Abstract
The Tubarão River rises in Santa Catarina, Brazil, and has been historically affected by coal mining activities around its springhead. To evaluate its water conditions, an investigation regarding a possible decontamination gradient associated with the increased river flow toward the estuary, as well as the influence of seasonality over this gradient was performed through a series of biomarkers (vitellogenin, comet assay, lipid peroxidation, protein carbonylation, gluthatione, gluthatione S-transferase, acetylcholinesterase, light microscopy in liver, and scanning electron microscopy in gills) and chemical analysis (polycyclic aromatic hydrocarbons (PAHs) in bile and metal analysis in sediment) in the cichlid Geophagus brasiliensis. Two collections (summer and winter) were made in four distinct sites along the river, while sediments were sampled between those seasons. As expected, the contamination linked exclusively to mining activities was not observed, possibly due to punctual inputs of contaminants. The decontamination gradient was not observed, although seasonality seemed to have a critical role in the responses of biomarkers and availability of contaminants. In the summer, the fish presented higher histopathological damages and lower concentrations of PAHs, while in the winter they showed both higher genetic damage and accumulation of PAHs. The Tubarão suffers impacts from diverse activities, representing health risks for wild and human populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid population growth together with the lack of efficient urban planning and economic development has brought about serious problems of environmental degradation. In particular, watersheds, natural reservoirs, and rivers receive pollutants from the most diverse human activities, limiting these resources and putting them in the spotlight of international agencies (IAP 2005; UN 2012).
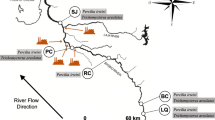
The Tubarão is an important river formed by the confluence of the Rocinha and Bonito rivers in the township of Lauro Müller, Santa Catarina (LMCH 2012). It presents a water flow that increases toward the estuary and a drainage area of 4,728 km2, covering 120 km up to the Santo Antônio lagoon (Fig. 1). The Tubarão River basin drains regions that suffered from the exploitation and processing of coal for over 50 years and which are still subject to the use of pesticides in crops, waste from intensive pig farming, as well as industrial and urban effluents (Amaral 1998; Bortoluzzi 2009; Silva et al. 2011a, b). In order to understand how the aquatic environments are impacted by anthropogenic activities and to better support environmental policies, constant water monitoring programs that associate biotic and abiotic factors are essential, since they generate precise answers about the presence and effects of contaminants (Van der Oost et al. 2003). Thus, to correlate both factors, it is necessary to use organisms, or bioindicators, as they constitute the main targets affected by imbalances in the environment.
Bioindicators are organisms, populations, or communities whose vital functions are closely correlated with certain environmental factors, being used as indicators in the evaluation of a particular ecosystem. Therefore, it is important that bioindicators have, besides other characteristics, low tolerance limits, i.e., sensitivity to small environmental changes, local abundance and low mobility, easy identification, and a well-known ecology (Powers 1989; Chovanec et al. 2003). Geophagus brasiliensis is a species of fish of the Cichlidae family with a distribution area that stretches from the states of northeastern Brazil to bordering areas with Uruguay. They are omnivorous fish with sexual dimorphism, reaching 15–30 cm in their natural habitats. This species has good tolerance to variation in temperature (15–30 °C), pH (5.5–8.0), and hypoxia, being a well-consolidated bioindicator of neotropical ecosystems (Wilhelm Filho et al. 2001; Paraguassu et al. 2005; Mazzoni and da Costa 2007; Madi and Ueta 2009; Clemente et al. 2010; Benincá et al. 2011).
Biomarkers are the measurable responses of the bioindicators (e.g., molecular, genetic, biochemical, histopathological) used to integrate the biotic (bioindicators) and abiotic (environmental characteristics) data, and to provide information on the monitored ecosystems (Van der Oost et al. 2003). It is known that a wide range of environmental pollutants such as heavy metals, pesticides, and polycyclic aromatic hydrocarbons can compromise the organism’s homeostasis and induce a series of damages (Bellassoued et al. 2012).
Vitellogenin (VTG) is a lipophosphoglycoprotein of high molecular weight, synthesized by oviparous females in reproductive season (Hiramatsu et al. 2005). Males also have the VTG gene, but it is typically silent. The exposure to compounds that exert estrogen activity is able to induce VTG expression in sexually immature female or male individuals (Matozzo et al. 2008; Moura Costa et al. 2010), which characterizes such an expression as a biomarker of estrogen endocrine disruption.
Oxidative stress acts through the generation of reactive oxygen species, which is commonly associated with cellular injuries, especially due to alterations in macromolecules, such as the DNA, lipids, and proteins. Several tests are known to quantify damage in those molecules (e.g., comet assay (CA) for DNA, lipid peroxidation (LPO) for membranes, and protein carbonylation (PCO) for proteins), thus having a high predictive value as biomarkers of this effect (Livingstone 2003; Ferreira et al. 2005; Filipak Neto et al. 2007; Guilherme et al. 2008; Dogan et al. 2011; Liebel et al. 2011; Nogueira et al. 2011). Also, metabolic and antioxidant enzymes, such as the reduced gluthatione and gluthatione S-transferase activity (GST), have been suggested as biomarkers of contaminant-mediated oxidative stress in a variety of organisms, and their induction reflects a specific response to xenobiotics (Sun et al. 2006; Almroth et al. 2010; Modesto and Martinez 2010; Hellou et al. 2012).
The inhibition of acetylcholinesterase (AChE) is widely studied as an organophosphate- and carbamate-specific neurotoxic biomarker, as well as heavy metals, organochlorines, and polycyclic aromatic hydrocarbons (PAHs) (Payne et al. 1996; Martinez-Tabche et al. 1997; Sturm et al. 1999).
Most of these damages can be observed in tissues such as the liver and gills and therefore be classified as histopathological damages, which reflect problems of both acute and chronological orders (e.g., inflammatory processes, necrosis of the tissue, appearance of neoplastic cells) (Rabitto et al. 2005, 2011; Romão et al. 2006; Miranda et al. 2008; Katsumiti et al. 2009; Silva et al. 2011a, b, c; Brito et al. 2012).
A number of works have recently put the Santa Catarina river basins in evidence, placing special emphasis on coal mining activities and human health conditions (Silva et al. 2009, 2011a, b). However, little data are available on the risks to biota or on the consequences to fishing activities in this area. Thus, the aim of the current study was to evaluate the water quality of the Tubarão river through a series of biomarkers (molecular, genetic, biochemical, and histopathological) and chemical analysis (polycyclic aromatic hydrocarbons) in the cichlid Neotropical freshwater fish G. brasiliensis, as well as metal analysis in sediment. In addition, we sought to investigate the presence of a possible decontamination gradient associated with the increased river flow toward the estuary and the influences of seasonality over this gradient.
