Abstract
The development of a complete set of extensive studies combining both the preparation factors of catalysts and photocatalytic experimental factors for the photodegradation of methylene blue, crystal violet, and Congo red using effective nano zinc oxide (ZnO) obtained from polysaccharides (chitosans, corn starch, and sodium alginate) as chelating agents was the main objective of this study. The influence of nature of polysaccharides, ratio of reactants, calcination temperatures during preparation process, and effects of photocatalytic experimental conditions on photodegradation was investigated. Corn starch and sodium alginate were found to be effective chelating agents and optimum preparation parameters were set as 3:3 % ratio of reactants and 450 °C calcination temperature to prepare nano ZnO with good photocatalytic activity. The order of organic dyes based on their photodegradation rates was arranged as crystal violet > methylene blue > Congo red. Our findings shed light on the optimization of both preparation conditions of photocatalysts and photocatalytic experimental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental problems associated with organic pollutants promote the development of fundamental and applied research in the area of environment (Ahmad et al. 2011). Synthetic dyes are the major industrial pollutants and water contaminants (Modirshahla et al. 2007). These dyes are used extensively in the textile industry for dying nylon, cotton, wool, and silk, as well as for coloring oils, fats, waxes, varnishes, and plastics. The paper, food, cosmetic, and leather industries are also major consumers of these dyes (Chen 2007). Excess use of various dyes in the textile industry has led to the severe water contamination by releasing the toxic and colored effluents, which are usually disposed by various physical and chemical methods, such as coagulation/flocculation (Allegre et al. 2004; Golob et al. 2005), electrocoagulation (Alinsafi et al. 2005), coagulation/carbon adsorption process (Papic et al. 2004), and so on. However, these methods barely transfer the pollutants from one phase to another without destruction or have the other limitations (Wang et al. 2007). Photocatalytic reaction is a promising and emerging process for the purification of water and air (Suzuki et al. 2008). In recent years, as a promising tool to surrogate the traditional wastewater treatment, semiconductor-assisted photocatalysis has fascinated the public concern for its ability to convert the pollutants into the harmless substances directly in the waste water. Till now, many kinds of semiconductors have been studied as photocatalysts including TiO2, ZnO, CdS, WO3, and so on (Liu et al. 1998; Konstantinou et al. 2001; Kwon et al. 2000; Lin et al. 2005). TiO2 is the most extensively used effective photocatalyst for its high efficiency, photochemical stability, nontoxic nature, and low cost. The widespread use of TiO2 as an effective photocatalyst in practical application, however, has been cramped by its optical property that TiO2 is only sensitive to UV light (Rao and Chu 2009). As a contrast, ZnO, a kind of semiconductor that has the similar band gap as TiO2, is not thoroughly investigated. However, the greatest advantage of ZnO is that it absorbs large fraction of the solar spectrum and more light quanta than TiO2 (Sakthivel et al. 2003). In addition, ZnO has more functions than TiO2 (Kamat et al. 2002) and, therefore, continued study of ZnO is necessary and quite needed. When illuminated with an appropriate light source, the photocatalyst generates electron/hole pairs with free electrons produced in the empty conduction band leaving positive holes in the valence band. The photogenerated electron/hole pairs are capable of initiating a series of chemical reactions at the catalyst surfaces, which involve adsorbed organic pollutants and surficial water species that result in the decomposition of the organic compounds. The formation of relatively harmless end products represents another attractive feature of this process (Rego et al. 2009). It is well known that the highly reactive OH˙ radicals and holes are generated on the surface of photocatalyst under the radiation of UV. Due to the fact that the photocatalytic property is a surface reaction (Farbod and Jafarpoor 2012), using nanostructures with high effective surface area, one can improve this property. Therefore, the surface characteristic of ZnO determined by the different fabrication methods will sway this property as well as the final degradation efficiency (Wang et al. 2007). Such speculation persuaded us to investigate the relationship between different ZnO nanostructures and their photocatalytic efficiency.
