Abstract
Although the long incubation time of biochemical oxygen demand (BOD7) measurements has been addressed by the use of microbial biosensors, the resulting sensor-BOD values gained from the measurements with specific industrial wastewaters still underestimates the BOD value of such samples. This research aims to provide fast and more accurate BOD measurements in the dairy wastewater samples. Unlike municipal wastewater, wastewater from the dairy industry contains many substrates that are not easily accessible to a majority of microorganisms. Therefore, a bacterial culture, Microbacterium phyllosphaerae, isolated from dairy wastewater was used to construct a semi-specific microbial biosensor. A universal microbial biosensor based on Pseudomonas fluorescens, which has a wide substrate spectrum but is nonspecific to dairy wastewater, was used as a comparison. BOD biosensors were calibrated with OECD synthetic wastewater, and experiments with different synthetic and actual wastewater samples were carried out. Results show that the semi-specific M. phyllosphaerae-based microbial biosensor is more sensitive towards wastewaters that contain milk derivates and butter whey than the P. fluorescens-based biosensor. Although the M. phyllosphaerae biosensor underestimates the BOD7 value of actual dairy wastewaters by 25–32 %, this bacterial culture is more suitable for BOD monitoring in dairy wastewater than P. fluorescens, which underestimated the same samples by 46–61 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dairy industry produces typically 1–2 m3 of wastewater per metric ton of milk processed (Omil et al. 2003; Vourch et al. 2008). The main contributors of organic load to these wastewaters are carbohydrates (i.e., lactose), proteins (i.e., casein), and lipids (i.e., fatty substances) originating from the milk. Carbohydrates (mainly lactose) are easily degradable, whereas proteins and lipids are more difficult to biodegrade (Kasapgil et al. 1994; Perle et al. 1995; Thassitou and Arvanitoyannis 2001). Appropriately adapted microorganisms are required to biodegrade these substances (Fang and Yu 2000; Janczukowicz et al. 2008).
Dairy effluent is an important issue in terms of organic load in wastewater treatment plants and as a potential source of environmental pollution (Perle et al. 1995). Therefore, it is necessary to determine the content of organic pollution in the wastewater going into treatment plants and to monitor the contaminants in the effluents before final discharge into the environment (Olaniran et al. 2008).
Biochemical oxygen demand (BOD) is most widely used for monitoring the organic pollution in wastewater (Tan and Lim 2005). According to the American or Swedish standards, the BOD test takes 5 or 7 days, respectively, to gain results (APHA 1985; SIS 1979). Therefore, this method is not suitable for in situ monitoring and online control of wastewater treatment systems. An alternative method based on microbial biosensors can be used for BOD measurements. A number of reports and reviews which focused on different microbial BOD sensors have been published (D’Souza 2001; Dhall et al. 2008; Kara et al. 2009; Kim and Park 2001; Rastogi et al. 2003; Sakaguchi et al. 2003; Su et al. 2011). Microbial biosensors have several advantages: they are cheap, give fast response, and are not labor-intensive. Nonetheless, the current BOD biosensors still have limitations including lifetime, stability, and accuracy (D'Souza 2001; Karube and Nakanishi 1994; Mello and Kubota 2002; Xu and Ying 2011). Su and coworkers (Su et al. 2011) reported that biosensors also suffer from poor selectivity because of the nonspecific cellular response to substrates. Therefore, a key component for constructing the microbial BOD sensor is the selection of microorganisms (Reshetilov 2005).
Since the composition of different industrial wastewaters often varies greatly, the universal microbial sensors are not suitable for the analysis of these specific wastewaters. Universal microorganisms used in biosensors are defined as microorganisms that use a limited substrate spectrum common to a majority of aerobic heterotrophic microorganisms. On the other hand, semi-specific microbial biosensors utilize microorganisms that, in addition to the universal substrate spectrum, can degrade some refractory compound or group of compounds specific to that particular microorganism. Thus, semi-specific biosensors allow us to detect refractory compounds that are found in industrial wastewaters, which would be undetected by universal biosensors (Raud et al. 2010; Raud et al. 2012a, b; Raudkivi et al. 2008). Therefore, it is advantageous to use semi-specific microbial BOD biosensors to determine the BOD in specific industrial wastewaters.