Materials and methods
Studied area
The Tubarão River basin is the largest in the state of Santa Catarina (Southern Brazil), with an area of 4,728 km2. It is part of the Atlantic drainage area RH9 and is limited by the following coordinates—27º48′00″ North, 28°48′08″ South, 48°8′18″ East, and 48º31′48″ West. Since 1985, much has occurred in the history of coal mining in the region, although no major concerns about the impacts that these activities could generate (from extraction and transport to storage and processing) were raised. Nowadays, most of the mines in the region are disabled, yet the undesirable effects of these activities, e.g., piles of waste, acid drainages, abandoned mines, and the acidification of water bodies, remain in full view (LMCH 2012).
For water quality assessment, fish and sediment were collected in four sites along the Tubarão River (Fig. 1). The first site (BN) is located at the confluence of the Tubarão River with the Braço do Norte river in a rural area of 29,000 inhabitants, which has been impacted by agricultural activities and the coal mining activity right upstream. The second site (T) is located in the city of Tubarão (97,000 inhabitants, 18 km from the site 1), which is more affected by urban pollution. The third site (CB) is located at the confluence with the Capivari de Baixo river (20,000 inhabitants, 5 km far from the site 2), affected by thermoelectric activity from the largest coal power plant in Latin America. Finally, site 4 (L) (Laguna city, 51,000 inhabitants, 30 km far from area 2) represents a complex of lagoons that are connected to the Atlantic Ocean through the Santo Antonio and Camacho lagoons. The water flow of the Tubarão River increases along its extension, with flows of 73.44 m3/s in BN, 76.24 m3/s in T, 110.07 m3/s in CB, and 110.33 m3/s in L (BNCH 2012; TCH 2012; CBCH 2012; LCH 2012).
Fish and sediment sampling
G. brasiliensis were sampled in the summer (December/2009) and winter (June/2010) seasons at four sites along the Tubarão River using fishing net throw (5–7 cm mesh) and fish traps. Sediments were sampled in March/2010 at the same areas alongside the river for determination of metals.
Biomarkers
Fish from the Tubarão River were collected in the four sites, stored in styrofoam boxes with local water and adequate aeration, and transported to a laboratory located in the city of Laguna. The journey from each collection site to the laboratory lasted approximately 40 min from BN site, 25 min from T site, 20 min from CB site, and 5 min from L site.
In the laboratory, 2 h after the fish were collected, they were anesthetized with 0.02 % benzocaine in water, weighed, and then measured. Blood was immediately sampled for the VTG and CA analysis; liver was used for biochemical (LPO, PCO, GSH, GST activity) and histopathological (light microscopy) analysis, brain and muscle for neurotoxic analysis (AChE activity), the second left gill arch obtained for scanning electron microscopy (SEM) analysis, and bile for PAH determination.
Endocrine disrupter biomarker
In VTG, blood was collected from the caudal vein of fish still alive, with heparinized syringe, and transferred to microtubes containing an anti-proteolytic solution of 1 mM of PMSF, 1:100 ratio (v/v). The blood was centrifuged in 4 °C, 4,000×g for 30 min to obtain the plasma, which was aliquoted and stored at −75 °C for further analysis. For a positive VTG expression control sample, 15 male fish received an intraperitoneal injection of 10 mg of 17α-ethinylestradiol (EE2 dissolved in canola oil) per kilogram of fish. After 15 days of exposure, the blood was collected as described above. To perform sample preparation, plasma proteins concentration was determined through Bradford (1976) method. Plasma proteins (100 μg) were prepared in sample buffer (10 % glycerol and 0.02 % bromophenol blue in 0.5 M Tris–HCl, pH 6.8) containing 0.1 % SDS (w/v) and 5 % β-mercaptoethanol (v/v), and heated at 100 °C for 5 min before loading. Discontinuous polyacrylamide gel electrophoresis was carried out in slabs of 1.5 mm thick, using a Mini Protean II (Bio-Rad). The resolving gel contained 8 % acrylamide for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the stacking gel contained 4 % of the same solution. The SDS-PAGE-separated proteins were transferred to nitrocellulose membranes (100 V, 1 h, 0 °C). The membranes were incubated in the blocking solution 5 % of non-fat dry milk in Tris-buffered saline (TBS) with 0.5 % Tween-20 at room temperature for 1 h. The membrane was then probed for VTG with polyclonal anti-Rhamdia quelen vitellogenin polyclonal antibodies (1:4,000; provided by Cell Toxicology Laboratory) in blocking solution, 4 °C, for 16 h. Bound antibodies were detected with goat anti-rabbit antibodies coupled to horseradish peroxidase (1:4,000) diluted in TBS. The reaction was developed using the Pierce chemiluminescent kit and detected with photograph Hyperformance film.
Genetic biomarker
In the comet assay, blood was sampled and diluted in fetal bovine serum and stored in ice (protected from light) for 24 h and then prepared for the comet assay (Singh et al. 1988; Ferraro et al. 2004). The microscope slides were prepared with the cell suspension (10 μl) in low-melting-point agarose (120 μl) at 37 °C followed by incubation in lysis solution at 4 °C for 7 days. After lysis, the slides were placed in solution of NaOH (10 M) and EDTA (200 mM), pH > 13 for 20 min for DNA denaturation. Electrophoresis was carried out at 25 V and 300 mA for 25 min at 4 °C, and slides were neutralized for 15 min with 0.4 M Tris, pH 7.5, fixed in 95 % ethanol for 5 min, and stained with ethidium bromide (0.02 μg/ml). DNA strand breaks were scored using a Leica® epifluorescence microscope at a magnification of ×400. For each fish, 100 nucleoids were visually analyzed according to the method described by Ramsdorf et al. (2009).
Biochemical biomarkers
Liver, brain, and muscle samples were frozen in dry ice and stored at −76 °C. Then, they were kept on ice and homogenized in ice-cold potassium phosphate buffer (pH 6.5 (liver), pH 7.5 (brain and muscle)). Homogenates were centrifuged at 10,000×g for 20 min at 4 °C. Aliquots of the supernatant were kept for analysis of LPO, PCO, GSH, GST activity, and AChE activity.
Lipid peroxidation (LPO)
The 200 μl of supernatant (phosphate buffer for the blank) and 800 μl of reaction solution (100 mM xylenol orange, 25 mM H2SO4, 4 mM BHT, butylated hydroxytoluene, and 250 μM FeSO4/NH4, ammonium ferrous sulfate in pure methanol) were added to 2-ml tubes. Tubes were kept at room temperature for 20 min and then centrifuged at 10,000×g for 10 min. Finally, 300 μl of clean supernatants were added to a 96-well microplate, and absorbances were measured at 570 nm. For determining the hydroperoxide concentrations, the apparent molar extinction coefficient for H2O2 and cumene hydroperoxide of 4.3 × 104 M−1 cm−1 was utilized (Jiang et al. 1992).