In this study, various nano ZnO with different surface characteristics were prepared using different polysaccharides as chelating agents and were compared for the photodegradation efficiency of different organic dyes such as methylene blue, crystal violet, and Congo red. Nano ZnO was prepared under calcination of zinc–polysaccharide complex formed by the interaction of polysaccharides with zinc metal salts. Few reports (Baskoutas et al. 2007) on synthesis of ZnO using polysaccharides are available; however, detailed studies regarding characterization of zinc–polysaccharide organic polymer intermediate, the morphology of obtained ZnO, effect of preparation conditions, and application of final ZnO are still to be addressed. Hence, the present methodology is a chemically simple, economic, toxic free, time saving, and clean synthesis technique that is performed at ambient temperature and involves sustainable treatment. Generally, polysaccharides are covered with hydroxyl or amino functional groups. The structural change of different polysaccharides makes it possible to produce various nano ZnO with different surface characteristics. The influences of size, morphology, and preparation method on the photocatalytic property of nano ZnO catalysts were studied. A ZnO-assisted photocatalytic degradation study of these different dyes under UV 365-nm light irradiation has been sparse and hence this research focuses on understanding the mechanistic details of the photodegradation of dyes in the UV/ZnO process as a foundation for future applications.
Materials and methods
Chemicals
Deionized distilled water was used to prepare all solutions. Zinc nitrate hexahydrate [Zn(NO3)2·6H2O] was commercially obtained from J.T. Baker. Low molecular weight chitosans, corn starch, and sodium alginate were obtained from Aldrich, commercially. The three dyes, namely methylene blue (C16H18N3ClS), crystal violet (C25H30ClN3), and Congo red (C32H22N6Na2O6S2), were obtained from Merck. All other chemicals and reagents used in this study were of analytical grade obtained from standard companies and used without any further purification.
Synthesis of ZnO using polysaccharides
In this present work, we have reported the application of nano ZnO obtained by varying the proportion of reactants at different calcination temperatures as photocatalysts for various dye degradations. A known amount (3 g) of different polysaccharides was added in 500 mL of deionized water heated at 60 °C and configured for different concentrations. Different ratio of zinc nitrate hexahydrate was dissolved in 100 mL water in a standard flask. To this zinc nitrate aqueous solution, different ratio of polysaccharide was added respectively and the reaction mixture was stirred constantly at 28 °C for 6 h. Then, the reaction mixture was filtered and the solid collected was dried in an oven at 105 °C to obtain zinc–polysaccharide organic polymers. Finally, this zinc–polysaccharide organic polymer was calcined at three different temperatures, namely 450, 650, and 850 °C, to obtain ZnO nanostructures which were referred as shown in Table 1.
Total zinc concentration analyses of catalysts
According to the heavy metal testing guidelines specified in NIEA R307.20T of the Environmental Protection Administration of Taiwan, catalyst samples were analyzed for the total concentration of zinc by using atomic absorption spectrometer (AAS) as follows: to 1.00 to 2.00 g of crushed and grounded (particle size less than 20 mesh) and dried sample at 105 °C in a conical flask, 10 mL of concentrated nitric acid was added and heated slowly to 95 °C. The reaction mixture was refluxed for 15 min and cooled. Then, 5 mL of nitric acid was added further and refluxed for 30 min and the flask was covered. After cooling the sample, a small amount of deionized water (2 mL) and 3 mL of 30 % hydrogen peroxide were added and heated slowly. After this, the mixture was cooled and slow addition of hydrogen peroxide at 1 mL rate was continued till the total volume of hydrogen peroxide was not exceeded 10 mL. After cooling the reaction mixture, the sample was filtered to remove the insoluble matters and then added to 100 mL of deionized water (or quantitatively suitable known volume) and subjected for AAS analysis. The total concentration of zinc can be calculated as follows:
where A is the zinc concentration (in milligram per liter) in the standard solution for calibration, W is the sample weight (in gram), D is the dilution factor, and L is the quantitative volume of the final sample (in liter). The total zinc concentration of nano ZnO obtained using polysaccharides is given in Table 1.
