Abstract
Chromite ore processing residues (COPR) is the source of the Cr(VI) contamination in the environment. Pannonibacter phragmitetus BB was used to treat two different types of COPRs in this research. The water-soluble Cr(VI) of COPR A and B is 3,982.9 and 1,181.4 mg/kg, respectively. In the column biotreatment process, P. phragmitetus BB can reduce Cr(VI) in the leachate to an undetectable level at the flow rate of 1 and 2 ml/min. In the direct biotreatment process, Cr(VI) in the liquid supernatant of COPR A and B decreased from 265 and 200 mg/l to 145 and 40 mg/kg after 240 h of incubation. In one-step and two-step biotreatment processes, Cr(VI) in the liquid supernatant of both COPRs can be reduced to an undetectable level. The toxicity characteristic leaching procedure results indicate that the Cr(VI) concentration of treated COPR A (3.48 mg/l) is lower than the identification standards for hazardous wastes of China (5 mg/l) (GB 5085.6-2007). The information obtained in this study has significance for the application of P. phragmitetus BB to remediate COPR contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromite ore processing residue (COPR) is produced by the high lime process where the chromite ore is roasted at very high temperatures to oxidize the chromium from trivalent to hexavalent state (Wazne et al. 2008; Tinjum et al. 2008). Although the production of COPR has been abandoned in some developed countries, the process is still being used in many developing countries, such as China, India, Pakistan, Kazakhstan, and Russia (Darrie 2001; Graham et al. 2006).
COPR contains large amounts of chromium, of which as much as 35 % may be hexavalent chromium (Thomas et al. 2001; Tinjum 2007). Hexavalent chromium is toxic and mutagenic to most organisms (Batool and Hasnain 2012; Poopal and Laxman 2009) and is known to cause various diseases (Ganguli and Tripathi 2002). Moreover, the water solubility of hexavalent chromium can make it leach into environment easily, which pollutes water and soil (Costa 1997; Wu et al. 2012). However, trivalent chromium is relatively less toxic and also considered to be biologically essential to mammals (Bagchi et al. 2002; Sharma and Forster 1994). Therefore, reduction of hexavalent chromium to trivalent chromium is an attractive and effective method to remediate hexavalent chromium pollution (Sultan and Hasnain 2007).
Traditional methods for remediation of hexavalent chromium in COPR include pyro-treatment with reductive agents at high temperature and hydro-treatment after Cr(VI) was extracted into aqueous solution (Whittleston et al. 2011; Zhang et al. 2009a). High-temperature reduction of hexavalent chromium is an effective method (Zhang et al. 2009b), but the energy consumption is the main obstacle (Zhang et al. 2009a). Reduction of Cr(VI) in solution extracted from COPR was carried out by many scientists (Moon et al. 2007, 2008; Dermatas et al. 2006; Panda and Sarkar 2012). The main disadvantage of this process is the ineffective release of Cr(VI) from COPR (Dermatas et al. 2006; Zhang et al. 2009b; Tinjum et al. 2008). Therefore, it is necessary to develop an effective and low-energy-consuming method to detoxify COPR.
Microbial reduction of toxic hexavalent chromium to less toxic trivalent chromium has emerged as a useful, promising, and cost-effective detoxification process. Many bacterial strains with capability of reducing hexavalent chromium have been isolated from various contaminated environments. The characteristics and the reduction ability of these bacteria also have been investigated under laboratory conditions (Xu et al. 2011; Elangovan et al. 2006; Liu et al. 2006). Many papers about bioremediation of Cr(VI)-contaminated soil and water have also been published in recent years (Polti et al. 2009; Losi et al. 1994). Pilot-scale experiment to bioremove Cr(VI) from wastewater also have been successively achieved by Ahmad et al. (2010). However, few papers have reported directly on detoxification of COPR by microorganism. The bacterial strain Pannonibacter phragmitetus BB have been isolated from chromium-containing slag, its morphology, Cr(VI) reduction capacity, Cr(VI) reduction product and its application in Cr(VI) removal in soils have been investigated in our previous experiment (Chai et al. 2009a, b). In the present study, P. phragmitetus BB was used to detoxify two different types of COPRs by using four different treatment processes. Scanning electron microscopy (SEM) and X-ray diffraction (XRD) were performed to investigate the morphology and mineralogical changes. The effectiveness of the treatment was evaluated by the toxicity characteristic leaching procedure (TCLP). The results of this research can provide significant information for the biotreatment of COPR.