Protein carbonylation (PCO)
The 200 μl of supernatant was mixed with 800 μl of 10 mM 2,4-dinitrophenylhydrazine in 2.0 M hydrochloric acid in tubes used in the reaction and with 800 μl of 2.0 M hydrochloric acid (without DNPH) in tubes used as blanks. Tubes were mixed in vortex for 5 min and kept at 30 °C for 1.5 h. Proteins were precipitated by addition of 1.0 ml of 28 % trichloroacetic acid and centrifuged at 9,000×g for 10 min. Pelleted proteins were washed three times by suspension in ethanol/ethyl acetate (1:1), mixed in vortex, and centrifuged. Proteins were then solubilized in 6.0 M guanidine hydrochloride, and tubes were centrifuged at 9,000×g for 5 min to remove any trace of insoluble material. The carbonyl content was spectrophotometrically determined at 360 nm using the molar absorption coefficient of 2.1 × 104 M−1 cm−1 for hydrazones (Levine et al. 1990; Quinlan and Gutteridge 2000).
Glutathione (GSH)
The 200 μl of supernatant (phosphate buffer for the blank) were mixed with 50 μl of 50 % trichloroacetic acid and centrifuged at 1,000×g for 15 min and 4 °C for protein precipitation. Then, 50 μl of supernatant and 230 μl of Tris (0.4 M, pH 8.9) were placed in a 96-well microplate, followed by the addition of 20 μl of 2.5 mM DTNB [5,5′-dithiobis(2-nitrobenzoic acid) in 25 % methanol]. The absorbance was determined at 415 nm, and GSH concentration was calculated by comparison with the standard curve for GSH. Although referred to as GSHs, other minor non-protein reduced thiols are also measured through this method (Sedlak and Lindsay 1968).
Glutathione S-transferase (GST activity)
The 50 μl of supernatant (phosphate buffer for the blank) and reaction medium (100 μl, 1.5 mM GSH, 2.0 mM CDNB, 0.1 M potassium phosphate buffer, pH 6.5) were placed in a 96-well microplate. Absorbance increase was immediately measured at 340 nm at intervals of 12 s, and the molar extinction coefficient for CDNB of 9.6 mM−1 cm−1 was utilized to calculate the enzymatic activity (Keen et al. 1976).
Acetylcholinesterase (AChE activity)
Supernatant of the brain and muscle were diluted (1:10, or 10 % v/v) in 0.1 M phosphate buffer, pH 7.5. After dilution, 50 μl were placed in a 96-well microplate, followed by 200 μl of DTNB (5,5-dithio-bis-benzoate 2nitro) and 50 μl of ACT (acetylthiocholine iodide). Absorbance was immediately measured at 415 nm at intervals of 5 s by the method of Ellman et al. (1961) modified for microplate by Silva de Assis (1998).
Protein content
Total protein content was quantified according to Bradford (1976). Briefly, 10 μl of supernatants and 250 μl of Bradford reagent (BioRad®) were added to a 96-well microplate. Then, the absorbance was measured at 595 nm for protein determination through comparison with standard bovine serum albumin curve. All absorbances above were spectrophotometrically measured (SUNRISE-TECAN).
Histopathological biomarkers
Light microscopy
Liver samples were fixed in ALFAC solution (70 % ethanol, 4 % formaldehyde, 5 % glacial acetic acid) for 16 h, dehydrated in a graded series of ethanol, diaphanized in xylene, and embedded in Paraplast-Plus (Sigma®). Sections of 5 μm were obtained, stained with hematoxylin/eosin, and observed under light microscope for histopathological analyses according to Bernet et al. (1999).
Scanning electron microscopy (SEM)
The second left gill arch was fixed with 3 % glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4), dehydrated in a graded series of ethanol (Merck®), and with liquid CO2, then metalized with gold for further analysis in a scanning electron microscope (JEOL JSM-6360LV).
Chemical analysis
Polycyclic aromatic hydrocarbons (PAHs)
Bile samples were diluted (1:300) in 48 % methanol, and PAHs were detected through different excitation/emission wavelengths (288/330, 267/309, 334/376, 364/406, and 380/422) corresponding to the number of rings of each PAH (2, 3, 4, 5, and 6 rings, respectively) in spectrophotometer (SUNRISE-TECAN). For quantification, a PAH mix (Cod. 47930-U, SUPELCO) was utilized to establish a PAH standard curve.
Metal analysis
Solid samples were stored in 500-ml polyethylene bottles. Samples were stored in closed plastic bags and transported to the laboratory soon after the collection, in order to prevent mineralogical changes. Samples were air-dried and split for multi-element geochemical composition analysis by inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma atomic-emission spectrometry (ICP-AES). All samples were acid-digested following a two-step digestion method devised to retain volatile elements in coal dissolution. Then, the resulting solution was analyzed by ICP-AES for major and selected trace elements and by ICP-MS for most trace elements. The digestion of international reference materials (SARM 19) and blanks was prepared following the same procedure. Analytical errors were estimated at <3 % for most of the elements and around 10 % for Cd and Mo. Hg analyses were made directly on solid samples using a LECO AMA 254 gold amalgam atomic absorption spectrometer (Smith et al. 2000; Querol et al. 1997, 2008).
Statistical analysis
Categorical data biomarkers CA and histopathological index (light microscopy) were reported as median ± min and max values, while continuous data biomarkers (LPO, PCO, GSH, GST activity, AChE activity) and PAHs concentration were reported as the mean ± standard deviation of the mean. All data were checked for normality and homoscedasticity before performing one-way analysis of variance. Nonparametric Kruskal–Wallis one-way analysis, followed by Dunn’s post hoc test, with a level of significance of 0.05 was performed. All field data biomarkers were analyzed with a multivariated statistical approach. A cluster analysis (single linkage rule, Bray–Curtis similarity measure) of the different collection sites, on the basis of the average biomarker values for each collection site, was also conducted and followed by a non-metric multidimensional scaling (nMDS) analysis conducted on the matrix obtained. A visual representation of the patterns of similarity among collection sites was provided by Cluster and nMDS. VTG, SEM, and metal analysis were reported descriptively.
Biomarkers were analyzed, and the average of their results was utilized to build a similarity matrix (Bray–Curtis index) intended to perform the cluster analysis (single-linkage) (Fig. 2). The cluster analysis showed the total similarity between the responses of biomarkers for all sampling sites. In order to achieve greater relevance and correlate them with the impacting sources, the sites were then referred to according to their predominant economic activity or characteristic. BN was referred to as Agr (standing for agriculture); T and CB were grouped into a single area and referred to as Urb (standing for urban) due to high similarity in the cluster analysis, economic characteristics, and distance between points. L was referred to as Est (standing for estuary). From this new grouping, biomarkers data were compared again with nonparametric Kruskal–Wallis one-way analysis, followed by Dunn’s post hoc test, with a significance level of 0.05 (Figs. 3 and 4).