Photocatalytic experiments and analyses
By measuring the photodegradation efficiency of three different dyes with a known concentration in aqueous solution under the illumination of UV, the photocatalytic activity of different nano ZnO was evaluated reliably. All the experiments were carried out in a Pyrex batch photoreactor. The radiation source was a UV lamp (λ max = 365 nm, 15 W, 270 lx, purchased from the Black-Ray), which was placed above a Pyrex reactor. Dye solutions were prepared dissolving the respective dye in deionized water at different concentrations in the 30–70-ppm range. The pH of solutions was adjusted in the range 6–8 with 1 N H2SO4 or 1 N NaOH, after the addition of the catalyst. For the photocatalytic degradation of dyes, a solution containing known concentration of the dye and ZnO was prepared and allowed to equilibrate for 30 min in the darkness, then 200 ml of the prepared suspension was transferred into the reactor, and then the lamp was switched on to initiate the reaction. During irradiation, the glass reactor was mounted on a magnetic stirrer to keep the suspension homogenous, and at every 15-min reaction intervals, 10 ml of sample was withdrawn and centrifuged, and the dye concentration was analyzed with a UV–vis spectrophotometer approximately at 664, 590, and 497 nm for methylene blue, crystal violet, and Congo red, respectively. A calibration plot based on Beer–Lambert’s law was established by relating the absorbance to the concentration. The changes in the absorption spectra of dyes at different irradiation times were recorded on a UV–vis spectrophotometer (Hitachi U-2800 A) in the wavelength range from 190 to 690 nm. The Zn2+ concentration was measured with Varian AA-400 AAS. The degradation percentage can be obtained by the following formula:
where C 0 is initial dye concentration (in milligram per liter) and C is dye concentration at time t (in milligram per liter).
Results and discussion
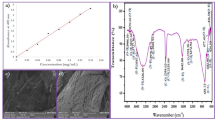
Nano ZnO was characterized using X-ray diffraction (XRD) (Bruker KAPPA APEX II instrument), scanning electron microscope (SEM) (HITACHI-S-800) and Brunauer–Emmett–Teller (BET) (Quantochrome NOVA 1000) analyses. The XRD patterns of nano ZnO obtained at different preparation conditions as shown in Fig. 1 indicated the nanocrystalline nature identical to the hexagonal phase with Wurtzite structure. As seen in Fig. 2, almost single-phase primary particle in spherical shape was obtained at low calcination temperature (450 °C), whereas in the case of high calcination temperature, particles with various shapes and sizes were obtained. It is confirmed from BET analyses that at optimum preparation conditions, nano ZnO obtained from sodium alginate (surface area = 13.4852 m2/g and pore size = 8.3392 nm pore volume) and corn starch (surface area = 13.7664 m2/g and pore size = 9.6948 nm) possessed larger surface area than that of chitosans (surface area = 11.4973 m2/g and pore size = 11.3392 nm).
The molecular structures and UV–vis absorption spectra of methylene blue, crystal violet, and Congo red are represented as shown in Fig. 3. Since the photocatalytic degradation of dyes occurs predominantly on the surface of photocatalysts, studies on the adsorption of the dyes from aqueous solution onto photocatalysts are relevant and important. To assess and confirm the role of photocatalysts (nano ZnO prepared and commercially available ZnO and TiO2) in the photocatalysis reaction, two sets of experiments were performed to compare dyes degradation rates. One set was performed with dyes exposed to photocatalysts. Then, the second set was performed by exposing dyes to photocatalysts in the presence of UV irradiation (the photocatalysis condition).
Adsorption of dyes on various photocatalysts
The dye adsorption results revealed that only small amount (about 2 to 3 %) of dyes was adsorbed on both the commercially available ZnO and TiO2. However, nano ZnO obtained from different polysaccharides showed enhanced adsorption of crystal violet (7.6 to 81 %) and low adsorption of methylene blue (2.3 to 37.5 %) and Congo red (0.9 to 4.7 %). Thus, the order of dye adsorption towards nano ZnO is crystal violet > methylene blue > Congo red. Even though the steric hindrance of both methylene blue and crystal violet is less than that of Congo red, the presence of S and N heteroatoms (Houas et al. 2001) in one of the rings of methylene blue structure makes the adsorption of dyes on photocatalysts feeble and this is the reason attributed to the low adsorption of methylene blue when compared to crystal violet. At the same time, nano ZnO obtained from sodium alginate and starch had higher adsorption capacity for methylene blue and crystal violet, whereas nano ZnO obtained from chitosans showed enhanced adsorption capacity for Congo red. This clearly depicted that nano ZnO obtained from starch and sodium alginate can act as an efficient adsorbent for methylene blue and crystal violet. However, for Congo red, nano ZnO obtained from starch and chitosans could be a suitable adsorbent. Hence, invariably in all cases, nano ZnO obtained from starch showed increased adsorption activity. But nano ZnO obtained from chitosans and sodium alginate displayed capricious activity in different situation (in the case of methylene blue and Congo red).
