Abstract
Acid mine drainage in the Iberian Pyrite Belt is probably the worst case in the world of surface water pollution associated with mining of sulphide mineral deposits. The Iberian Pyrite Belt is located in SW Iberian Peninsula, and it has been mined during the last 4,500 years. The central and eastern part of the Iberian Pyrite Belt is drained by the Tinto and Odiel rivers, which receive most of the acidic leachates from the mining areas. As a result, the main channels of the Tinto and Odiel rivers are very rich in metals and highly acidic until reaching the Atlantic Ocean. A significant amount of the pollutant load transported by these two rivers is delivered during the rainy season, as is usual in rivers of Mediterranean climate regions. Therefore, in order to have an accurate estimation of the pollutant loads transported by the Tinto and Odiel rivers, a systematic sampling on a weekly basis and a high temporal resolution sampling of floods events were both performed. Results obtained show that metal fluxes are strongly dependent on the study period, highlighting the importance of inter-annual studies involving dry and wet years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid drainage is one of the biggest environmental problems caused by mining of sulphide-rich mineral deposit. Acid mine drainage (AMD) is responsible for the pollution and degraded water quality in groundwater, streams, rivers and whole river basins. Johnson and Hallberg (2005) estimated that in 1989, approximately 19,300 km of rivers and 72,000 ha of lakes and reservoirs throughout the world were severely affected by mining effluents. Mine drainage results from the oxidation of pyrite and other sulphide minerals generally associated with coal and metal-bearing mineral deposits. Mine drainage is most often acidic and contains high concentrations of metals and metalloids. In southwestern Spain, thousands of years of mining in the Iberian Pyrite Belt (IPB) (Davis et al. 2000; Leblanc et al. 2000; Nocete et al. 2005) has resulted in enormous metal-rich wastes that seriously degrade two of the most important basins in the Huelva province, the Odiel and Tinto watersheds (Olías et al. 2004; Sanchez-España et al. 2005; Cánovas et al. 2007; Sarmiento 2008; Sarmiento et al. 2009, 2011). These two rivers discharge into the Gulf of Cadiz. The pollution load transported by these rivers to the ocean has motivated a large number of studies to assess the processes that control the metal fate and fluxes during estuarine mixing (Elbaz-Poulichet and Dupuy 1999; Grande et al. 1999; Borrego et al. 2002; Sainz et al. 2004; Nieto et al. 2007). Olías et al. (2006) estimated that the Odiel and Tinto rivers transport enormous amounts of dissolved contaminants: 7,922 t/year of Fe, 5,781 t/year of Al, 3,475 t/year of Zn, 1,721 t/year of Cu, 1,615 t/year of Mn and minor quantities of other metals (71 t/year of Co, 27 t/year of Pb, 35 t/year of As, etc.). Sarmiento et al. (2009) estimated that the load transported by both rivers represents 3 % of the global gross flux of Cu and 15 % of the Zn, coming mainly from the Odiel load; however, this estimation should also take into account the contribution of intense rainfall events. Most published works on pollutant transport deal with annual budgets without considering the study of rain events. These type of events need to be studied with a high temporal sampling resolution as geochemical and hydrological factors (i.e. dilution, mineral dissolution/precipitation, sorption processes, changes in AMD sources and rainfall distribution) have a great variability during floods. In addition, the interaction of these factors provokes frequently discharge/concentration hysteresis (Evans and Davies 1998) making the study of these events difficult. Some studies have been performed on the Tinto and Odiel rivers which highlighted the importance of these events in metal transport from rivers to oceans (Cánovas et al. 2008, 2010, 2012a). Therefore, the objective of this study is to estimate the flux of metals from the Tinto and Odiel rivers into the Ría of Huelva and the Atlantic Ocean, considering also the contribution of intense rainfall events that are common for rivers in Mediterranean climate regions. This paper reviews the work of our group during the last 10 years on acid mine drainage in the Iberian Pyrite Belt, summarising and updating our previous work that is widespread in several publications since 2004.