In this study, several bacterial cultures were isolated from the dairy wastewater of the Valio Eesti AS Laeva dairy plant (Estonia) in order to obtain the bacterial culture with semi-specific properties towards the compounds found in dairy wastewaters. The bacterial culture, Microbacterium phyllosphaerae, was finally selected for semi-specific biosensor construction because it has the best sensitivity towards lactose and other milk constituents. Comparison measurements were conducted with the nonspecific Pseudomonas fluorescens-based biosensor.
Materials and methods
Isolation, cultivation, and identification of microorganism
A sample of dairy wastewater was collected from the Valio Eesti AS Laeva dairy plant (Estonia) in order to isolate semi-specific microorganisms which are able to degrade lactose and other milk constituents. Using serial dilutions, the sample was spread on the milk (30 g L−1 agar, 500 mg L−1 milk) and lactose (15 g L−1 lactose, 5 g L−1 agar, 100 mL L−1 M9 10× salt solution, 2,500 μL L−1 vitamins) containing selective solid medium plates. The plates were incubated in room temperature (22 ± 1 °C) for a few days until colonies developed. Chosen colonies were indexed and simultaneously restreaked on fresh milk and lactose selective plates. The colonies that grew on both types of selective plates within 3 days were chosen and restreaked. This procedure was repeated until pure bacterial strains were gained. Finally, the isolated culture indexed as 17.3 was chosen based on rapid growth rate and better immobilization characteristics. The culture was identified as M. phyllosphaerae by 16S rDNA sequence comparison. The cells of the nonspecific bacterial culture of P. fluorescens were used to bring out the advantages of the semi-specific biosensor (Raud et al. 2010; Raud et al. 2012a, b; Raudkivi et al. 2008). The bacterial culture was isolated, identified, and cultivated in the Institute of Technology of the University of Tartu.
Cultivation
Both bacterial cultures were cultivated under aerobic conditions in a rotating shaker for 18 h at 30 °C and at 210 rpm in 3 mL of Luria–Bertani (LB) broth (10 g L−1 tryptone, 5 g L−1 yeast extract, and 5 g L−1 NaCl). The gained bacterial suspension was used to inoculate 150 mL of the LB broth. The broths, inoculated with M. phyllosphaerae and P. fluorescens, were cultivated under the same conditions up to 14 and 8 h, respectively. The optical densities of the microbial suspensions were measured with a spectrophotometer (HP 4853) at 600 nm to obtain the late exponential growth phase of the cells (M. phyllosphaerae OD600 = 3.7; P. fluorescens OD600 = 3.8). The cells were harvested by centrifugation at 4,000 rpm for 20 min and washed twice with 10 mL of phosphate buffer (7.04 g L−1 NaH2PO4 × H2O and 6.0 g L−1 Na2HPO4; pH = 6.86).
Immobilization and preconditioning
The cells of M. phyllosphaerae or P. fluorescens were immobilized in an agarose gel matrix to form microbial membranes. The mixture of 7.5 mL of phosphate buffer and 180 mg of agarose (type I-A, low EEO, Sigma, Germany) was heated to 80 °C to completely dissolve agarose. The homogenous agarose solution was cooled to 50 °C to minimize the heat shock for the introduced bacteria. Then, 800 μl of the previously prepared bacterial suspension was added to the solution. After stirring, the suspension was cast onto the polypropylene net discs (Scrynel, PP 500 HD) and allowed to cool in ambient temperature until the solid gel was formed. The concentration of bacteria in the gel matrix was kept constant in order to have biomembranes within a comparable linear working range.
The resulting microbial membranes were preconditioned by placing them into the phosphate buffer solution dosed with the Organization for Economic Cooperation and Development (OECD) synthetic wastewater solution with a BOD7 of 30 mg L−1 for 2 weeks. According to the recipe, the OECD synthetic wastewater contained 1.6 g L−1 peptone, 1.1 g L−1 meat extract, 0.30 g L−1 carbamide, 0.28 g L−1 K2PO4, 0.07 g L−1 NaCl, 0.04 g L−1 CaCl2 × 2H2O, and 0.02 g L−1 MgSO2 × 7H2O (OECD 1984). During the preconditioning and experimental period, the membranes containing the immobilized bacteria were stored in phosphate buffer solution at 4 °C.