Effect of different polysaccharides used to obtain nano ZnO on photocatalysis
This study mainly focused on the evaluation of photocatalytic efficacy of nano ZnO towards different dyes. Since different polysaccharides were utilized to obtain nano ZnO, it is necessary to compare the effect of these polysaccharides yielding the better structural properties for nano ZnO to assist the photodegradation of dyes. Hence, the photocatalytic degradation of these dyes was carried out as shown in Fig. 4, using the nano ZnO obtained from these polysaccharides at same experimental conditions (3–3 % of reactants and calcination at 450 °C). It can be observed that nano ZnO obtained from sodium alginate and corn starch enhanced the photodegradation of methylene blue and crystal violet than that of chitosans. But for Congo red, the photocatalytic activity of nano ZnO obtained from chitosans is significantly higher than others. The reason for such discrepancy could be attributed to the structural changes due to different orientation of hydroxyl groups and different functional groups of different polysaccharides (Guibal 2004; Massicotte et al. 2008). To different dyes, the same catalysts obtained from different polysaccharides had different catalytic effect. For methylene blue and Congo red, nano ZnO obtained from sodium alginate, corn starch, and chitosans almost showed similar degradation rate. But for crystal violet, surprisingly, the photodegradation effect of nano ZnO obtained from corn starch and sodium alginate is quite good. The degradation rate is very fast and even 100 % removal of crystal violet was achieved within 1 h. It is noteworthy to mention that both sodium alginate and corn starch are efficient chelating and structural directing agents to prepare nano ZnO when compared to chitosans. Among three dyes, Congo red has the lowest photodegradation rate and the order of photodegradation efficiency is crystal violet > methylene blue > Congo red.
Effect of preparation conditions of nano ZnO on photocatalysis
We have also assumed that different preparation conditions of nano ZnO such as the ratio of polysaccharide and zinc nitrate, the calcination temperatures would influence the photocatalytic degradation of dyes. Based on this speculation, photocatalytic degradation of these dyes was carried out using nano ZnO prepared at different conditions. The effect of the ratio of polysaccharide and zinc nitrate during the preparation of nano ZnO on photodegradation is shown in Fig. 5. On the basis of experimental results, it was proposed that both homogenous (evenly) and heterogeneous (unevenly) gradual percentage variation of both polysaccharide and zinc nitrate resulted into diverse photodegradation of dyes. For example, the photocatalytic efficiency of nano ZnO obtained when the ratio of both polysaccharide and zinc nitrate is 3 %, and the enhanced photodegradation of methylene blue (about 76 %) occurred. If the percentage of polysaccharide and zinc nitrate either increased (5 %) or decreased (1 %), during the synthesis of nano ZnO, then the photodegradation of methylene blue decreased to 64 and 50 %, respectively, for nano ZnO obtained using 1 and 5 % of polysaccharide and zinc nitrate. The photodegradation efficiency of nano ZnO obtained by using both same ratio (3 % both polysaccharide and zinc nitrate) and different ratio (3 % polysaccharide and 6 % zinc nitrate) is almost constant and there is no significant difference. Also, experiment results concluded that an increase in the ratio of polysaccharide and zinc nitrate above 3 % did not favor the degradation process and decreased the photodegradation of dyes as shown in Fig. 5. Thus, it is confirmed that proper portion of reactants during the preparation of nano ZnO significantly affected the photocatalytic efficiency of nano ZnO, and in this study, the optimum ratio of polysaccharide and zinc nitrate to produce efficient nano ZnO is 3 %.