Site description
Geology
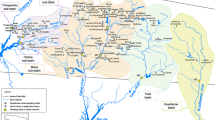
Most of the Odiel and Tinto basins flow across the IPB (Fig. 1). The IPB extends east to west from Seville Province, across Huelva Province, into Portugal. It is considered to be one of the larger polymetallic massive sulphide deposit in the world with a length of over 200 km, a width of about 40 km and estimated original reserves in the order of 1,700 mt of sulphide ore (Saez et al. 1999). The IPB belongs to the South Portuguese Zone of the Hercynian Iberian Massif and is formed by upper Palaeozoic materials that can be divided into three lithological groups: (1) the Phyllite–Quartzite Group, formed by a thick sequence of shales and sandstones, (2) the volcano–sedimentary complex (VSC), including a mafic–felsic volcanic sequence interstratified with shales and (3) the Culm group where shales, sandstones and conglomerates prevail. The southern part of both rivers flows across Neogene marly sediments from the Guadalquivir Basin. In the northern part of the area, plutonic and metamorphic rocks from the Ossa-Morena Zone also outcrop.
There are many polymetallic massive sulphide deposits (>80) associated with the VSC. Pyrite (FeS2) is the main mineral in these deposits, together with lower quantities of sphalerite (ZnS), galena (PbS), chalcopyrite (CuFeS2), arsenopyrite (FeAsS) and other sulphides that contain minor quantities of Cd, Sn, Ag, Au, Co, Hg, etc. Figure 1 shows several mines draining into the Odiel and Tinto basins.
Hydrology and climatology
Odiel River comes from Sierra de Aracena and Tinto River from Peña del Hierro mine. Both meet in a common estuary, known as the Ría of Huelva. The Odiel River, which is 140 km long, is the longest. The surface of its drainage basin is 2,300 km2. Its annual flow has been estimated at about 500 hm3 year−1. The Tinto River (length 100 km, surface of the watershed 720 km2), has an average annual flow of 100 hm3 year−1. Marked variations from this mean occur in both rivers due to the Mediterranean climate, which includes long periods of drought and intense rainy events. The mean annual rainfall in the zone is 812 mm, 50 % of which occurs between October and January.
The Odiel drainage network has a total of 1,149 km of streams. The most important tributaries in the basin are the Olivargas, Oraque and Meca on the right bank and the Agrio and Villar on the left bank. Several reservoirs have been built in the Odiel River basin. The biggest water reservoirs in the basin are Olivargas and Sancho, with a capacity of 29 and 58 hm3, respectively (Fig. 1).
Methodology
Sampling
Several samplings were performed throughout the whole basins at 24 stations in the Odiel River and at ten stations in the Tinto River between 2002 and 2006 (Fig. 1). A total of 266 water samples were collected.
Flood events were monitored in Tinto and Odiel rivers at points T8 and O13 (Fig. 1), respectively, from 2004 to 2006. Samplings were carried out by a Teledyne ISCO® autosampler, with a sample container holding up to 24 bottles and an outlet pipe made of polyethylene. Samples were pumped by a peristaltic pump, with a schedule purge stage between samplings to avoid cross-contamination (Cánovas et al. 2010). Detailed information about rainfall and river flow data during the period of sampling can be seen in Cánovas et al. (2008, 2010, 2012a, b). Pollutant loads were estimated by establishing relationships between flow and element concentrations. When these relationships were not simple to set, the following equation was applied:
where L T is the total load, C i the pollutant concentration in sample i and Q T is the discharge. For detailed information about estimation methods see Olias et al. (2006) and Cánovas et al. (2012a).
All water samples were filtered in field through 0.22 μm Millipore filters fitted on Sartorius polycarbonate filter holders. Samples for cations and metal analysis were acidified in the field to pH < 2 with Suprapur HNO3 (2 %) and then stored in the dark at 4 °C in polyethylene bottles until analysis. Samples collected for alkalinity and anion determinations were filtered but not acidified. Samples for Fe(II) determination were filtered through 0.1 μm, and the Fe(II) was made complex by adding 0.5 % (w/w) of 1,10-phenanthroline chloride solution after buffering to pH = 4.5 with an ammonium acetate/acetic buffer (Rodier et al. 1996).