Experimental procedure
The biosensor was constructed according to the description in our previous paper (Raud et al. 2010). The Clark-type oxygen sensor CellOx 325 (WTW, Germany) was used to construct the biosensor. The microbial membrane was attached to the oxygen sensor with a special collar and insertion ring. The signal was detected and transformed using the InoLab 740 converter (WTW, Germany) and Multilab Pilot program. The experiments were carried out in phosphate buffer solution at room temperature. An aerator unit and magnetic stirrer were used to ensure the oxygen saturation and homogeneity of the measurement solution.
Constructed BOD biosensors were calibrated using the OECD synthetic wastewater with 2,000 mg L−1 of BOD7 as a standard solution. The steady-state method was used to evaluate the signal changes resulting from the changes in the BOD7 of the measurement solution. The biosensor was inserted into an aerated phosphate buffer solution, and after the initial steady-state signal (S 0) was achieved, a certain amount of the sample was inserted into the measurement solution. After the addition of substrate, the immobilized microorganisms started to degrade the organic compounds. Therefore, the consumption of oxygen increased, and oxygen measured with the dissolved oxygen sensor decreased. The sensor signal decreased until a new steady state (S t) was achieved. The substrate was inserted into the measurement solution using a step-by-step adding method in order to change the BOD7 of the measurement solution gradually with each addition. BOD7 of the measurement solution was elevated until the output from the oxygen sensor was zero or did not change after the addition of the substrate into the measurement solution (Raud et al. 2012a). The output signal of the oxygen sensor at a certain BOD value was normalized according to Eq. 1:
where S N is the normalized signal, S 0 is the stabilized signal at BOD7 = 0 mg L−1, and S t is the stabilized signal at a certain BOD value. The normalized output signal was plotted against the BOD7 of the solution, and the resulting calibration curve was used to calculate the sensor-BOD values of the sample solutions. The obtained results were plotted against the results of the respective BOD7 analysis (sensor-BOD vs. BOD7), and the accuracy of the BOD biosensors were evaluated. All measurements were carried out with six parallel microbial membranes for both bacterial cultures.
Wastewater samples
Measurements with different synthetic and actual wastewater samples were carried out to evaluate the suitability of the semi-specific BOD biosensor for the analysis of dairy wastewater. Synthetic wastewater samples with a composition similar to industrial wastewaters were based on the OECD synthetic wastewater to which different refractory components were added. To gain a synthetic wastewater sample similar to the wastewater from a dairy industry (sample A), 1 mL of sterilized milk was added to 1 L of OECD synthetic wastewater. Experiments were also carried out with OECD synthetic wastewater dosed with different additives (0.5 mL milk, 0.5 g cellulose, and 0.084 g phenol to 1 L of standard solution; sample B) to mimic mixed industrial wastewaters. In this case, half of the OECD wastewater components described in the previous paragraph were used. The concentrations of additional compounds were chosen in a way that each would make up for 20 % of the BOD7 of the OECD synthetic wastewater. As the conventional BOD7 analyses could not determine the BOD7 value of the cellulose solution (Raud et al. 2012b), the cellulose part in the solution was accounted by half of the chemical oxygen demand (COD) value. The COD was determined by Dr. Lange COD test (Cuvette Test LCK114, Dr. Bruno Lange GmbH, Düsseldorf, Germany). The measurements were also performed with two wastewater samples from the Valio Eesti AS Laeva dairy plant (samples C and D). Before the measurements the samples were processed in an ultrasonic bath for sterilization. Conventional BOD7 analysis of the above mentioned solutions was performed according to the APHA method in triplicate (APHA 1985).