Effect of ratio of reactants during synthesis on a photodegradation of methylene blue by nano ZnO obtained from sodium alginate, b photodegradation of methylene blue by nano ZnO obtained from corn starch, c photodegradation of methylene blue by nano ZnO obtained from chitosans, and d photodegradation of crystal violet by nano ZnO obtained from chitosans
Similarly, during the synthesis of nano ZnO, the wide range of calcination temperature was involved and different calcination temperatures necessarily affect the physical characteristics of the final product. The specific surface area (Sakthivel et al. 2003) which is one of the factors that affects the photocatalytic behavior of nano ZnO is being altered at different calcination temperatures. In order to gain more information about the effect of calcination temperature during the synthesis of nano ZnO on photodegradation of dyes, photocatalysis was carried out using different nano ZnO obtained at different calcination temperatures (450, 650, and 850 °C) as shown in Fig. 6. In all cases, it is seen that low calcination temperature enhanced the photodegradation rate, and if the calcination temperature was increased, the photodegradation rate was decreased and the better calcination temperature to synthesize nano ZnO was optimized as 450 °C. The above-mentioned experimental studies clearly explained that the different surface morphologies (Wang et al. 2007) associated with nano ZnO obtained from different polysaccharides and different preparation conditions are the main factors that control the photocatalytic efficiency of nano ZnO towards different dyes. Hence, it is confirmed that the preparation method of nano ZnO is a crucial factor that affects the final photodegradation efficiency also and indicated clearly that the fabrication method rather than size was the most important factor that determined the photocatalytic activity of nano ZnO (Wang et al. 2007).
Effect of calcination temperatures during synthesis on a photodegradation of methylene blue by nano ZnO obtained from sodium alginate (3:3 %), b photodegradation of methylene blue by nano ZnO obtained from sodium alginate (1:1 %), and c photodegradation of Congo red by nano ZnO obtained from sodium alginate (3:3 %)
The effect of structural morphologies on the photodegradation efficiency can be ascribed (Wang et al. 2007) to three reasons: when the size and structural morphologies of nano ZnO varies, (1) the amount of the dispersion particles per volume in the solution will also vary, resulting in changes in the photon absorbance; (2) at the same time, the surface area of ZnO photocatalyst will vary, which will affect the adsorption dye molecules on the surface; (3) furthermore, the simple couple of photoexcited electron hole pairs will be altered. However, studies performed with different nano ZnO with different morphologies indicated that crystallinity also played the key role and morphology for the different preparation method (Li and Haneda 2003) will be definitely altered.
Effect of total zinc ion concentration of photocatalysis
In order to understand the influence of total zinc ion concentration, the total zinc ion concentration of all the catalysts obtained in this study was compared as shown in Table 1. From Table 1, it is clear that total zinc ion concentration increased with an increase in proportion of the zinc nitrate and also with calcination temperatures. However, photocatalytic studies indicated that an increase in total zinc ion concentration of catalyst did not favor the enhanced catalytic activity and it has no direct correlation at all. This can be explained by the example that nano ZnO obtained using chitosans with highest zinc ion concentration showed decreased catalytic activity compared to sodium alginate and corn starch. Catalysts obtained using sodium alginate and corn starch under 3:3 % ratio and 450 °C calcination temperature showed enhanced catalytic activity but still contained low zinc ion concentration than that of high proportion of zinc nitrate, high calcination temperature, and also that of obtained using chitosans at all conditions. This is because of the reason that photocatalysis mechanism is mainly based on the formation of electron and hole pairs and it is independent on zinc ion as shown in the following equations.
The above-mentioned experimental results clearly indicated that the preparation process of photocatalysts could produce noticeable changes on final photodegradation of dyes. However, apart from this, the photocatalytic experimental conditions will definitely also have a profound dominated effect on photocatalytic degradation process, and hence, it is necessary to have an insight into those factors affecting the photocatalysis.
Effect of temperature
A series of experiments were carried out at three different temperatures (20, 25, and 30 °C) to assess the optimum catalyst temperature. The results showed that higher the temperature, the higher the degradation rate will be and the degradation efficiencies were about 90, 82, and 78 % when the temperatures of the experiments were 30, 25, and 20 °C, respectively. Thus, 30 °C was fixed as the optimum temperature for the enhanced photocatalytic activity. This is due to the fact that the fast and easy regeneration of radicals occurs at a higher temperature. Usually, for many chemical reactions, the higher the temperature is, the greater the reaction rate.