Analytical determinations
Several physicochemical parameters were measured in the field. Temperature, pH and specific conductance were measured using a portable MX300 multimeter (Mettler Toledo). Dissolved oxygen was analysed using a Hanna meter, and redox potential was determined using a meter with Pt and Ag/AgCl electrode (Crison). The pH meter was calibrated using Hanna pH standard buffer solutions (pH 4.01 and pH 7.01) and redox potential was checked using Hanna standard buffer solutions (240 and 470 mV).
Anions were determined by ion chromatography using a Dionex DX-120 fitted with an AS 9-HC of 4 × 250 mm column and a 4-mm ASRS-ULTRA suppressing membrane. Detection limits were 0.1 mg L−1 for fluoride, nitrate and chloride, and 0.5 mg L−1 for sulphate. Alkalinity was determined by the titration method (Standard Methods for the Examination of Water and Wastewater no. 2320) with standardised HCl and bromcresol green indicator.
The concentrations of dissolved Al, As, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Pb, Sb, Se, Si, Sn and Zn were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES Jobin-Ybon Ultima2) using a protocol especially designed for AMD samples (Tyler et al. 2004). Analysis was performed at the Central Research Services of the University of Huelva. Multielement standard solutions prepared from single certified standards supplied by SCP SCIENCE were used for calibration. They were run at the beginning and at the end of each analytical series. Certified reference material NIST SRM-1640 fresh-water-type and inter-laboratory standard IRMM-N3 wastewater test material, from the European Commission Institute for Reference Materials and Measurements, were also used to analyse the samples. Detection limits were calculated by average, and standard deviations, from ten blanks. Detection limits were as follows: 200 μg L−1 for Al, Fe, Mn, Mg, Na, K and Si; 500 μg L−1 for Ca; 50 μg L−1 for Zn; 5 μg L−1 for Cu; 2 μg L−1 for As and 1 μg L−1 for the other trace elements.
Fe(II) was determined using colorimetry at 510 nm with a Shimadzu UVmini-1240 spectrophotometer. The detection limit was 0.3 mg L−1 and the precision was higher than 5 %.
Results and discussion
Hydrochemical characteristics in both rivers
Mean chemical composition and range of the AMD-polluted samples for the Odiel and Tinto basins are listed in Table 1. Representative values of AMD-unpolluted samples for both basins are also included (samples O1 and T6, Fig. 1). The results obtained from analysis of the Odiel samples are characterised by large variations of pH (2.2–7.3), electrical conductivity (0.16–16 mS cm−1) and redox potential (253–813 mV). These large variations are the result of the different degree of mixing of AMD-polluted and AMD-unpolluted waters in the drainage basin. The main water course of the Tinto River is the only affected stream in the whole Tinto Basin. These waters have low pH (1.2–3.0) and high redox potential (583–832 mV); however, the electrical conductivity shows large variations (1.3–37 mS cm−1). All samples from two basins show an excellent correlation (R 2 = 0.74; ρ < 0.05) between pH and redox potential (Fig. 2). The Eh–pH conditions most commonly found in these waters were acidic and oxidising; however, the Tinto samples are more acids and oxidants in comparison with the Odiel waters.
Relationships between redox potential and a pH (diamond), b specific conductance (circle), c dissolved oxygen (triangle) and d Fe2+/Fet ratio (square) for the contaminated samples in the Tinto and Odiel rivers (Modified from Sarmiento et al. 2009)
In the Odiel basin mean concentrations of the main pollutants are the following: Fe (153 mg L−1), Zn (51 mg L−1), Cu (12 mg L−1), As (0.2 mg L−1), Co (1 mg L−1) and sulphate (2,122 mg L−1). By comparison, the conditions in the Tinto River are more extreme than in the Odiel River, with mean sulphate content of 15,164 mg L−1; Fe, 3,954 mg L−1; Zn, 90 mg L−1; Cu, 88 mg L−1; As, 4.7 mg L−1; and lower values of Cd, Co, Ni and Pb. The mean contents of pollutants in the Odiel River are much lower than in the Tinto River, especially Fe and As contents (26 and 22 times lower than the Tinto, respectively). Concentrations of Cr, Cu and sulphates are around eight times lower than the Tinto; however, the mean Zn and Ni concentrations are only twice larger in the Tinto that the Odiel River (Table 1).