Results and discussion
Calibration of the BOD biosensors
The calibrations of both biosensors were carried out with OECD synthetic wastewater. Although the glucose–glutamic acid solution has been widely used as a reference for the conventional BOD analysis, using OECD synthetic wastewater for calibrating the BOD biosensors has shown better agreement between the conventional BOD analysis and biosensor BOD measurements (Liu and Mattiasson 2002; Tanaka et al. 1994). The calibration curve for a M. phyllosphaerae-based biosensor was calculated on an average of nine parallel measurements (standard deviation (SD) 6.3 %), and for the P. fluorescens-based biosensor, the calibration curve was calculated on an average of eight parallel measurements (SD 3.7 %).
Linear range and sensitivity of the BOD biosensors
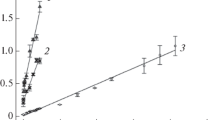
The linear range of the BOD biosensor is defined as the range of substrate concentration where the change in the biosensor output signal is proportional to the BOD7 of the sample (Liu and Mattiasson 2002; Thévenot et al. 2001). The linear ranges of M. phyllosphaerae- and P. fluorescens-based BOD sensors were up to 40 mg L−1 of BOD7, while the lowest detection limit used was 5 mg L−1 of BOD7 (Fig. 1). The sensitivity of the biosensor is represented by the slope of the calibration curve (Liu and Mattiasson 2002; Thévenot et al. 2001). The sensitivities of M. phyllosphaerae- and P. fluorescens-based sensors were 0.0220 and 0.0213, respectively. The linear range and sensitivity of the microbial biosensor depend on the amount of immobilized bacteria and, more importantly, on the amount of active bacterial cells in the bacterial suspension. Generally, biosensors with higher active cell concentrations have a narrower linear range but are more sensitive.
Service life and stability of the BOD biosensors
The service life and operational stability of BOD microbial biosensors are primarily related to the stability of the immobilized microorganisms but depend on the method of immobilization as well (Liu and Mattiasson 2002; Wang et al. 2010). The operational stabilities of M. phyllosphaerae and P. fluorescens BOD biosensors were determined during calibration with OECD synthetic wastewater (at a BOD7 value of 25 mg L−1 and 15 mg L−1, respectively). A biosensor was considered to be stable when deviation from the average sensor response was below 15 % (Raud et al. 2010).
During the preconditioning period, the sensitivity of the M. phyllosphaerae biosensor increased (Fig. 2), and then, after reaching the maximum sensitivity, it stabilized at slightly lower values by day 35. In the case of the P. fluorescens biosensor, the sensitivity of the sensor was highest at the beginning of the preconditioning period. Then, the sensor response slowly decreased until it stabilized at lower values by day 42. After stabilization, M. phyllosphaerae and P. fluorescens BOD biosensors produced stable and reproducible results up to day 310 (Fig. 2). This shows that the biosensor based on the pure microbial culture will yield a long and stable operational period.
Service life and stability of M. phyllosphaerae and P. fluorescens biosensors when calibrating with OECD synthetic wastewater at BOD7 values of 25 mg L−1 and 15 mg L−1, respectively. The dotted lines indicate the time when a biosensor was considered to be stable and reproducible results could be obtained. The arrows indicate the respective lengths of service life for the biosensors
Response time and recovery time of the BOD biosensors
Using the steady-state measuring method, the response time of the BOD biosensor is the time taken to achieve a stable output signal after the substrate addition (Liu and Mattiasson 2002; Thévenot et al. 2001). During the preconditioning period, the response time of both microbial biosensors was 45–50 min. When the operational stability was obtained, the response time shortened to 20–30 min.
Recovery time of a biosensor is defined as the period needed for the biosensor to gain the initial output signal after the experiment (Thévenot et al. 2001). During the preconditioning period, the recovery time of both BOD biosensors was 1 week. After obtaining the operational stability of the biosensor, the recovery time was 2–3 days. Due to the internal supplies of nutrients that were still in the bacterial cells, it was impossible to measure using the same BOD biosensor for 2 consecutive days.
BOD measurements in wastewater samples
Measurements with four different wastewaters were conducted, and sensor-BOD values using the M. phyllosphaerae- and the P. fluorescens-based BOD biosensors were gained. Conventional BOD7 analysis was conducted, and the results of the two methods were compared to evaluate the accuracy of the biosensors.