Genetic and biochemical biomarkers in G. brasiliensis from the Tubarão River. a Score of genetic damage in blood cells (Kruskal–Wallis = 48.00). b Lipid peroxidation, LPO (Kruskal–Wallis = 47.10). c Protein carbonylation, PCO (Kruskal–Wallis = 33.58). d Glutathione, GSH (Kruskal–Wallis = 20.61). e Glutathione S-transferase, GST activity (Kruskal–Wallis = 66.54). f Acetytlcholinesterase, AChE activity in brain (Kruska–Wallis = 6.09), and in muscle (Kruskal–Wallis = 31.14). Comparisons between the areas are represented by Latin letters (a, b) for the summer and by Greek letters (α, β) for the winter. Numbers (1, 2) were utilized for comparisons between seasons (summer versus winter) in the same sampling area. Agr agriculture area; Urb urban area; Est estuary area
Results
Seasonality was noticeably observed through physical parameters of temperature and rainfall (Table 1). The summer had high temperatures with low rainfall, which maintained the level of the river in normal conditions. In the winter, the low temperatures and the unusually high rainfall for this season made the river level rise and present one of the highest floods of its history. A total of 139 male and female fish were collected, 85 in the summer and 54 in the winter (Table 2).
From all the individuals collected in the Tubarão River, only those from Urb area showed vitellogenin expression (Fig. 3). It was observed that two females (20 %) and five males (50 %) presented the VTG in the plasma (Table 3). These individuals demonstrated a lower concentration of this protein when compared with individuals exposed to 10 mg EE2 (positive control).
DNA damage was higher in fish from all the areas sampled during the winter in comparison with the summer collection (Fig. 4a). Lipid peroxidation was lower in Est (summer and winter) when compared with Agr and Urb (summer and winter). No seasonal differences between the areas were observed (Fig. 4b). Protein carbonylation was higher in Urb (summer) in comparison with Est (summer) and higher in Urb (winter) when compared with Agr and Est (winter). There were no seasonal differences between the areas (Fig. 4c). Glutathione concentration was significantly higher in Est (summer) compared with Agr and Urb (summer). No seasonal differences were observed for this biomarker (Fig. 4d). GST activity was higher in Agr (summer) in comparison with Urb and Est (summer) and Agr (winter) (Fig. 4e).
AChE activity in the brain did not present any differences while its activity in the muscle was lower in Urb (winter) when compared with Est (winter). In regard to the seasonal comparison, its activity was lower in Est (summer) in comparison with Est (winter) (Fig. 4f).
The histopathological index of the liver (Table 4 and Fig. 5) was significantly higher in Urb and Est (summer) compared with Urb and Est (winter). Figure 5 shows the relevant histopathological alterations such as necrosis, inflammatory processes, neoplastic cells, and parasites (Fig. 6). Gills present the primary and secondary lamellas as described for teleosts. The secondary lamellas are laterally disposed on both sides of the primary lamella and have the surface area for gas exchange (Fig. 7a1). For both samplings, the Agr areas showed incidence of necrosis in the cells of the secondary lamella (Fig. 7a, b). Epithelial cells discharged from primary lamella surface were visible and common in the majority of fish (Fig. 7c, d). The Urb areas had large regions of cellular disarrangement of primary lamella followed by epithelial cells discharges (Fig. 8a, e). Hyperplasic regions were observed in primary lamella (Fig. 8b) as well as some pre-neoplastic-like lesions with tissue disarrangement (Fig. 8d). The secondary lamella of fish from this area presented large damaged regions (Fig. 8c), with the presence of parasites (Fig. 8f). Gills of fish from the Est areas presented regions of necrosis with cellular disarrangements in the primary lamella (Fig. 9b) and aspects of hyperplasic process in the secondary lamella with posterior lamellar fusions (Fig. 9d).
Histopathological index in the liver of G. brasiliensis (Kruskal–Wallis = 29.53). Comparisons between the areas are represented by Latin letters (a, b) for the summer and by Greek letters (α, β) for the winter. Numbers (1, 2) were utilized for comparisons between seasons (summer versus winter) in the same sampling area. Agr agriculture area; Urb urban area; Est estuary area
Histopathological findings in the liver of G. brasiliensis. a Normal aspect of the tissue; arrows show pancreatic tissues. In detail (a1), healthy hepatocytes are observed. b The arrows point a large necrosis area. In detail (b1), vacuolization within hepatocyte cytoplasms is observed. c Pre-neoplastic area (black arrow) surrounded by a leucocitary infiltration (white arrow). d Apicomplexa-type parasites. e Nematode-type parasites (white arrow) surrounded by a large melanomacrophage center (black arrow). f Acanthocephala-type parasite (black arrow) and a large area of pancreatic tissue damage (white arrows). Scale bar = 50 μm. Hematoxilin and eosin stains
Morphological alterations in the gills of G. brasiliensis from the Agr areas. a1 Normal aspect of gill, primary lamella (L1), and secondary lamella (L2). Scale bar = 20 μm. a Necrotic processes of epithelial cells (arrows). Scale bar = 100 μm. b Necrosis areas (arrows) of secondary lamellas without reposition of cells. Scale bar = 50 μm. c Microridges on epithelial cells surface with arrows showing cell discharges. Scale bar = 10 μm. d Area lacking in reposition (arrows) after cells discharges (arrowhead). Scale bar = 10 μm
Morphological alterations in the gills of G. brasiliensis of the Urb areas. a Area showing cellular disarrangement of primary lamella with epithelial cells discharges. Scale bar = 20 μm. b Observe areas of hyperplasia in the primary lamella. Scale bar = 20 μm. c Damaged areas of the secondary lamella. Scale bar = 20 μm. d A preneoplastic-like damage with disarrangement of the gill tissue. Scale bar = 50 μm. e Cellular death with cells discharges. Scale bar = 20 μm. f Fixation point of an ectoparasite in the secondary lamella. Scale bar = 20 μm
Morphological alterations in the gills of G. brasiliensis of the Est areas. a Normal aspect of gill, primary lamella (L1), and secondary lamella (L2). Scale bar = 100 μm. b Necrosis area (arrowhead) with cellular disarrangements (arrows). Scale bar = 20 μm. c Normal aspect of gill, primary lamella (L1), and secondary lamella (L2). Scale bar = 100 μm. d Areas of epithelial damage regarding hyperplastic processes and lamellar fusion. Scale bar = 20 μm
PAH determination (Table 5) showed that two-ringed PAH concentration was higher in Urb (summer) than Est (summer) and in Est (winter) than Urb (winter) and Est (summer). Three-ringed PAHs did not show differences between the areas or seasonal effects. Four- and five-ringed PAHs concentration was significantly higher in Est (winter) than Est (summer). Six-ringed PAHs were significantly higher in Urb and Est (winter) in comparison with Urb and Est (summer), respectively, and in Urb (winter) than Agr (winter).
Metals (As, Cd, Co, Cr, Cu, Hg, Mo, Ni, Pb, Se, V, Zn) in sediment did not reveal themselves to be of primordial importance since metals concentration was lower than or very close to the prevention values established by CETESB resolution nº 195-2005-E (Table 6) except for Se in the Urb area.
An nMDS ordination of data of these three areas was made (Fig. 10) obeying the same previous parameters. Areas grouped on the left-hand side of the ordination graph account for the summer and represent areas with higher histopathological damage and lower DNA damage, whereas the sites grouped on the right-hand side, regarding the winter, had higher DNA damage and lower histopathological index, corresponding to the biomarkers data observed.