Most of the samples are high in sulphate concentration with values between 13 mg L−1 and 114 g L−1, dependent on the pH values. Sulphate concentration also shows a high correlation (R 2 = 0.96; ρ < 0.05) with the specific conductance, and with most of the measured metal and metalloids, like Al, Fe, Cu, Co, Zn and As.
The high acidity generated during the sulphide oxidising process gives rise to accelerated hydrolysis of the other minerals in the waste materials and country rocks, mobilising large quantities of their constituent elements. Concentrations of Al, Ca, Mg and Mn are especially high (e.g. Al 195 mg L−1, Ca 82 mg L−1, Mg 199 mg L−1 and Mn 23 mg L−1 in the Odiel River; Al 519 mg L−1, Ca 154 mg L−1, Mg 530 mg L−1 and Mn 48 mg L−1 in the Tinto River).
Figure 2 shows the relationships between redox potential and pH, specific conductance, dissolved oxygen and Fe2+/Fet ratio for the contaminated samples in both basins. Sarmiento et al. (2009) observed that the distribution of the Fe species may be described by a Boltzmann model for the Odiel basin samples. Including the Tinto basins samples (black symbols in Fig. 2), the result holds (Fig. 2d). In relation to the composition and physicochemical parameters, four groups of streams affected by AMD can be differentiated based on their origin, level of influence of oxidation of sulphides and extent of neutralisation of the acidity generated.
A first group of samples are from streams strongly affected by oxidation of sulphides and are at or near the AMD sources. These samples are essentially acidic leachates which have not undergone dilution or precipitation processes. They all show similar characteristics in connection with the elements associated to the oxidation of sulphides, carrying high quantities of metals and sulphates, including the highest concentrations of Fe (28 g L−1), Cu (379 mg L−1), Zn (584 mg L−1), Ni (8 mg L−1), Co (24 mg L−1), As (45 mg L−1) and Cd (3 mg L−1). These samples show redox potential between 650 and 750 mV, are extremely acidic (pH below 3), have high specific conductance and have elevated Fe2+/Fet ratio.
A second group of samples show redox potential higher than 750 mV (Fig. 2). This group of streams is strongly affected by AMD but is not so close to the mining sites. They are strongly oxic and acidic waters (pH below 3). Fe(II) is oxidised to Fe(III), so the ratio Fe(II)/Fe decreases. The specific conductance is elevated but lower than the previous group due in part to precipitation of Fe(III)-containing species.
Samples included in a third group show redox potentials between 650 and 750 mV and dissolved oxygen saturation higher than 60 % (Fig. 2c). These streams are strongly affected by AMD but have undergone dilution and/or neutralisation processes. Carbonate minerals are practically non-existent in the IPB, so the neutralisation is basically due to the dilution by uncontaminated water (Cánovas et al. 2007) and to the hydrolysis of aluminosilicate minerals from the country rocks. In these samples pH increases (up to 4, Fig. 2a), and specific conductance decreases (generally less than 4 mS/cm, Fig. 2b). Fe concentration is moderately low (less than 100 mg L−1), and Fe2+/Fe ratio shows large variations according to the dissolved oxygen saturation (Fig. 2d). The most relevant characteristic of these streams are the high concentrations of Ca, Al, Mg and Mn and relatively low Fe contents, which indicates that significant processes involving the precipitation of Fe compounds have occurred.
The last group of samples shows redox potentials of less than 650 mV. These streams are slightly affected by AMD or are strongly diluted/neutralised. Iron concentration is very low and generally only present as Fe(II). The pH values are close to neutrality, and specific conductance and toxic element concentrations are low.
As it can be observed in Fig. 2, Tinto samples have higher conductivity values and are more acidic than the Odiel samples; however, according to the relationship between redox potentials and dissolved iron species, samples from both rivers show the same distribution pattern.