Measurements showed that the M. phyllosphaerae BOD biosensor underestimated the BOD7 of sample A only by 8 %. However, the P. fluorescens-based biosensor underestimated it by 24 % (Fig. 3a). This is approximately the amount of BOD that was added to the synthetic wastewater by milk addition. It shows that the bacterial culture of M. phyllosphaerae has the required enzymes to degrade the refractory compounds found in milk, while the bacterial culture of P. fluorescens lacks them.
The correlation between the BOD7 and sensor-BOD from semi-specific M. phyllosphaerae and nonspecific P. fluorescens biosensors in synthetic wastewater samples with milk and mixed additives, respectively, in a sample A and b sample B. The center line on the graph is the 1/1 correlation between the sensor-BOD and BOD7
Figure 3b shows that the results obtained with the M. phyllosphaerae biosensor differed from the BOD7 of sample B by 5 %, while the P. fluorescens BOD sensor underestimated the BOD7 of the same sample by 22 %. According to the study conducted by Salmerón-Alcocer (Salmerón-Alcocer et al. 2007), M. phyllosphaerae is able to degrade phenol. Our studies showed that this bacterial culture can assimilate the refractory compounds found in milk as well. Therefore, it is expected that the M. phyllosphaerae-based biosensor can estimate the BOD7 of the synthetic wastewater with different additives (milk, cellulose, and phenol) more accurately than the biosensor based on the nonspecific P. fluorescens.
Measurements with actual wastewater samples from the Valio Eesti AS Laeva dairy plant (samples C and D) showed that neither of the used biosensors was able to estimate the BOD7 within the allowed control limits of 15.4 % (APHA 1985). However, the semi-specific M. phyllosphaerae biosensor (underestimated samples C and D by 32 and 25 %, respectively) was more sensitive towards the wastewater samples than the nonspecific P. fluorescens biosensor (underestimated samples C and D by 61 and 46 %, respectively) (Fig. 4).
The correlation between the BOD7 and sensor-BOD from semi-specific M. phyllosphaerae and nonspecific P. fluorescens biosensors in wastewater samples from the Valio Eesti AS Laeva dairy plant with BOD7 values of 1,950 and 3,050 mg L−1, respectively, in a sample C and b sample D. The center line on the graph is the 1/1 correlation between the sensor-BOD and BOD7
Wastewater from dairy industries generally has high concentrations of fats, casein, and lactose and also contains various kinds of chemicals (acids, alkalis, and detergents) (Ozturk et al. 1993; Perle et al. 1995). Most of these chemicals are coming from the cleaning systems. These compounds inhibit the assimilation of organic substrates by the immobilized bacteria which leads to the lower sensor-BOD values. Moreover, since during the milk processing and protein curdling of the substrates in dairy wastewaters which are not only in dissolved form but also in the form of colloids and particulate matter (Schwarzenbeck et al. 2005), these compounds are not easily degraded by microorganisms in a short measuring period. Results could be improved by a pretreatment of wastewater samples which leads to the dissolution of colloids. The decomposed compounds would be assimilated faster by microorganisms, resulting in an increase in the accuracy of the biosensor (Chee et al. 2007).
It should be noted that actual wastewater samples C and D were taken in different times; therefore, these samples also differed in composition. Since the dairy industry produces different products (like milk, butter, yogurt, cheese, etc.), the characteristics of wastewaters also greatly vary in time, depending on the kind of products produced by the factory (Rico Gutierrez et al. 1991). Sample D was taken when cheese was produced, and the production of cheese is related to the production of whey. While producing the cheese, the curdling part is taken away, and the content of the curdling protein is probably less in the dairy wastewater which in turn means less use of detergents. Thus, it may be assumed that sample D contained less washing liquids and more specific refractory compounds which were more easily degradable to M. phyllosphaerae.
The absence of enzymes necessary to degrade milk derivates in the P. fluorescens biosensor can be the reason for the lower sensitivity compared with the M. phyllosphaerae biosensor. Therefore, the microbial biosensor based on M. phyllosphaerae exhibits semi-specific properties towards a high lipid, protein, and carbohydrate content, whereupon it is more appropriate for analyzing dairy industry wastewaters than the microbial biosensor based on the universal bacterial culture of P. fluorescens.