Effect of initial dye concentration
It is important from an application point of view to study the dependence of removal efficiency on the initial concentration of the dye. Therefore, the effect of dye concentration on the degradation efficiency was investigated at different concentrations of dyes. It can be stated that the degradation decreases with the increasing initial concentration of the dyes. The negative effects of the initial dye concentration are ascribed to the competence between dye and hydroxyl radical (OH˙) adsorption on the surface of catalyst. If the initial concentration of the dye is increased, the hydroxyl concentration on surface of catalyst is depressed and remains constant for all dye molecules which results in the reduction of the formation of hydroxyl radicals. Hence, the removal rate decreases and this could be the presumed reason for the observation. The higher concentration of the pollutants necessarily induced the degree of increased light source, i.e., once the concentration of the pollutants is increased, and it also causes the dye molecules to absorb more light and photons that never reach the photocatalyst surface, and thus, the photodegradation efficiency decreases (Sakthivel et al. 2003; Peternel et al. 2007; Daneshvar et al. 2005).
Effect of the photocatalyst concentration and irradiation time
It is noteworthy to say that the initial concentration of photocatalyst will also affect the photocatalytic efficiency. It is clear that with an increased catalyst concentration from 0.25 to 1 g/L, the percentage of degradation increased. The increase in the amount of catalyst increased the number of active sites on the photocatalyst surface, which in turn increased the number of hydroxyl radicals (Joseph et al. 2000). However, when the concentration of ZnO catalyst was increased above the limiting value, the degradation rate may be decreased in some cases due to an increase in the turbidity of the suspension and a decrease in UV light penetration as a result of increased scattering effect. The duration of light irradiation also determined the efficiency of photodegradation. At longer exposure time to light, the photodegradation efficiency was enhanced as shown in Table 2. Also surprisingly in the case of photodegradation of crystal violet by photocatalysts obtained from sodium alginate and corn starch, 100 % degradation was achieved in 1 h irradiation time, and thus, the photocatalyst obtained in this study could also save the light energy. Hence, the desired degradation could be achieved easily in short duration of time using the photocatalysts developed in this work.
Effect of pH
The pH of the photocatalytic systems will definitely influence the generation of hydroxyl radicals and thus photodegradation efficiency. So, it is important to monitor the pH of the systems during photocatalysis and Table 3 includes the pH of the different systems containing the reactants in aqueous solution. From Table 3, it can be seen that the pH of the pollutants in aqueous solution is varied upon the addition of catalysts. However, this pH should be maintained steadily upon long time. In order to test this, long time monitoring of pH of systems containing pollutants and catalysts was carried out as shown in Fig. 7, which indicated that photocatalysts obtained using polysaccharides did not change the original pH and could be able to maintain the original pH for long time, whereas commercially available ZnO did not maintain the original pH initially but reached steady pH after sometime only. Hence, to study the influence of pH, photocatalysts obtained using polysaccharides could be found suitable to maintain the different pH values appropriately throughout the experiments.
ZnO can be dissolved both at acidic and alkaline environments. Hence, the effect of pH was studied from pH 3 to 9 in this study as shown in Fig. 8, and at low pH, the photocatalytic effect of catalyst is greatly reduced as shown in Table 4. The photodegradation efficiency was almost enhanced when the pH of the system was increased from 3 to 9. But photodegradation of Congo red showed discrepancy in some cases as shown in Table 4 which has no explanation. In all cases, the photodegradation efficiency was more at pH 9. At all pH values, the catalysts obtained from sodium alginate and corn starch exhibited increased catalytic activity than that of catalyst obtained using chitosans. Similarly, crystal violet and methylene blue degradation rates were faster than that of Congo red except in the case of nano ZnO obtained from chitosans. This is due to the reason that nano ZnO obtained using chitosans showed greater affinity towards Congo red rather than that of methylene blue and crystal violet as discussed earlier. In all cases, photodegradation of Congo red did not even occur at pH 3. The surface characteristics of nano ZnO will be altered appropriately at different pH values. At higher pH values, the surface of the ZnO is negatively charged by adsorbing OH−, which favors the formation of hydroxyl radicals. However, at lower pH value, the ZnO surface is preferentially covered by dye molecules (Wang et al. 2007), and hence, the adsorption of hydroxyl radicals at catalyst surface was depressed. The decrease in the photocatalytic degradation at acidic pH may be also due to dissolution of ZnO at low pH (Behnajady et al. 2006). Therefore, a higher pH value can provide more hydroxyl radicals on the increase of pH value of the systems and consequently enhancing the photodegradation efficiency.