Figure 3 shows the polluted samples plotted in the Ficklin diagram (Plumlee et al. 1992) for the composition of mine drainages. The Ficklin diagram classifies waters based on their pH and the sum of base metals Zn, Cu, Pb, Cd, Co and Ni. The four types of samples described above represent the different degrees of natural attenuation of AMD (Lambeth 1999). Leachates originating from sulphide oxidation have low values of pH and dissolved oxygen, carrying in solution high quantities of Fe(II) and toxic elements (samples from first group). When water contacts atmospheric oxygen, Fe(II) is oxidised to Fe(III), involving Fe(III) precipitation, so redox potential increases and metal concentration decreases (second group). When these leaches are mixed with uncontaminated waters or neutralised by the interaction with country rocks, the pH increases and the dissolved Fe(III) precipitates (third group). If the dilution/neutralisation processes are strong and pH is near neutrality, most of the Fe and toxic metals precipitate (fourth group).
Ficklin diagram showing the sum of dissolved base metals (Zn, Cu, Cd, Pb, Co and Ni) against pH for contaminated samples from Odiel and Tinto basins (Modified from Sarmiento et al. 2009)
According to this diagram (Fig. 3), in the Odiel Basin, different types of polluted water can be found, from highly acidic waters with extreme metal content to near-neutral waters with low metal content. However, the Tinto river polluted waters are all highly acidic, with a high or extreme metal content.
Dissolved contaminant load transported by the Odiel and Tinto rivers into the Ría of Huelva
Effects of floods on the pollution load assessment
In order to show the importance of flood events monitoring on pollution load estimation, hydrochemical data from Cánovas et al. (2008, 2010, 2012a) of monitored floods under different hydrological conditions are presented. As in other AMD systems (e.g. Keith et al. 2001), dissolved concentrations increase dramatically (Fig. 4b, c) following the first rainfall event of the season due to the progressive dissolution of soluble evaporitic salts and pore waters accumulated during summer on mine areas and the riverbed. Once this mineral store is depleted, a general decrease in concentrations is observed when freshwater dilution processes became increasingly dominant as the winter progresses (Fig. 4e, f). However, not all elements follow the same tendency during flood events as evidenced by Cánovas et al. (2010); a decrease in Na and Sr concentration is observed concomitant to the increase in most elements during the soluble evaporitic salts dissolution. Also, As and Pb did not follow the behaviour of most elements; both elements peaked later than the rest of metals suggesting a geochemical/mineralogical control.
Flood events studied in the Tinto (a–c) and Odiel (d–f) rivers. Evolution of electrical conductivity (EC) and discharge before and during monitored floods (a, d) and sulphate (b, e) and Fe (c, f) concentrations during monitored floods in the Tinto (red square) and the Odiel (blue circle) rivers respectively (Modified from Cánovas et al. 2010, 2012a)
The importance of the Tinto and Odiel rivers as metal sources for the Atlantic Ocean has led to a number of estimates of the dissolved metal loads transported by both rivers (Braungardt et al. 2003; Sainz et al. 2004; Olías et al. 2006). However, despite the large data sets used, these estimates are based at best on a weekly basis and rarely have considered flood events. As previously pointed out in Cánovas et al. (2008), rivers in Mediterranean climate regions, such as the Tinto and Odiel rivers, have alternate long drought periods and short but intense rainfall events during which most of the water discharge and dissolved contaminant and suspended matter transport occur.
Table 2 shows the pollutant load carried by the Tinto and Odiel rivers during two consecutive hydrologic years (2004/2005 and 2005/2006). High-resolution samplings (HRS) during floods allow capturing the sharp hydrochemical changes that takes place in these events. In the case of the Tinto River, the scarcity of rainfalls gave rise to intense wash out processes of soluble salts with the first rainfall after summer. In systematic samplings, based on a weekly basis at best, these data are usually ignored, which underestimated the pollutant loads between 20 and 40 % (Table 2) in both hydrological years. Only both Mn and As estimates provided similar figures. In the case of the Odiel River, the only flood event monitored (Cánovas et al. 2012a) corresponds to a sampling point placed upstream from that used in synoptic samplings, which does not allow the direct comparison of both sampling approaches. Considering the similar characteristics of both adjacent catchments, synoptic sampling in the Odiel may provide similar underestimation than in the Tinto River. However, both hydrological years were especially dry and the dominant process during monitored flood events was the wash out of soluble salts. When rainfalls are intense along the year, different flood episodes take place and dilution became dominant, and therefore, synoptic samplings may overestimate the pollutant load in both rivers.