Discussion
The current study investigated if the history of neglected environmental impact still appears in areas downstream Lauro Müller, since local biodiversity of small and medium streams can be considerably affected by coal mining activities (Gray and Delaney 2008; Ostrofsky and Schworm 2011). It also sought to observe if there would be some sort of decontamination gradient associated with the increased river flow toward the estuary, as well as the influence of seasonality over this gradient. From these assumptions, it is expected that the Agr areas are the most impacted, given that they have experienced a long period of mining activities right upstream, while both Urb and Est areas have a healthier status in relation to coal damage.
Endocrine disturbances were evaluated by the expression of vitellogenin in males of G. brasiliensis and were only observed in the Urb area. The dumping of municipal sewage without proper treatment in urbanized centers is common, increasing the variety of compounds available for the aquatic organisms as well as the potential of estrogenic activity. VTG expression in male fish represents strong evidence that this area has substances able to act as endocrine disrupters with estrogenic activity, such as the EE2. This compound is vastly produced by the pharmaceutical industry and induces VTG expression in fish (Henry et al. 2009; Strömqvist et al. 2010). Some studies show that VTG expression is dose-dependent (Pait and Nelson 2003; Moura Costa et al. 2010), and exposure to concentrations as low as 5 ng/l of EE2 in the water is still able to induce VTG expression (Allner et al. 1999; Rose et al. 2002). Even though VTG was not expressed in Agr and Est areas, we cannot state that those areas are free of such compounds.
In the summer, the low rainfall and high atmospheric temperature registered for the period contributed to lowering the depth of the river. In this season, the fish generally presented higher histopathological index (particularly due to parasitism and inflammatory processes) and lower concentrations of PAHs in bile. These data indicate that the contaminants, especially PAHs, may be present in the environment and bioavailable to fish exposure, despite being less concentrated in the organism in comparison with the winter collection.
Agr and Urb areas had similar responses for all biomarkers except for GST. GST activity was higher in Agr, which means increased cell demand for antioxidant or conjugation activities. The histopathological alterations and high GST activity can be partially explained by oxidative stress (supported by LPO and PCO responses) and the PAH exposure. Necroses were the most evident alteration, though leukocyte infiltration and macrophage grouping also have important implications. Inflammatory responses, in particular, those potentiated by macrophages recruitment and activation, can lead to extensive hepatic damage through oxidative burst (Forlenza et al. 2008) and release of proteases that damage membrane lipids, proteins, and DNA, ultimately leading to cell death by necrosis. This hypothesis is corroborated by the increase of LPO, which means damages in biological membranes, whereas the decrease of PCO leads to the activation of proteasome and autophagic processes which, in turn, remove irreversibly damaged proteins (Strahler et al. 2007). In addition, the bioactivation of PAHs by phase I enzymes, such as cytochrome P450, can lead to the generation of reactive metabolites and oxygen species that damage cell biomolecules (Fasulo et al. 2010; Wessel et al. 2010).
The Est area (summer) presented the lowest occurrence of necrosis, despite the presence of five- and six-ringed PAHs and other histopathological alterations, which shows that cell defense mechanisms such as GSH are being effective in avoiding cell death in the presence of such xenobiotics. In addition, it is possible that the constant dilution of contaminants found in the lagoons directly connected with the open sea has led to better health conditions of the fish in this area than the ones upstream.
The second part of the study was developed during the winter, with low atmospheric temperatures and an atypically rainy season, characterized by one of the biggest floods in the history of the Tubarão River. Fish collected during this season presented higher levels of biological impact, observed in the genetic biomarker responses as well as in the chemical analysis. The Agr area (winter) presented a misbalance of oxidative conditions in hepatic cells, and high numbers of macrophages centers were found. As previously stated, the increased incidence of DNA lesion may be due to mutagenic PAHs, particularly five- and six-ringed, or to the insufficient detoxifying activity of GST of the PAHs metabolites, associated with insufficient DNA repair.
The Urb area (winter) presented the worst health condition to G. brasiliensis. The three groups of biomolecules (lipids, proteins, and DNA) were affected by chemical stress, exceeding cell defense mechanisms, and organisms’ physiological accommodation capacity. Almroth et al. (2008) observed similar responses in rainbow trout exposed to sewage with high levels of PAHs. The particularly important high PCO, compared with the other sites in the winter, indicated the accumulation of degradation-resistant protein aggregates, whereas the high DNA damage may be related to the high exposure of five- and six-ringed PAHs associated with unaltered GSH concentration. Yet, the presence of large necrotic areas and high incidence of macrophage centers and parasites reinforce the high potential of aquatic environmental degradation by urban and industrial discharges (Violante-Gonzalez et al. 2007; Takemoto et al. 2009; Ryan 2010; Barugahare et al. 2011).
Unexpectedly, Est area (winter) presented the highest concentrations of PAHs in fish, combined with unaltered histopathological index, LPO, and GST. It is possible that the cells’ defenses mechanisms such as GSH might be protecting membrane lipids from chemical stress, but PAH bioactivation continued to damage DNA (Calliani et al. 2009). Also, the accumulation of PAHs in fish from this area could be explained by the increase in the river flow recurrent from the high rainfall of the season. The rain could be acting both as a leaching agent, carrying PAHs from the soil to the water, and as a mobilizing agent, increasing the river flow which, in turn, mobilizes the bottom sediment of the river, bringing the PAHs back to the flowing water, and making them bioavailable to re-enter the food chain.
Concerning the neurotoxic parameters, the activity of AChE in the muscle is generally higher than in the brain (Üner et al. 2006; Golombieski et al. 2008; Modesto and Martinez 2010; Rodrigues et al. 2012), and according to Durieux et al. (2010), the AChE activity is usually higher in hotter than in colder waters. The maintenance of the cerebral activity of AChE during both seasons and the decrease observed in the muscle during the summer collection, especially in the Est area, may be an indicative of pollutants effects on this tissue. Presumably, some of these pollutants are unable to reach the brain tissue due to the blood–brain barrier (Adedeji 2011; Eliceiri et al. 2011).
The gills are organs involved in key parameters for a good development and health of the fish, such as gas exchange, acid/base balance, osmoregulation processes, excretion of nitrogenous compounds, and sensorial function. Alterations in these structures can cause imbalances in vital functions, compromising the survival and development of the organism (Hughes 1984; Evans 1987). Fish from all studied areas presented lesions or alterations in gills, which shows that this tissue is a primary target of water contamination. Hyperplasia can cause disarrangements of secondary lamellae and lead to lamellar fusions which hamper gas exchange, while necrosis and neoplastic processes jeopardize most of the functions of the organ (Nero et al. 2006; Oliveia Ribeiro et al. 2000, 2005). The presence of parasites may be related to an inefficient immune defense and contributes to the impairment of gill function. These findings are an important indicative of severe disturbances in fish from the Tubarão River and seem to have correlation with acute and chronic intoxication (Rabitto et al. 2011; Brito et al. 2012; Silva et al. 2011a).