Conclusions
The bacterial culture of M. phyllosphaerae, isolated from the dairy wastewater of the Valio Eesti AS Laeva dairy plant, was used as a biological recognition system in the semi-specific microbial BOD biosensor. The selection of the microorganism was based on its ability to use lactose as a substrate because lactose and milk protein concentrations are high in a dairy wastewater. The nonspecific P. fluorescens was used in a universal microbial biosensor for comparison. To investigate the applicability of the semi-specific M. phyllosphaerae BOD biosensor, actual wastewater samples were used to test the correlation between the sensor-BOD and BOD7 values.
The semi-specific M. phyllosphaerae biosensor enabled to measure the BOD in wastewaters containing milk and milk derivates, both synthetic and industrial, more accurately than a nonspecific P. fluorescens biosensor. The M. phyllosphaerae biosensor underestimated the BOD7 of actual wastewater samples up to 32 %, while the P. fluorescens biosensor underestimated the same samples up to 61 %. Therefore, it can be concluded that the microbial biosensor of M. phyllosphaerae has semi-specific properties towards the degradation of lactose and milk proteins; thus, it is more appropriate for measuring BOD in dairy wastewater than the microbial biosensor based on the universal bacterial culture of P. fluorescens.
References
APHA (1985) Standard methods for examination of water and wastewater, 16th edn. American Public Health Association, Washington D.C, pp 525–531
Chee G-J, Nomura Y, Ikebukuro K, Karube I (2007) Stopped-flow system with ozonizer for the estimation of low biochemical oxygen demand in environmental samples. Biosens Bioelectron 22:3092–3098
D’Souza SF (2001) Microbial biosensors. Biosens Bioelectron 16:337–353
Dhall P, Kumar A, Joshi A, Saxsena TK, Manoharan A, Makhijani SD, Kumar R (2008) Quick and reliable estimation of BOD load of beverage industrial wastewater by developing BOD biosensor. Sensors Actuators B 133:478–483
Fang HHP, Yu HQ (2000) Effect of HRT on mesophilic acidogenesis of dairy wastewater. J Environ Eng 126:1145–1148
Janczukowicz W, Zielinski M, Debowski M (2008) Biodegradability evaluation of dairy effluents originated in selected sections of dairy production. Bioresour Technol 99:4199–4205
Kara S, Keskinler B, Erhan E (2009) A novel microbial BOD biosensor developed by the immobilization of P. syringae in micro-cellular polymers. J Chem Technol Biotechnol 84:511–518
Karube I, Nakanishi K (1994) Immobilized cells used for detection and analysis. Curr Opin Biotechnol 5:54–59
Kasapgil B, Anderson GK, Ince O (1994) An investigation into the pretreatment of dairy wastewater prior to aerobic biological treatment. Water Sci Technol 29:205–212
Kim MN, Park KH (2001) Klebsiella BOD sensor. Sensors Actuators B 80:9–14
Liu J, Mattiasson B (2002) Microbial BOD sensors for wastewater analysis. Water Res 36:3786–3802
Mello LD, Kubota LT (2002) Review of the use of biosensors as analytical tools in the food and drink industries. Food Chem 77:237–256
OECD (1984) Activated sludge, respiration inhibition test. OECD guidelines for the testing of chemicals. OECD, Paris
Olaniran AO, Motebejane RM, Pillay B (2008) Bacterial biosensors for rapid and effective monitoring of biodegradation of organic pollutants in wastewater effluents. J Environ Monit 10:889–893
Omil F, Garrido JM, Arrojo B, Méndez R (2003) Anaerobic filter reactor performance for the treatment of complex dairy wastewater at industrial scale. Water Res 37:4099–4108
Ozturk I, Eroglu V, Ubay G, Demir I (1993) Hybrid upflow anaerobic sludge blanket reactor (HUASBR) treatment of dairy effluents. Water Sci Technol 28:77–85
Perle M, Kimchie S, Shelef G (1995) Some biochemical aspects of the anaerobic degradation of dairy wastewater. Water Res 29:1549–1554