The values obtained in the hydrological year 2004–2005 have been compared with the global gross flux based on the compilation made by Gaillardet et al. (2003) for the mean values of trace elements input to estuaries and the ocean by selecting the largest rivers of the world (Table 3). The global gross flux of SO42− was based on the result obtained by Meybeck (2003) for the estimation of anthropogenic SO42− input to the ocean (Table 3).
The load transported by both rivers represents 0.8 % of the global gross flux of Cu and 2.8 % of the Zn, coming mainly from the Odiel load (Table 3). Estimates performed by synoptic sampling (at a weekly basis) fail to capture flood events and provide lower figures for Zn and Cu (0.5 and 2.0 %, respectively), highlighting the importance of these events in metal fluxes into the ocean.
These calculated metal fluxes are substantially lower than those obtained by Olías et al. (2006). Values of contaminant load for both rivers are notably lower than those reported by Olias et al. (2006); however, the hydrologic years monitored were considerably drier (around 300 and 600 mm, respectively; Table 2) than those considered by these authors (average rainfall of 981 mm from 1995 to 2002). On the other hand, as these estimates were performed on a weekly basis and flood events were not studied in detail, it is reasonable to expect similar errors than those reported in Table 2.
Particulate metal fluxes must also be considered in AMD environments. Cánovas et al. (2012a) detected high total (dissolved + particulate) concentrations of As, Fe, Pb, Cr, Ti, V and Ba during rainy events in the Odiel River due to Fe-rich sediment remobilization, and direct precipitation to a lesser extent. The transported total Fe by the Odiel River was 37 times higher than the dissolved total Fe after rainy events in 2006. Lower values were observed for Pb (seven times higher) and Cr (five times higher), although the most striking finding was recorded for As which was totally transported by particulate matter. These figures highlight the importance of the particulate pollutant load during rainy conditions in AMD-affected river systems. If considered, the very dry season monitored by these authors (maximum discharge of 2.3 m3 s−1), a higher particulate metal load may be expected in more wet seasons.
Conclusions
The Tinto and Odiel rivers drain the central and eastern parts of the Iberian Pyrite Belt. As a result of intense sulphide oxidation processes in the watershed of these two rivers, both of them are acidic and very rich in metals and metalloids. Mean concentrations of the main pollutants are Fe (153 mg L−1), Zn (51 mg L−1), Cu (12 mg L−1), As (0.2 mg L−1) and sulphate (2,122 mg L−1), for the Odiel River; and Fe (3,954 mg L−1), Zn (90 mg L−1), Cu (88 mg L−1), As (4.7 mg L−1) and sulphate (15,164 mg L−1), for the Tinto River.
The four groups of polluted samples can be distinguished in the Tinto and Odiel rivers based on the relationships between redox potential and pH, specific conductance, dissolved oxygen and Fe2+/Fet ratio, ranging from strongly affected streams near the AMD sources that are highly acidic and have not undergone dilution or precipitation processes to slightly affected or strongly neutralised streams. In the Ficklin diagram for the chemical classification of mine drainages, different types of polluted water can be found in both rivers. In the case of the Odiel River, mine drainages range from highly acidic waters with extreme metal content to near-neutral waters with low metal content; while in the case of the Tinto River, polluted waters are all highly acidic with a high or extreme metal content.
For rivers in Mediterranean climate regions, as is the case of the Tinto and Odiel rivers, a significant amount of the annual river discharge can be concentrated in short but intense rainfall events. Therefore, the dissolved contaminant load transported by the Tinto and Odiel rivers into the Ría of Huelva and the Atlantic Ocean is strongly dependent on an accurate estimation of the contribution of the flood events associated with these intense rainfalls. These events should be studied with a high temporal sampling resolution in order to accurately estimate all factors controlling the water chemistry.