Materials and methods
COPR, bacterial strain, and culture condition
COPR A was newly produced from a chromate-producing process in a chemical company in Hubei Province, China. COPR B was collected from a bankrupted chromate manufactory in Hunan Province, China, and COPR has been deposited for more than 20 years. P. phragmitetus BB was a bacterium previously isolated from chromium-containing slag and cultured in Luria–Bertani (LB) medium. The optimum pH and temperature for bioreduction of Cr(VI) were 10.0 and 30 °C, respectively (Chai et al. 2009a).
COPR characterization
To determine the element composition of the two COPRs, the samples were air-dried, grounded, and sieved with a 200 mesh (75 μm). Total element composition of COPRs was determined by X-ray fluorescence. Water-soluble Cr(VI) concentration was determined according to the following procedure: 10 g COPR was suspended with 100 ml ultra-purified water and shaken on a rotary shaker at 175 rpm for 2 h and then filtrated; Cr(VI) concentration in the filtrate was measured with a spectrophotometer. The elemental composition and main heavy metal (total Cr, Cr(VI)) of the two COPRs are given in Table 1.
Biotreatment experiment
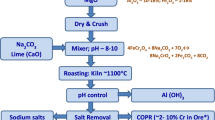
P. phragmitetus BB cells were grown in LB medium at 30 °C on a rotary shaker (175 rpm), harvested after 48 h, washed three times with sterilize water, and resuspended in the same water to an OD600 of 2.0. The experimental design of the biotreatment process was shown in Fig. 1.
The COPRs used for column biotreatment were air-dried, grounded, and sieved with an 8 mesh (2.36 mm). In the column biotreatment process, 200 g of COPR was placed in a column of 5 cm in diameter and 30 cm in height, and a plastic mesh (d = 0.2 mm) was placed at the bottom of the column to retain COPR. For COPR A, 800 ml of LB medium and 200 ml of cell suspension were mixed as circulation leachate. The pH of the mixture was adjusted to 10 with 5 mol/l of NaOH and passed through the column of COPR A by a peristaltic pump at flow rate of 1.0, 2.0, and 4.0 ml/min. The leachate was collected and withdrawn at 3- or 12-h intervals for determining Cr(VI) concentration. The experiment was carried out at 30 °C. For COPR B, 400 ml of LB medium was mixed with 100 ml of cell suspension as circulation leachate. All other procedures were the same as that of COPR A. The leachate was collected and spray-circulated the COPRs for 7 days after Cr(VI) in the leachate was completely reduced.
Direct biotreatment was carried out in a 1-L beaker at 30 °C. One hundred grams of COPR A and 200 ml of cell suspension were added into 800 ml of fresh LB medium, and 100 g of COPR B and 100 ml of cell suspension were added into 400 ml of fresh LB medium. The mixture was incubated at 30 °C. During the incubation procedure, a certain volume of liquor samples was withdrawn at 3- or 12-h intervals, and the samples were used for determining Cr(VI) concentration and cell density.
In the one-step biotreatment process, 800 and 400 ml water were added into 100 g of COPR A and COPR B, respectively. The mixtures were stirred with a magnetic stirring apparatus for 2 h and then filtered. The COPR and filtrate were collected respectively. Cell suspension of 200 and 100 ml and LB medium powder of 25 and 12.5 g were added into the filtrate of COPR A and B, respectively, and then incubated at 30 °C till Cr(VI) in the filtrate was completely reduced. Thereafter, the filtrate was added into COPRs, stirred for 2 h, and then incubated at 30 °C. The suspension was withdrawn at 3- or 12-h intervals, and the samples were used for determining Cr(VI) concentration and cell density.