Effect of molecular structures of dyes
As a general trend, the experiments revealed that, irrespective of the catalyst nature, the efficiency in the removal of the dyes was comparable. The different extent of dye removal could be explained on the basis of an intrinsically different reactivity of compounds. Among three dyes, Congo red displayed the decreased degradation rate due to the presence of SO3 − groups which are weakly anchored to the surface of ZnO (Comparelli et al. 2005). Additionally, it can be suggested (Joseph et al. 2000; Guillard et al. 2003) that planar or non-planar geometry of the dye molecule may also in turn affect the dye reactivity towards hydroxyl radicals. The smallest rate constant of Congo red can also be explained by the steric hindrance of a large aromatic molecule which leads to a smaller number of Congo red molecule adsorbed on catalyst surface.
Conclusion
The application of nano ZnO obtained using polysaccharides (corn starch, sodium alginate, and chitosans) as photocatalysts for degradation of methylene blue, crystal violet, and Congo red in the light of various possible factors was investigated. A complete set of experimental studies indicated that nature of polysaccharides used for the preparation of nano ZnO and preparation conditions also affected the final photocatalytic studies along with photocatalytic experimental conditions. Thus, a combined study of preparation conditions as well as photocatalytic experimental conditions was studied extensively in a single work which is the highlight of this task. The obtained results could confirm that among polysaccharides, corn starch and sodium alginate are the efficient chelating agents for the development of effective nano ZnO photocatalysts. The percentage ratio of reactants during preparation and calcination temperatures are also important factors that influence the final photocatalytic activity of nano ZnO and the optimum preparation conditions were set to be 3:3 % ratio and 450 °C calcination temperature. The effect of total zinc ion concentration of catalysts indicated that it has no direct correlation with photocatalytic activity since an increase in zinc ion concentration did not increase the catalytic activity. The dyes crystal violet and methylene blue displayed good degradation capacity when compared to Congo red. A comparative study with commercially available ZnO and TiO2 indicated that nano ZnO obtained in this work exhibited super photocatalytic ability to degrade organic dyes promising as effective photocatalysts for organic dyes.
References
Ahmad M, Shi Y, Nisar A, Sun H, Shen W, Wei M, Zhu J (2011) Synthesis of hierarchical flower-like ZnO nanostructures and their functionalization by Au nanoparticles for improved photocatalytic and high performance Li-ion battery anodes. J Mater Chem 21:7723–7729
Alinsafi A, Khemis M, Pons MN, Leclerc JP, Yaacoubi A, Benhammou A, Nejmeddine A (2005) Electro-coagulation of reactive textile dyes and textile wastewater. Chem Eng Process 44:461–470
Allegre C, Maisseu M, Charbit F, Moulin P (2004) Coagulation-flocculation decantation of dye house effluents: concentrated effluents. J Hazard Mater B 116:57–64
Baskoutas S, Giabouranis P, Yannopoulos SN, Dracopoulos V, Toth L, Chrissanthopoulos A, Bouropoulos N (2007) Preparation of ZnO nanoparticles by thermal decomposition of zinc alginate. Thin Solid Films 515:8461–8464
Behnajady MA, Modirshahla N, Hamzavi R (2006) Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J Hazard Mater B 133:226–232
Chen C-C (2007) Degradation pathways of ethyl violet by photocatalytic reaction with ZnO dispersions. J Mol Catal A Chem 264:82–92
Comparelli R, Fanizza E, Curri ML, Cozzoli PD, Mascolo G, Agostiano A (2005) UV-induced photocatalytic degradation of azo dyes by organic-capped ZnO nanocrystals immobilized onto substrates. Appl Catal B Environ 60:1–11
Daneshvar N, Rabbani M, Modirshahla N, Behnajady MA (2005) Photooxidative degradation of Acid Red 27 in a tubular continuous-flow photoreactor: influence of operational parameters and mineralization products. J Hazard Mater 118:155–160