Systematic samplings (based on a weekly basis) in the Tinto and Odiel rivers usually do not show the sharp hydrochemical changes that take place during flood events and generally underestimate the pollutant loads of most elements between 20 and 40 %.
Comparison of the dissolved contaminant load transported by the Tinto and Odiel rivers to the ocean with estimated global gross flux compilations for the largest rivers of the world shows that for the hydrological year 2004–2005, the load transported by both rivers represents 0.8 % of the global gross flux of Cu and 2.8 % of the Zn. These calculated metal fluxes are substantially lower than those obtained in previous studies because the hydrologic years monitored were considerably drier (rainfall between 300 and 600 mm) than those considered in the previous studies (mean rainfall of 981 mm), highlighting the importance of long-time studies involving dry and wet years and detailed sampling of flood events in order to accurately calculate mean annual contaminant loads in the Mediterranean climate regions.
References
Borrego J, Morales JA, de la Torre ML, Grande JA (2002) Geochemical characteristics of heavy metal pollution in surface sediments of the Tinto and Odiel river estuary (southwestern Spain). Environ Geol 41:785–796
Braundgardt CB, Achterberg EP, Elbaz-Poulichet F, Morley NH (2003) Metal geochemistry in a mine polluted estuarine system in Spain. Appl Geochem 18:1757–1771
Cánovas CR, Olías M, Nieto JM, Sarmiento AM, Cerón JC (2007) Hydrogeochemical characteristics of the Tinto and Odiel rivers (SW Spain). Factor controlling metal contents. Sci Total Environ 373:363–382
Cánovas CR, Hubbard CG, Olías M, Nieto JM, Black S, Coleman ML (2008) Hydrochemical variations and contaminant load in the Río Tinto (Spain) during flood events. J Hydrol 350:24–40
Cánovas CR, Olías M, Nieto JM, Galván L (2010) Wash-out processes of evaporitic sulfate salts in the Tinto River: hydrogeochemical evolution and environmental impact. Appl Geochem 25:288–301
Cánovas CR, Olías M, Sarmiento AM, Nieto JM, Galván L (2012a) Pollutant transport processes in the Odiel River (SW Spain) during rain events. Water Resour Res 48. doi: 10.1029/2011WR011041
Cánovas CR, Olías M, Vázquez-Suñé E, Ayora C, Nieto JM (2012b) Influence of releases from a freshwater reservoir on the hydrochemistry of the Tinto River (SW Spain). Sci Total Environ 416:418–428
Davis RA, Welty AT, Borrego J, Morales JA, Pendon JG, Ryan JG (2000) Rio Tinto Estuary (Spain): 5000 years of pollution. Environ Geol 39:1107–1116
Elbaz-Poulichet F, Dupuy C (1999) Behavior of rare earth elements at the freshwater-seawater interface of two acid mine rivers: the Tinto and Odiel (Andalucia, Spain). Appl Geochem 14:1063–1072
Evans C, Davies TD (1998) Causes of concentration/discharge hysteresis and its potential as a tool for analysis of episode hydrochemistry. Water Resour Res 34:129–137
Gaillardet J, Viers J, Dupré B (2003) Trace elements in river waters. In: Drever, J.I. (Ed.), Surface and Ground Water, Weathering, and Soils. In: Holland, H.D., Turekian, K.K. (Exec. Eds.), Treatise on Geochemistry, vol. 5. Elsevier, pp. 225–272
Grande JA, Borrego J, Morales JA (1999) A study of heavy metal pollution in the Tinto-Odiel estuary in southwestern Spain using factor analysis. Environ Geol 39:1095–1101
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Keith DC, Runnells DD, Esposito KJ, Chermak JA, Levy DB, Hannula SR, Watts M, Hall L (2001) Geochemical models of the impact of acidic groundwater and evaporative sulfate salts on Boulder Creek at Iron Mountain, California. Appl Geochem 16:947–961