Conclusions
According to the biomarker responses, the three areas studied in the Tubarão River are impacted by chemicals in concentrations/associations enough to affect health conditions of G. brasiliensis. However, it does not seem to be related with the historical coal mining activity or other specific factor of the region. Future studies considering different conditions of exposure such as in situ exposure of caged fish would be of great value to understand the real impact of human activities in the river.
Considering the hypothesis of the pollution gradient, the results show that the Agr and Urb areas present similar conditions of impacts. The consequences of the high influx of untreated urban and industrial effluents must be better investigated, mainly because the Urb area was the only one to present the occurrence of endocrine disruptor agents. Notably, adaptative processes seemed to buffer biomarkers responses in the fish from the Est areas. In conclusion, punctual influx of contaminants in all three areas studied might be responsible for the absence of a pollution gradient.
In regard to seasonality, the winter season could be responsible for a higher input of PAHs in the atmosphere, since it is a colder period, and thus, a larger amount of energy is spent in house heating and lighting. In order to obtain this energy, the thermoelectric power plant in the Urb area might be consuming more coal to produce additional energy, and, in consequence, more PAHs are generated from its burning. From this high influx of PAHs, the heavy rain in this period could help these contaminants to enter the aquatic system, which explains why their concentration is higher in the Urb and Est areas (downstream the power plant) but not in the Agr area (upstream).
References
Adedeji OB (2011) Response of acetylcholinesterase activity in the brain of Clarias gariepinus to sublethal concentration of diazinon. J Appl Sci Environ Sanit 6:137–141
Allner B, Wegener G, Knacker T, Stahlschmidt-Allner P (1999) Electrophoretic determination of estrogen-induced protein in fish exposed to synthetic and naturally occurring chemicals. Sci Total Environ 233:21–31
Almroth BC, Albertsson E, Sturve J, Förlin L (2008) Oxidative stress, evident in antioxidant defences and damage products, in rainbow trout caged outside a sewage treatment plant. Ecotoxicol Environ Saf 70:370–378
Almroth BC, Johansson A, Förlin L, Sturve J (2010) Early-age changes in oxidative stress in brown trout, Salmo trutta. Comp Biochem Physiol B 155:442–448
Amaral E (1998) Feasibility study on the recovery of mined areas in southern Santa Catarina, JICA report, Secretaria de Estado do Desenvolvimento Urbano e Meio Ambiente-SDM, Florianópolis (in Portuguese)
Barugahare R, Dennis M, Becker JA, Slapeta J (2011) Detection of Cryptosporidium molnari oocysts from fish by fluorescent-antibody staining assays for Cryptosporidium spp. affecting humans. Appl Environ Microbiol 77:1878–1880
Bellassoued K, Hamza A, Van Pelt J, Elfeki A (2012) Seasonal variation of Sarpa salpa fish toxicity, as related to phytoplankton consumption, accumulation of heavy metals, lipids peroxidation level in fish tissues and toxicity upon mice. Environ Monit Assess. doi:10.1007/s10661-012-2621-1
Benincá C, Ramsdorf W, Vicari T, Oliveira Ribeiro CA, Almeida MI, Silva de Assis HC, Cestari MM (2011) Chronic genetic damages in Geophagus brasiliensis exposed to anthropic impact in estuarine lakes at Santa Catarina Coast–Southern of Brazil. Environ Monit Assess 184:2045
Bernet D, Schimidt H, Meier W, Burkhard-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34
Bortoluzzi, IP (2009) Project of protection and recovery of springs, Technical Report, Comitê de Gerenciamento da Bacia Hidrográfica do Rio Tubarão e Complexo Lagunar, Tubarão (In Portuguese)
Braço do Norte City Hall (2012) http://www.bracodonorte.sc.gov.br/ (in Portuguese; last accessed in 08/01/2012)
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brito IA, Freire CA, Yamamoto FY, Silva de Assis HC, Souza-Bastos LR, Cestari MM, Ghisi NC, Prodocimo V, Filipak Neto F, Oliveira Ribeiro CA (2012) Monitoring water quality in reservoirs for human supply through multi-biomarker evaluation in tropical fish. J Environ Monit 14:615–625
Calliani I, Porcelloni S, Mori G, Frenzilli G, Ferraro M, Marsili L, Casini S, Fossi MC (2009) Genotoxic effects of produced waters in mosquito fish (Gambusia affinis). Ecotoxicology 18:75–80
Capivari de Baixo city hall (2012) http://www.capivaridebaixo.sc.gov.br/ (In Portuguese; last accessed in 08/01/2012)
Chovanec A, Hofer R, Schiemer F (2003) Fish as bioindicators. In: Markert BA, Breure AM, Zechmeister HG (eds) Bioindicators and biomonitors: principles, concepts and applications. Elsevier, Amsterdam, pp 639–675
Clemente Z, Busato RH, Oliveira Ribeiro CA, Cestari MM, Ramsdorf WA, Magalhães VF, Wosiack AC, Silva de Assis HC (2010) Analyses of paralytic shellfish toxins and biomarkers in a southern Brazilian reservoir. Toxicon 55:396–406
Companhia de Tecnologia de Saneamento Ambiental, CETESB. Decisão da Diretoria Nº 195-2005- E, de 23 de novembro de 2005. São Paulo. Brazil (in Portuguese)
Dogan D, Can C, Kocyigit A, Dikilitas M, Taskin A, Bilinc H (2011) Dimethoate-induced oxidative stress and DNA damage in Oncorhynchus mykiss. Chemosphere 84:39–46
Durieux EDH, Farver TB, Fitzgerald PS, Eder KJ, Ostrach DJ (2010) Natural factors to consider when using acetylcholinesterase activity as neurotoxicity biomarker in Young-of-Year striped bass (Morone saxatilis). Fish Physiol Biochem 37:21–29
Eliceiri BP, Gonzalez AM, Baird A (2011) Zebrafish model of the blood–brain barrier: morphological and permeability studies. Meth Mol Biol 686:371–378
Ellman GL, Courtney KD, Andreas VJ, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88
Evans DH (1987) The fish gill: site of action and model for toxic effects of environmental pollutants. Environ Heal Perspect 71:47–58
Fasulo S, Marino S, Mauceri A, Maisano M, Giannetto A, D’Agata A, Parrino V, Minutoli R, De Domenico E (2010) A multibiomarker approach in Coris julis living in a natural environment. Ecotoxicol Environ Saf 73:1565–1573
Ferraro MVM, Fenocchio AS, Mantovani MS, Oliveira Ribeiro CA, Cestari MM (2004) Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests. Genet Mol Biol 27:103–107
Ferreira M, Moradas-Ferreira P, Reis-Henrique MA (2005) Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat Toxicol 71:39–48