Farbod M, Jafarpoor E (2012) Fabrication of different ZnO nanostructures and investigation of morphology dependence of their photocatalytic properties. Mater Lett 85:47–49
Golob V, Vinder A, Simonic M (2005) Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dyes Pigments 67:93–97
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Separ Purif Tech 38:43–74
Guillard C, Lachheb H, Houas A, Ksibi M, Elaloui E, Herrmann JM (2003) Influence of chemical structure of dyes, of pH and of inorganic salts on their photocatalytic degradation by TiO2 comparison of the efficiency of powder and supported TiO2. J Photochem Photobiol A 158:27–36
Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann J-M (2001) Photocatalytic degradation pathway of methylene blue in water. Appl Catal B Environ 31:145–157
Joseph JM, Destaillats H, Hung H-M, Hoffmann MR (2000) The sonochemical degradation of azobenzene and related azo dyes: rate enhancements via Fenton’s reactions. J Phys Chem A 104:301–307
Kamat PV, Huehn R, Nicolaescu R (2002) A “sense and shoot” approach for photocatalytic degradation of organic contaminants in water. J Phys Chem B 106:788–794
Konstantinou I, Sakellarides T, Sakkas V, Albanis T (2001) Photocatalytic degradation of selected S-triazine herbicides and organophosphorus insecticides over aqueous TiO2 suspensions. Environ Sci Technol 35:398–405
Kwon YT, Song KY, Lee WI, Choi GJ, Do YR (2000) Photocatalytic behavior of WO3-loaded TiO2 in an oxidation reaction. J Catal 191:192–199
Li D, Haneda H (2003) Morphologies of zinc oxide particles and their effects on photocatalysis. Chemosphere 51:129–137
Lin H, Liao S, Hung S (2005) The dc thermal plasma synthesis of ZnO nanoparticles for visible-light photocatalyst. J Photochem Photobiol A Chem 174:82–87
Liu B, Torimoto T, Yoneyama H (1998) Photocatalytic reduction of CO2 using surface-modified CdS photocatalysts in organic solvents. J Photochem Photobiol A Chem 113:93–97
Massicotte LP, Baille WE, Mateescu MA (2008) Carboxylated high amylase starch as pharmaceutical excipients structural insights and formulation of pancreatic enzymes. Int J Pharmaceut 356:212–223
Modirshahla N, Behnajady MA, Ghanbary F (2007) Decolorization and mineralization of C.I. Acid Yellow 23 by Fenton and photo-Fenton processes. Dyes Pigments 73:305–310
Papic S, Koprivanac N, LoncaricBozic A, Metes A (2004) Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dyes Pigments 62:291–298
Peternel IT, Koprivanac N, Božić AML, Kušić HM (2007) Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J Hazard Mater 148:477–484
Rao YF, Chu W (2009) Reaction mechanism of linuron degradation in TiO2 suspension under visible light irradiation with the assistance of H2O2. Environ Sci Technol 43:6183–6189
Rego E, Marto J, São Marcos P, Labrincha JA (2009) Decolouration of Orange II solutions by TiO2 and ZnO active layers screen-printed on ceramic tiles under sunlight irradiation. Appl Catal A General 355:109–114
Sakthivel S, Neppolian B, Shankar MV, Arabindoo B, Palanichamy M, Murugesan V (2003) Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. Sol Energy Mater Sol C 77:65–82
Suzuki H, Amano T, Toyooka T, Ibuki Y (2008) Preparation of DNA-adsorbed TiO2 particles with high performance for purification of chemical pollutants. Environ Sci Technol 42:8076–8082
Wang H, Xie C, Zhang W, Cai S, Yang Z, Gui Y (2007) Comparison of dye degradation efficiency using ZnO powders with various size scales. J Hazard Mater 141:645–652
Acknowledgments
We thank the National Central University and National Science Council (NSC; grant no.: NSC99-2221-E-008-025-MY3), Taiwan, Republic of China, for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Thirumavalavan, M., Yang, FM. & Lee, JF. Investigation of preparation conditions and photocatalytic efficiency of nano ZnO using different polysaccharides. Environ Sci Pollut Res 20, 5654–5664 (2013). https://doi.org/10.1007/s11356-013-1575-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1575-3