Lambeth RH (1999) Natural attenuation of acidic drainage from sulfidic tailings at a site in Washington State. In: Filipek LH, Plumlee GS (eds) The environmental geochemistry of mineral deposits, Part B. Case studies and research topics. Society of Economic Geologists, Rev. Econ. Geol. vol. 6, pp 479–491
Leblanc M, Morales JA, Borrego J, Elbaz-Poulichet F (2000) 4500-year-old mining pollution in Southwestern Spain: long-term implications for modern mining pollution. Econ Geol 65:655–662
Meybeck M (2003) Global occurrence of major elements in rivers. In: Drever, J.I. (Ed.), Surface and Ground Water, Weathering, and Soils. In: Holland, H.D., Turekian, K.K. (Exec. Eds.), Treatise on Geochemistry, vol. 5. Elsevier, pp. 207–223
Nieto JM, Sarmiento AM, Olías M, Cánovas CR, Riba I, Kalman J, Delvalls TA (2007) Acid mine drainage pollution in the Tinto and Odiel Rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva estuary. Environ Int 33:445–455
Nocete F, Saez R, Nieto JM, Cruz-Aunon R, Cabrero R, Alex E, Bayona MR (2005) Circulation of silicified oolitic limestone blades in South-Iberia (Spain and Portugal) during the third millennium b.c. an expression of a core/periphery framework. J Anthropol Archaeol 24:62–81
Olías M, Nieto JM, Sarmiento AM, Cerón JC, Canovas CR (2004) Seasonal water quality variations in a river affected by acid mine drainage: the Odiel river (south west Spain). Sci. Total Environ 333:267–281
Olías M, Cánovas C, Nieto JM, Sarmiento AM (2006) Evaluation of the dissolved contaminant load transported by the Tinto and Odiel rivers (South West Spain). Appl Geochem 21:1733–1749
Plumlee GS, Smith KS, Ficklin WH, Briggs PH (1992) Geological and geochemical controls on the composition of mine drainages and natural drainages in mineralized areas. In: Kkaraka YK, Maest AS (eds) Proceedings of the 7th International Symposium on Water-Rock Interaction, Park City, UT, 13–18 July, 1992, pp 419–422
Rodier J, Broutin JP, Chambon P, Champsaur H, Rodi L (1996) L’analyse De L’eau. Dunod, Paris
Sáez R, Pascual E, Toscano M, Almodovar GR (1999) The Iberian type of volcano-sedimentary massive sulphide deposits. Mineral Deposita 34:549–570
Sainz A, Grande JA, de la Torre ML (2004) Characterisation of heavy metal discharge into the Ría of Huelva. Environ Int 30:557–566
Sánchez-España J, López Pamo E, Santofimia E, Reyes J, Barettino D (2005) Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications. Appl Geochem 20:1320–1356
Sarmiento AM (2008) Study of the pollution by acid mine drainage of the surface waters in the Odiel basin (SW Spain). Ph.D. Thesis, University of Huelva, Spain. UMI ProQuest, Publ. No.: AAT 3282346. Ann Arbor,USA
Sarmiento AM, Olías M, Nieto JM, Cánovas CR (2009) Hydrochemical characteristics and seasonal influence on the pollution by acid mine drainage in the Odiel river Basin (SW Spain). Appl Geochem 24:697–714
Sarmiento AM, DelValls A, Nieto JM, Salamanca MJ, Caraballo MA (2011) Toxicity and potential risk assessment of a river polluted by acid mine drainage in the Iberian Pyrite Belt (SW Spain). Sci Total Environ 409:4763–4771
Tyler G, Carrasco R, Nieto JM, Perez R, Ruiz MJ, Sarmiento AM (2004). Optimization of mayor and trace element determination on acid mine drainage samples by ultrasonic nebulizer-ICP-OES (USN-ICP-OES). Pittcon Conference 7–12 March 2004. Chicago, USA. 9000/1000
Acknowledgments
This study has been funded by the Spanish Ministry of Science and Technology through the project CTM2010-21956-C02 and by the Environmental Council of the Andalusia Regional Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Nieto, J.M., Sarmiento, A.M., Canovas, C.R. et al. Acid mine drainage in the Iberian Pyrite Belt: 1. Hydrochemical characteristics and pollutant load of the Tinto and Odiel rivers. Environ Sci Pollut Res 20, 7509–7519 (2013). https://doi.org/10.1007/s11356-013-1634-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1634-9