Filipak Neto F, Zanatta SM, Silva de Assis HC, Bussolaro D, Ferraro MVM, Randi MAF, Alves Costa JRM, Cestari MM, Roche H, Oliveira Ribeiro CA (2007) Use of hepatocytes from Hoplias malabaricus to characterize the toxicity of a complex mixture of lipophilic halogenated compounds. Toxicol Vitr 21:706–715
Forlenza M, Scharsack JP, Kachamakova NM, Taverne-Thiele AJ, Rombout JHWM, Wiefertjes GF (2008) Differential contribution of neutrophilic granulocytes and macrophages to nitrosative stress in a host–parasite animal model. Mol Immunol 45:3178–3189
Golombieski JI, Marchesan E, Camargo ER, Salbego J, Baumart JS, Loro VL, Machado LSO, Zanella R, Baldisserotto B (2008) Acetylcholinesterase enzyme activity in carp brain and muscle after acute exposure to diafuran. Sci Agric 65:340–345
Gray NF, Delaney E (2008) Comparison of benthic macroinvertebrate indices for the assessment of the impact of acid mine drainage on an Irish river below an abandoned Cu-S mine. Environ Pollut 155:31–40
Guilherme S, Válega M, Pereira ME, Santos MA, Pacheco M (2008) Antioxidant and biotransformation responses in Liza aurata under environmental mercury exposure—relationship with mercury accumulation and implications for public health. Mar Pollut Bull 56:845–859
Hellou J, Ross NW, Moon TW (2012) Glutathione, glutathione S-transferase, and glutathione conjugates, complementary markers of oxidative stress in aquatic biota. Environ Sci Pollut Res 19:2007–2023
Henry TB, Mcpherson JT, Rogers ED, Heah TP, Hawkins SA, Layton AC, Sayler GS (2009) Changes in the relative expression pattern of multiple vitellogenin genes in adult male and larval zebrafish exposed to exogenous estrogens. Comp Biochem Physiol Physiol 154:119–126
Hiramatsu N, Cheek AO, Sullivan CV, Matsubara T, Hara A (2005) Vitellogenesis and endocrine disruption. BiochemMol Biol Fish 6:431–471
Hughes GM (1984) General anatomy of the gills. Fish Physiol, Part A 10:1–72
Instituto Ambiental do Paraná, IAP (2005) Monitoring of the water quality of rivers of the metropolitan region of Curitiba in the period of 1992 to 2005. Curitiba, Brasil (in Portuguese)
Jiang ZY, Hunt JV, Wolf SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389
Katsumiti A, Valdez Domingos FX, Azevedo M, Da Silva MD, Damian RC, Almeida MIM, Silva de Assis HC, Cestari MM, Randi MAF, Oliveira Ribeiro CA, Freire CA (2009) An assessment of acute biomarker responses in the demersal catfish Cathorops spixii after the Vicuña oil spill in a harbour estuarine area in Southern Brazil. Environ Monit Assess 152:209–222
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S-transferases. J Biol Chem 251:6183–6188
Laguna City Hall (2012) http://www.laguna.sc.gov.br/ (In Portuguese; last accessed in 08/01/2012)
Lauro Müller City Hall (2012) http://www.lauromuller.sc.gov.br/ (In Portuguese; last accessed in 08/01/2012)
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidativelymodified proteins. Methods Enzymol 186:464–478
Liebel S, Oliveira Ribeiro CA, Silva RC, Ramsdorf WA, Cestari MM, Magalhães VF, Garcia JRE, Esquivel BM, Filipak Neto F (2011) Cellular responses of Prochilodus lineatus hepatocytes after cylindrospermopsin exposure. Toxicol Vitr 25:1493–1500
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev Med Vet 154:427–430
Madi RR, Ueta MT (2009) The role of Ancyrocephalinae (Monogenea: Dactylogyridae), parasite of Geophagus brasiliensis (Pisces: Cichlidae), as an environmental indicator. Rev Bras Parasitol Vet 18:38–41
Martinez-Tabche L, Ramírez BM, German-Faz C, Galar CI, Madrigal OM, Ulloa GV, Orozco FM (1997) Toxic effect of sodium dodecilbencensulphonate, lead, petroleum and their mixtures on the activity of acetylcholinesterase of Moina macrocopa in vitro. Environ Toxicol 12:1–5
Matozzo V, Gagné F, Marin MG, Ricciardi F, Blaise C (2008) Vitellogenin as a biomarker of exposure to estrogenic compounds in aquatic invertebrates: a review. Environ Int 34:531–545
Mazzoni R, Da Costa LDS (2007) Feeding ecology of stream-dwelling fishes from a coastal. Braz Arch Biol Tech 50:627–635
Miranda AL, Roche H, Randi MAF, Menezes ML, Oliveira Ribeiro CA (2008) Bioaccumulation of chlorinated pesticides and PCBs in the tropical freshwater fish Hoplias malabaricus: histopathological, physiological, and immunological findings. Environ Int 34:939–949
Modesto KA, Martinez CBR (2010) Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299
Moura Costa DD, Neto FF, Costa MDM, Morais RN, Garcia JRE, Esquivel BM, Oliveira Ribeiro CA (2010) Vitellogenesis and other physiological responses induced by 17-β-estradiol in males of freshwater fish Rhamdia quelen. Comp Biochem Physiol C 151:249–257
Nero V, Farwell A, Lister A, Van Der Kraak G, Lee LEJ, Van Meer T, MacKinnone MD, Dixon DG (2006) Gill and liver histopathological changes in yellow perch (Perca flavescens) and goldfish (Carassius auratus) exposed to oil sands process-affected water. Ecotoxicol Environ Saf 63:365–377
Nogueira L, Rodrigues ACF, Trídico CP, Fossa CE, Almeida EA (2011) Oxidative stress in Nile tilapia (Oreochromis niloticus) and armored catfish (Pterygoplichthys anisitsi) exposed to diesel oil. Environ Monit Assess 180:243–255
Oliveia Ribeiro CA, Pelletier E, Pfeiffer WC, Rouleau C (2000) Comparative uptake, bioaccumulation, and gill damages of inorganic mercury in tropical and Nordic freshwater fish. Environ Res 83:286–292
Oliveira Ribeiro CA, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat Toxicol 74:53–69
Ostrofsky ML, Schworm AE (2011) A history of acid mine contamination, recovery, and eutrophication in Sandy Lake, Pennsylvania. J Paleolimnol 46:229–242
Pait AS, Nelson JO (2003) Vitellogenesis in male Fundulus heteroclitus (killifish) induced by selected estrogenic compounds. Aquat Toxicol 64:331–342
Paraguassu AR, Alves DR, Luque JL (2005) Metazoan parasites of the acará, Geophagus brasiliensis (Quoy & Gaimard, 1824) (Osteichthyes: Cichlidae) from the Lajes reservoir, Rio de Janeiro. Rev Bras Parasitol Vet 14:35–39 (In portuguese)
Payne JF, Mathieu A, Melvin W, Fancey LL (1996) Acetylcholinesterase, and old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundland. Mar Pollut Bull 32:225–230
Powers DA (1989) Fish as model systems. Science 246:352–358
Querol X, Whateley MKG, Fernandez-Turiel JL, Tuncali E (1997) Geological controls on the mineralogy and geochemistry of the Beypazari lignite, central Anatolia, Turkey. Int J Coal Geol 33:255–271