For the two-step biotreatment process, water is added into COPRs and stirred for 2 h. Then cell suspension and LB medium powder were added into the filtrate and incubated at 30 °C till Cr(VI) in the filtrate was completely reduced. Then both reduced filtrates were added into washed COPRs and stirred at room temperature for 2 h and then filtered. The filtrates were incubated at 30 °C till Cr(VI) in the filtrate was completely reduced. Thereafter, the filtrates were added into COPRs, stirred for 2 h, and then incubated at 30 °C. The suspension was withdrawn at 3- or 12-h intervals, and the samples were used for determining Cr(VI) concentration and cell density.
All experiments were performed in triplicate, and the microbial biomass in the culture was determined by cell counting using a counting chamber under a microscope.
SEM and XRD detection
In order to investigate the bacterial influence on the COPR biotreatment, the morphology of the untreated and treated COPRs by the two-step biotreatment process was observed by SEM (Nova NanoSEM 230). The crystallographic compositions of the untreated and treated COPRs were also characterized by XRD (D/max 2550 VB + 18 kW).
TCLP test
To evaluate the effectiveness of COPR biotreatment, TCLP test was performed according to USEPA Method 1311 (USEPA 1992).
Analytical methods
Cr(VI) concentration was determined according to the standard method described by Han et al. (2007). The absorbance of the purple complex formed from reacting Cr(VI) with S-diphenylcarbazide was measured at λ = 540 nm by a UV spectrophotometer (Rayleigh Analytical Instrument Corp., China).
Results and discussion
Column biotreatment
The results of column biotreatment and control samples are showed in Fig. 2. In the control experiment, Cr(VI) concentration increased sharply in the initial 21 h. The maximum Cr(VI) concentration in the leachate at the flow rate of 1 and 2 ml/min was similar and reached about 307 mg/ml. Thereafter, the change of the Cr(VI) concentration in the leachate was not obvious. However, Cr(VI) concentration in leachate of COPR A increased sharply in the first 10 h and remained a stable concentration between 10 and 30 h (Fig. 2a). Thereafter, it decreased remarkably to an undetectable level at 48 h. The change trend of Cr(VI) concentration in leachate of COPR B (Fig. 2b) was similar to that of COPR A. Meanwhile, the maximum Cr(VI) concentration in the leachate at the flow rate of 2 ml/min was higher than that at 1 ml/min since P. phragmitetus BB needed more time to reduce Cr(VI) in leachate at the flow rate of 2 ml/min. Moreover, high flow rate (4 ml/min) caused the low permeability of the COPRs (data not shown). The results implied that P. phragmitetus BB could completely reduce Cr(VI) in the leachate within 60 h at the flow rate of 1 and 2 ml/min.
Direct biotreatment
As shown in Fig. 3a, in the control experiment, the initial concentration of Cr(VI) increased continuously from 165 to 280 mg/ml within 36 h, and then the change of the Cr(VI) concentration was not obvious (Fig. 3a). Cell density in the control experiment increased slightly, indicating that the microorganism in the environment entered into the reaction system and their growth was restricted by the rigor condition. Moreover, direct biotreatment could not reduce Cr(VI) in the liquid supernatant completely. The concentration of Cr(VI) in the liquid supernatant of COPR A increased from 260 to 265 mg/l after 3 h of incubation and then decreased to 145 mg/l after additional 240 h of incubation. The cell density in the liquid supernatant of COPR A increased from 1.1 × 109 to 8.2 × 109 cells/ml after 24 h of incubation and then followed by a slow decline and with a final density of 5.7 × 109 cells/ml (Fig. 3b). Similar trend was also found in COPR B (Fig. 3c, d). However, Cr(VI) concentration and the maximum cell density in COPR B were lower than that in the liquid supernatant of COPR A. It was reported that many minerals were formed during COPR production process, and Cr(VI) was enwrapped in these minerals, which resulted in a slow releasing of Cr(VI) from COPR (Graham et al. 2006; Tinjum et al. 2008; Zhang et al. 2009a). Therefore, the concentration of Cr(VI) in liquid supernatant of COPR A and B increased when it was mixed with fresh medium and cell suspension.