Rastogi S, Rathee P, Saxena TK, Mehra NK, Kumar R (2003) BOD analysis of industrial effluents: 5 days to 5 min. Curr Appl Phys 3:191–194
Raud M, Linde E, Kibena E, Velling S, Tenno T, Talpsep E, Kikas T (2010) Semi-specific biosensors for measuring BOD in dairy wastewater. J Chem Technol Biotechnol 85:957–961
Raud M, Tenno T, Jõgi E, Kikas T (2012a) Comparative study of semi-specific Aeromonas hydrophila and universal Pseudomonas fluorescens biosensors for BOD measurements in meat industry wastewaters. Enzym Microb Technol 50:221–226
Raud M, Tutt M, Jõgi E, Kikas T (2012b) BOD biosensors for pulp and paper industry wastewater analysis. Environ Sci Pollut Res 19:3039–3045
Raudkivi K, Tutt M, Talpsep E, Kikas T (2008) Pseudomonas putida P67.2 and Pseudomonas fluorescens P75 based microbial sensors for biochemical oxygen demand (BOD) measurements in phenolic wastewaters of oil shale industry. Oil Shale 25:376–386
Reshetilov AN (2005) Microbial, enzymatic, and immune biosensors for ecological monitoring and control of biotechnological processes. Appl Biochem Microbiol 41:442–449
Rico Gutierrez JL, Garcia Encina PA, Fdz-Polanco F (1991) Anaerobic treatment of cheese-production wastewater using a UASB reactor. Bioresour Technol 37:271–276
Sakaguchi T, Kitagawa K, Ando T, Murakami Y, Morita Y, Yamamura A, Yokoyama K, Tamiya E (2003) A rapid BOD sensing system using luminescent recombinants of Echerichia coli. Biosens Bioelectron 19:115–121
Salmerón-Alcocer A, Ruiz-Ordaz N, Juárez-Ramírez C, Galíndez-Mayer J (2007) Continuous biodegradation of single and mixed chlorophenols by a mixed microbial culture constituted by Burkholderia sp., Microbacterium phyllosphaerae, and Candida tropicalis. Biochem Eng J 37:201–2011
Schwarzenbeck N, Borges JM, Wilderer PA (2005) Treatment of dairy effluents in an aerobic granular sludge sequencing batch reactor. Appl Microbiol Biotechnol 66:711–718
SIS (1979) Water analysis—determination of biochemical oxygen demand, BOD, of water—dilution method. The Swedish Standards Institute, Stockholm, pp 1–9
Su L, Jia W, Hou C, Lei Y (2011) Microbial biosensors: a review. Biosens Bioelectron 26:1788–1799
Tan TC, Lim EWC (2005) Thermally killed cells of complex microbial culture for biosensor measurement of BOD of wastewater. Sensors Actuators B 107:546–551
Tanaka H, Nakamura E, Minamiyama Y, Toyoda T (1994) BOD biosensor for secondary effluent from wastewater treatment plants. Water Sci Technol 30:215–227
Thassitou PK, Arvanitoyannis IS (2001) Bioremediation: a novel approach to food waste management. Trends Food Sci Technol 12:185–196
Thévenot DR, Toth K, Durst RA, Wilson GS (2001) Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron 16:121–131
Vourch M, Balannec B, Chaufer B, Dorange G (2008) Treatment of dairy industry wastewater by reverse osmosis for water reuse. Desalination 219:190–202
Wang J, Zhang Y, Wang Y, Xu R, Sun Z, Jie Z (2010) An innovative reactor-type biosensor for BOD rapid measurement. Biosens Bioelectron 25:1705–1709
Xu X, Ying Y (2011) Microbial biosensors for environmental monitoring and food analysis. Food Rev Int 27:300–329
Acknowledgments
We gratefully acknowledge the support of Estonian Science Foundation (ETF 9136) and the target-financed project of the Estonian Ministry of Education and Research NoSF0180135s08 named “Processes in macro- and microheterogeneous and nanoscale systems and related technological applications.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kibena, E., Raud, M., Jõgi, E. et al. Semi-specific Microbacterium phyllosphaerae-based microbial sensor for biochemical oxygen demand measurements in dairy wastewater. Environ Sci Pollut Res 20, 2492–2498 (2013). https://doi.org/10.1007/s11356-012-1166-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1166-8