Environ Pollut 157:1862–1866
Leita L, Margon A, Sinicco T, Mondini C (2011) Glucose promotes the reduction of hexavalent chromium in soil. Geoderma 164:122–127
Liu D, Zou J, Wang M, Jiang W (2008) Hexavalent chromium uptake and its effects on mineral uptake, antioxidant defence system and photosynthesis in Amaranthus viridis L. Bioresour Technol 99:2628–2636
Losi ME, Amrhein C, Frankenberger WT Jr (1994) Factors affecting chemical and biological reduction of hexavalent chromium in soil. Environ Toxicol Chem 13:1727–1735
Oliver DS, Bowman RS, Brockman FJ, Kieft TL (2003) Microbial reduction of hexavalent chromium under vadose zone conditions. J Environ Qual 32:317–324
Owlad M, Aroua MK, Daud WAW, Baroutian S (2009) Removal of hexavalent chromium-contaminated water and wastewater: a review. Water Air Soil Pollut 200:59–77
Parfitt RL (1978) Anion adsorption by soils and soil materials. Adv Agron 30:1–50
Park D, Ahn CK, Kim YM, Yun YS, Park JM (2008) Enhanced abiotic reduction of Cr(VI) in a soil slurry system by natural biomaterial addition. J Hazard Mater 160:422–427
Park D, Yun YS, Park JM (2004) Reduction of hexavalent chromium with the brown seaweed Ecklonia biomass. Environ Sci Technol 38:4860–4864
Park D, Yun YS, Lee DS, Lim SR, Park JM (2006) Column study on Cr(VI)-reduction using the brown seaweed Ecklonia biomass. J Hazard Mater 137:1377–1384
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW (2011) Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J Hazard Mater 185:549–574
Qiu Y, Cheng H, Xu C, Sheng GD (2008) Surface characteristics of crop-residue-derived black carbon and lead (II) adsorption. Water Res 42:567–574
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press Pty Ltd, Melbourne
Schwab P, Zhu D, Banks MK (2007) Heavy metal leaching from mine tailings as affected by organic amendments. Bioresour Technol 98:2935–2941
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Skinner WM, Prestidge CA, Smart RSC (1996) Irradiation effects during XPS studies of Cu (II) activation of zinc sulphide. Surf Interface Anal 24:620–626
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310
Tokunaga SR, Firestone MK, Olson KR, Wan J, Sutton TK, Lanzirotti A, Hazen TC, Herman DJ (2003) In situ reduction of chromium (VI) in heavily contaminated soils through organic carbon amendment. J Environ Qual 32:1641–1649
Uluozlu OD, Sari A, Tuzen M, Soylak M (2008) Biosorption of Pb (II) and Cr (III) from aqueous solution by lichen Parmelina tiliaceae biomass. Bioresour Technol 99:2972–2980
USEPA, Chromium, Hexavalent (colorimetric) (1995) Test methods for evaluating solid waste, physical/chemical methods. SW–846. In USEPA: USA
Weng CH, Huang CP, Sanders PF (2002) Transport of Cr (VI) in soils contaminated with chromite ore processing residue (COPR). Pract Period Hazard Toxic Radioactive Waste Manage 6:6–13
Yang L, Chen JP (2008) Biosorption of hexavalent chromium onto raw and chemically modified Sargassum sp. Bioresour Technol 99:297–307
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Comm Soil Sci Plant Anal 18:131–146
Zhitkovich A (2011) Chromium in drinking water: sources, metabolism and cancer risks. Chem Res Toxicol 24(10):1617–1629
Zhong L, Yang J (2012) Reduction of Cr(VI) by malic acid in aqueous Fe-rich soil suspensions. Chemosphere 86:973–978
Acknowledgments
The authors are grateful to the Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC CARE), Australia for funding this research work in collaboration with University of South Australia. The Postdoctoral fellowship program (PJ008650042012) at the National Academy of Agricultural Science, Rural Development Administration, Republic of Korea, supported Dr Kunhikrishnan’s contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 114 kb)
Rights and permissions
About this article
Cite this article
Choppala, G., Bolan, N., Kunhikrishnan, A. et al. Concomitant reduction and immobilization of chromium in relation to its bioavailability in soils. Environ Sci Pollut Res 22, 8969–8978 (2015). https://doi.org/10.1007/s11356-013-1653-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1653-6