One-step biotreatment process
The results of the one-step biotreatment process are showed in Fig. 4. Cr(VI) concentration in the filtrate of COPR A (with water washing) reached about 190 mg/l after 2-h stirring, then Cr(VI) was reduced completely after 12 h of incubation (Fig. 4a). When the reduced filtrate was mixed with the washed COPR A, Cr(VI) concentration in the liquid supernatant reached up to 55 mg/l, and then Cr(VI) was reduced completely within 45 h. The variation of cell density in the liquid supernatant of COPR A is shown in Fig. 4b. The lag phase of P. phragmitetus BB was about 9 h, and then the cell density increased sharply with the maximum cell density up to 9.03 × 109 cells/ml at 18 h. Thereafter, the cell density remained stable. This result also indicated that mixing the washed COPR A with reduced filtrate has no effect on the growth of P. phragmitetus BB. In the control experiment, Cr(VI) concentration in the filtrate of COPR A almost have no change, and the cell density just increased slightly.
For COPR B, Cr(VI) concentration in the filtrate (with water washing) was 100 mg/l, and Cr(VI) was reduced completely with 9 h. When the washed COPR B was mixed with the reduced filtrate, Cr(VI) concentration in filtrate reached up to 173 mg/l, and then Cr(VI) was reduced completely after 237 h of incubation (Fig. 4c). This result indicates that the metabolic products of P. phragmitetus BB probably enhanced the leaching of Cr(VI) from COPR. The cell density in the liquid supernatant of COPR B increased sharply after P. phragmitetus BB and medium were added into the filtrate, but the cell density decreased obviously after the washed COPR B was mixed with the reduced filtrate (Fig. 4d). In fact, many materials existing in COPR B have the adsorption capacity to bacteria, such as carbon and silica (Kwon et al. 2006; Rivera-Utrilla et al. 2001), probably resulting a decrease in the cell density. In the control experiment, Cr(VI) concentration in the filtrate of COPR B was stable at about 100 mg/l within 240 h, and the increase of the cell density was not obvious.
Two-step biotreatment
During the two-step biotreatment process, COPRs were washed twice with water and the reduced filtrate. As shown in Fig. 5a, Cr(VI) in the filtrate of COPR A was reduced completely within 33 h both in the first cycle and the second cycle. Then the reduced filtrated was mixed with the washed COPR A, and the concentration of Cr(VI) in the filtrate declined to 11 mg/l. Cell density variation in the two-step biotreatment process of COPR A (Fig. 5b) was similar to that of COPR B (Fig. 4b). When COPR B was mixed with reduced filtrate and stirred for 2 h for the second cycle, Cr(VI) concentration in the liquid supernatant still could reach up to 155 mg/l (Fig. 5c), which was obviously higher than that of COPR A. Although the difference of total chromium between COPR A and B is not significant, the content of water-soluble chromium in COPR A was about three times of COPR B (Table 1). Some special materials (such as carbon, which is identified in the “Morphology and crystallographic compositions of COPR” section) may exist in COPR B which inhibited the leaching of Cr(VI) from COPR B. When mixing water-washed COPR B with reduced filtrate, more Cr(VI) can be leached into the reduced filtrate. This can be attributed to the fact that some of the metabolic products of P. phragmitetus BB can induce the leaching of Cr(VI). Therefore, Cr(VI) concentrations in subsequent filtrate (173 mg/l) and liquid supernatant (155 mg/l) were higher than that in the initial filtrate (100 mg/l). During the second cycle, the decrease of the cell density was not obvious (Fig. 5d), which may be ascribed to the saturation of bacteria adsorption onto the existing COPR materials. In the control experiment, the change of Cr(VI) concentration in the filtrate of both COPR A and B was slight, and the increase of the cell density was not obvious.