Querol X, Izquierdo M, Monfort E, Alvarez E, Moreno T, Alastuey A, Zhuang X, Lud W, Wang Y (2008) Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int J Coal Geol 75:93–104
Quinlan GJ, Gutteridge JMC (2000) Carbonyl assay for oxidative damage to proteins. In: Experimental protocols for reactive oxygen and nitrogen species, Oxford University Press, New York, 257–258
Rabitto I, Alves Costa JRM, Akaishi FM, Silva de Assis HC, Pelletier E, Oliveira Ribeiro CA (2005) Effects of dietary Pb(II) and tributyltin on neotropical fish, Hoplias malabaricus: histopathological and biochemical findings. Ecotoxicol Environ Saf 60:147–156
Rabitto IS, Bastos WR, Almeida R, Adilson A, Holanda IBB, Galvão RCF, Filipak Neto F, Menezes ML, Santos CAM, Oliveira Ribeiro CA (2011) Mercury and DDT exposure risk to fish-eating human populations in Amazon. Environ Int 37:56–65
Ramsdorf WA, Guimarães FSF, Ferraro MVM, Gabardo J, Trindade ES, Cestari MM (2009) Establishment of experimental conditions for preserving samples of fish blood for analysis with both comet assay and flow cytometry. Mutat Res 673:78–81
Rodrigues S, Antunes SC, Brandão FP, Castro BB, Gonçalves F, Nunes B (2012) Effects of anticholinesterase drugs on biomarkers and behavior of pumpkinseed, Lepomis gibbosus (Linnaeus, 1758). J Environ Monit 14:1638–1644
Romão S, Donatti L, Freitas MO, Teixeira J, Kusma J (2006) Blood parameter analysis and morphological alterations as biomarkers on the health of Hoplias malabaricus and Geophagus brasiliensis. Braz Arch Biol Tech 49:441–448
Rose J, Holbech H, Lindholst C, Norum U, Korsgaard B, Bjerregaard P (2002) Vitellogenin induction by 17beta-estradiol and 17alpha-ethinylestradiol in male zebrafish (Danio rerio). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 131:531–539
Ryan U (2010) Cryptosporidium in birds, fish and amphibians. Exp Parasitol 124:113–120
Sedlak J, Lindsay R (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Silva de Assis HC (1998) The use of biomarkers for the detection of total water pollution. Thesis, Berlin Technical University, Germany, pp. 99 (in German)
Silva LFO, Oliveira MLS, da Boit KM, Finkelman RB (2009) Characterization of Santa Catarina (Brazil) coal with respect to human health and environmental concerns. Environ Geochem Heal 31:475–485
Silva GS, Filipak Neto F, Silva de Assis HC, Bastos WR, Oliveira Ribeiro CA (2011a) Potential risks of natural mercury levels to wild predator fish in an Amazon reservoir. Environ Monit Assess 8:4815–4827
Silva LFO, Querol X, da Boit KM, Vallejuelo SFO, Madariaga JM (2011b) Brazilian coal mining residues and sulphide oxidation by Fenton s reaction: an accelerated weathering procedure to evaluate possible environmental impact. J Hazard Mater 186:516–525
Silva LFO, Wollenschlager M, Oliveira MLS (2011c) A preliminary study of coal mining drainage and environmental health in the Santa Catarina region, Brazil. Environ Geochem Heal 33:55–65
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Smith KS; Ramsey CA; Hageman PL (2000) Sampling strategy for the rapid screening of mine-waste dumps on abandoned mine lands. In: ICARD 2000, Proc 5th Int Conf Acid Rock Drainage, Society for Mining, Metallurgy, and Exploration, Littleton, CO, 1:1453–1461
Strahler J, Philipp E, Brey T, Broeg K, Abele D (2007) Physiological aging in the Icelandic population of the ocean quahog Arctica islandica. Aquat Biol 1:77–83
Strömqvist M, Tooke N, Brunström B (2010) DNA methylation levels in the 5-flanking region of the vitellogenin I gene in liver and brain of adult zebrafish (Danio rerio)-Sex and tissue differences and effects of 17α-ethinylestradiol exposure. Aquat Toxicol 98:275–281
Sturm A, Silva de Assis HC, Hansen D (1999) Cholinesterases of marine teleost fish: enzymological characterization and potential use in the monitoring of neurotoxic contamination. Mar Environ Res 47:389–398
Sun Y, Yu H, Zhang J, Yin Y, Shi H, Wang X (2006) Bioaccumulation, depuration and oxidative stress in fish (Carassius auratus) under phenanthrene exposure. Chemosphere 63:1319–1327
Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Moreira LHA, Ceschini TL, Bellay S (2009) Diversity of parasites of fish from the upper Paraná river floodplain, Brazil. Braz J Biol 69:691–705
Tubarão City Hall (2012) http://www.tubarao.sc.gov.br/ (in Portuguese; last accessed in 08/01/2012).
Üner N, Oruç EF, Sevgiler Y, Sahin N, Durmaz H, Usta D (2006) Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol 21:241–245
United Nations, UN (2012) Department of Economic and Social Affairs, Population Division. http://www.un.org/esa/population (last accessed in 08/01/2012)
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Violante-Gonzalez J, Aguirre-Macedo ML, Mendonza-Franco EF (2007) A checklist of metazoan parasites of fish from Tres Palos Lagoon, Guerrero, Mexico. Parasitol Res 102:151–161
Wessel N, Santos R, Menard D, Le Menach K, Buchet V, Lebayon N, Loizeau V, Burgeot T, Budzinski H, Akcha F (2010) Relationship between PAH biotransformation as measured by biliary metabolites and EROD activity, and genotoxicity in juveniles of sole (Solea solea). Mar Environ Res 69:71–73
Wilhelm Filho D, Torres MA, Tribess TB, Pedrosa RC, Soares CHL (2001) Influence of season and pollution on the antioxidant defenses of the cichlid fish acará (Geophagus brasiliensis). Braz J Med Biol Res 34:719–726
Acknowledgments
This study was approved by the Ethical Council of the Universidade Federal do Parana, and the authors gratefully acknowledge the Catarinense Institute of Environmental Research and Human Development (IPADHC) for all their financial, logistic, and technical support assistance; the Information Center for Hydrometeorology and Environmental Resources of Santa Catarina (EPAGRI/CIRAM) for the meteorological data; and the Electron Microscopy Center of Federal University of Parana (CME) for their technical assistance. Also, the authors thank all researchers, students, and citizens who helped in the collections of the specimens and material analysis, especially Mr. Jorge from Capivari de Baixo and Mr. Daniel Pamplona from Curitiba who were of prime importance for this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Osório, F.H.T., Silva, L.F.O., Piancini, L.D.S. et al. Water quality assessment of the Tubarão River through chemical analysis and biomarkers in the Neotropical fish Geophagus brasiliensis . Environ Sci Pollut Res 21, 9145–9160 (2014). https://doi.org/10.1007/s11356-013-1512-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1512-5