Morphology and crystallographic compositions of COPR
The surface properties of COPRs could clearly be observed at higher magnifications (Fig. 6). The crystal grains of COPR A and B with different sizes and rough surface are shown in Fig. 6a, b, respectively. The crystal structure of these treated COPRs was destroyed to small grains (Fig. 6c, d). This indicates that P. phragmitetus BB or its metabolites could induce the destruction of the crystal structure of the COPRs.
The crystallographic compositions and XRD patterns of the untreated and treated COPRs are shown in Fig. 7. The result showed that both untreated and treated COPRs contain periclase, brownmillerite, brucite, and calcite. Aluminum hydrogen sulfate was generated in both COPRs after treatment. Besides, carbon also appeared in the untreated COPR B, which may attribute to the incomplete combustion of the carbon in COPR-producing process. Many researchers have reported that periclase, brownmillerite, brucite, and calcite are the main composition of COPR (Chrysochoou and Dermatas 2007; Geelhoed et al. 2003). Brownmillerite and hydrogarnet may be associated with physical entrapment of Cr(VI) in the interior of nodules, which inhibited the leaching of Cr(VI) (Chrysochoou et al. 2009). Carbon has strong adsorption capacity to heavy metals, organic compounds, and pigments (Meena et al. 2005; Karanfil et al. 1999). The presence of carbon can inhibit the leaching of Cr(VI) from COPR B and lead to the water-soluble Cr(VI) at an abnormal low concentration.
TCLP test
TCLP was performed to identify the treatment effectiveness of COPRs by P. phragmitetus BB (Table 2). In the column biotreatment process, leachates were circulated further for 7 days after Cr(VI) in the leachates was reduced completely. TCLP result shows that Cr(VI) in the leachates of COPR A was 6.67 and 19.29 mg/l at the flow rate of 1 and 2 ml/min, respectively, and 21.23 and 24.95 mg/l in the leachates of COPR B. In the direct biotreatment process, Cr(VI) in the liquid supernatant was not reduced completely, and Cr(VI) concentrations of TCLP for COPR A and B maintained 64.38 and 40.67 mg/l, respectively, which were higher than the identification standards for hazardous wastes of China (GB 5085.6-2007). In the one-step biotreatment process, although Cr(VI) in the liquid supernatant was reduced to an undetectable level, Cr(VI) concentration of TCLP still cannot meet the allowable limit of the identification standards for hazardous wastes of China (GB 5085.6-2007). In the two-step biotreatment process, Cr(VI) concentration in TCLP of COPR A decreased to 3.48 mg/l, which was lower than the identification standards for hazardous wastes of China (GB 5085.6-2007). Therefore, the two-step biotreatment process was an effective way to treat COPRs by using P. phragmitetus BB. Although there were several literatures on the detoxification of COPR with different ways, few of them can decrease Cr(VI) concentration under allowable limit (5 mg/l), or the remediation needs a long time (18 months) (Moon et al. 2008). The results of this research provided an innovative way to remediate COPR contamination.
Conclusion
In conclusion, this study investigated the detoxification of two different types of COPRs by P. phragmitetus BB. COPRs were treated by different biotreatment processes, including column biotreatment, direct biotreatment, one-step biotreatment, and two-step biotreatment. The two-step biotreatment process was a potential approach to treat COPRs by P. phragmitetus BB, and column biotreatment process also can detoxify Cr(VI) of COPRs to a low concentration. The further research is to investigate the metabolic products of P. phragmitetus BB and the influence mechanism of metabolites on Cr(VI) leaching.
Reference
Ahmad WA, Zakaria ZA, Khasim AR, Alias MA, Ismail S (2010) Pilot-scale removal of chromium from industrial wastewater using the ChromeBac™ system. Bioresour Technol 101:4371–4378
Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG (2002) Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 180:5–22
Batool R, Hasnain S (2012) Hexavalent chromium reduction by bacteria from tannery effluent. J Microbiol Biotechnol 22:547–554
Chai L, Huang S, Yang ZH, Peng B, Chen YH (2009a) Hexavalent chromium reduction by Pannonibacter phragmitetus BB isolated from soil under chromium-containing slag heap. J Environ Sci Heal A 44:615–622
Chai L, Huang S, Yang Z, Peng B, Huang Y, Chen Y (2009b) Cr(VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J Hazard Mater 167:516–522
Chrysochoou M, Dermatas D (2007) Application of the Rietveld method to assess chromium (VI) speciation in chromite ore processing residue. J Hazard Mater 141:370–377
Chrysochoou M, Fakra SC, Marcus MA, Moon DH, Dermatas D (2009) Microstructural analyses of Cr (VI) speciation in chromite ore processing residue (COPR). Environ Sci Technol 43:5461–5466
Costa M (1997) Toxicity and carcinogenicity of Cr (VI) in animal models and humans. Crit Rev Biotechnol 27:431–442
Darrie G (2001) Commercial extraction technology and process waste disposal in the manufacture of chromium chemicals from ore. Environ Geochem Health 23:187–193
Dermatas D, Chrysochoou M, Moon DH, Grubb DG, Wazne M, Christodoulatos C (2006) Ettringite-induced heave in chromite ore processing residue (COPR) upon ferrous sulfate treatment. Environ Sci Technol 40:5786–5792
Elangovan R, Abhipsa S, Rohit B, Ligy P, Chandraraj K (2006) Reduction of Cr (VI) by a Bacillus sp. Biotechnol Lett 28:247–252
Ganguli A, Tripathi AK (2002) Bioremediation of toxic chromium from electroplating effluent by chromate-reducing Pseudomonas aeruginosa A2 Chr in two bioreactors. Appl Microbiol Biotechnol 58:416–420
Geelhoed JS, Meeussen J, Roe MJ, Hillier S, Thomas RP, Farmer JG, Paterson E (2003) Chromium remediation or release? Effect of iron (II) sulfate addition on chromium (VI) leaching from columns of chromite ore processing residue. Environ Sci Technol 37:3206–3213
Graham MC, Farmer JG, Anderson P, Paterson E, Hillier S, Lumsdon DG, Bewley RJ (2006) Calcium polysulfide remediation of hexavalent chromium contamination from chromite ore processing residue. Sci Total Environ 364:32–44
Han X, Wong Y, Wong M, Tam N (2007) Biosorption and bioreduction of Cr (VI) by a microalgal isolate, Chlorella miniata. J Hazard Mater 146:65–72
Karanfil T, Kitis M, Kilduff JE, Wigton A (1999) Role of granular activated carbon surface chemistry on the adsorption of organic compounds. 2. Natural organic matter. Environ Sci Technol 33:3225–3233
Kwon KD, Green H, Bjöörn P, Kubicki JD (2006) Model bacterial extracellular polysaccharide adsorption onto silica and alumina: quartz crystal microbalance with dissipation monitoring of dextran adsorption. Environ Sci Technol 40:7739–7744
Liu YG, Xu WH, Zeng GM, Li X, Gao H (2006) Cr (VI) reduction by Bacillus sp. isolated from chromium landfill. Process Biochem 41:1981–1986
Losi ME, Amrhein C, Frankenberger WT (1994) Bioremediation of chromate-contaminated groundwater by reduction and precipitation in surface soils. J Environ Qual 23:1141–1150
Meena AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN (2005) Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater 122:161–170
Moon DH, Wazne M, Dermatas D, Christodoulatos C, Sanchez AM, Grubb DG, Chrysochoou M, Kim MG (2007) Long-term treatment issues with chromite ore processing residue (COPR): Cr6+ reduction and heave. J Hazard Mater 143:629–635
Moon DH, Wazne M, Jagupilla SC, Christodoulatos C, Kim MG, Koutsospyros A (2008) Particle size and pH effects on remediation of chromite ore processing residue using calcium polysulfide (CaS5). Sci Total Environ 399:2–10
Panda J, Sarkar P (2012) Bioremediation of chromium by novel strains Enterobacter aerogenes T2 and Acinetobacter sp. PD 12 S2. Environ Sci Pollut Res 19:1809–1817
Polti MA, García RO, Amoroso MJ, Abate CM (2009) Bioremediation of chromium (VI) contaminated soil by Streptomyces sp. MC1. J Basic Microb 49:285–292
Poopal AC, Laxman RS (2009) Chromate reduction by PVA-alginate immobilized Streptomyces griseus in a bioreactor. Biotechnol Lett 31:71–76
Rivera-Utrilla J, Bautista-Toledo I, Ferro-García MA, Moreno-Castilla C (2001) Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J Chem Technol Biotechnol 76:1209–1215
Sharma DC, Forster CF (1994) The treatment of chromium wastewaters using the sorptive potential of leaf mould. Bioresour Technol 49:31–40
Sultan S, Hasnain S (2007) Reduction of toxic hexavalent chromium by Ochrobactrum intermedium strain SDCr-5 stimulated by heavy metals. Bioresour Technol 98:340–344
Thomas RP, Hillier SJ, Roe MJ, Geelhoed JS, Graham MC, Paterson E, Farmer JG (2001) Analytical characterization of solid-and solution-phase chromium species at COPR-contaminated sites. Environ Geochem Health 23:195–199
Tinjum JM (2007) Mineralogical properties of chromium ore processing residue and chemical remediation strategies. Ph.D. dissertation, University Wisconsin–Madison, Madison
Tinjum JM, Benson CH, Edil TB (2008) Mobilization of Cr (VI) from chromite ore processing residue through acid treatment. Sci Total Environ 391:13–25
USEPA (1992) Method 1311: toxicity characteristic leaching procedure, In: Office of Solid Waste and Emergency Response (Ed.), Test methods for evaluating solid waste, SW-846, U.S. Environmental Protection Agency, Washington, DC
Wazne M, Jagupilla SC, Moon DH, Christodoulatos C, Koutsospyros A (2008) Leaching mechanisms of Cr (VI) from chromite ore processing residue. J Environ Qual 37:2125–2134
Whittleston RA, Stewart DI, Mortimer R, Tilt ZC, Brown AP, Geraki K, Burke IT (2011) Chromate reduction in Fe (II)-containing soil affected by hyper alkaline leachate from chromite ore processing residue. J Hazard Mater 194:15–23
Wu P, Li S, Ju L, Zhu N, Wu J, Li P, Dang Z (2012) Mechanism of the reduction of hexavalent chromium by organo-montmorillonite supported iron nanoparticles. J Hazard Mater 219:283–288
Xu L, Luo M, Li W, Wei X, Xie K, Liu L, Jiang C, Liu H (2011) Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 stimulated with external electron donors under alkaline conditions. J Hazard Mater 185:1169–1176
Zhang D, He S, Dai L, Hu X, Wu D, Peng K, Bu G, Pang H, Kong H (2009a) Treatment of chromite ore processing residue by pyrolysis with rice straw. Chemosphere 77:1143–1145
Zhang D, Kong H, Wu D, He S, Hu Z, Hu X (2009b) Remediation of chromite ore processing residue by pyrolysis process with sewage sludge. Bioresour Technol 100:2874–2877
Acknowledgments
This work was supported by the National Funds for Distinguished Young Scientist (50925417), the National Natural Science Foundation of China (51074191), and the National “Twelfth Five-Year” Plan for Science and Technology Support (2012BAC09B04).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wang, Y., Yang, Z., Peng, B. et al. Biotreatment of chromite ore processing residue by Pannonibacter phragmitetus BB. Environ Sci Pollut Res 20, 5593–5602 (2013). https://doi.org/10.1007/s11356-013-1526-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1526-z