Abstract
Acetochlor is a widely used herbicide in maize fields; however, the ecological risk of its residue in the soil–plant system remains unknown. We investigated the dissipation dynamics of field dose acetochlor and clarified its impact on microbial biomass and community structure both in the rhizosphere and bulk soil over 1 month after its application. Soil microbial parameters such as quantities of culturable bacteria and fungi represented by colony-forming units, soil microbial biomass carbon (SMBC), and phospholipid fatty acids (PLFAs) were determined across different sampling times. The results showed that the dissipation half-lives of acetochlor were, respectively, 2.8 and 3.4 days in the rhizosphere and bulk soil, and 0.02–0.07 μg/g residual acetochlor could be detected in the soil 40 days after its application. Compared to the bulk soil, microbial communities in the rhizosphere soil were inclined to be affected by the application of acetochlor: SMBC content and bacterial growth were most likely to be increased; however, fungal growth was prone to be inhibited. The principal component analysis of PLFAs, as well as the comparisons of fungi/bacteria and cy17:0/C16:1ω9c ratios between different treatments over sampling time, revealed that the soil microbial community composition was significantly affected by acetochlor at its early application stage (at day 15); thereafter, the effects of acetochlor were attenuated or even could not be detected. Our results suggested that residual acetochlor did not confer a long-term impairment on viable bacterial groups in the rhizosphere and bulk soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acetochlor, i.e., 2-chloro-N-(ethoxymethyl)-N-(2-ethyl-6-methylphenyl) acetamide, is a widely used preemergent and early postemergent chloroacetanilide herbicide in corn fields, which prevents the growth of annual grasses and broadleaf weeds by inhibiting the photosynthetic electron transport of the host (Nemeth-Konda et al. 2002; Foley et al. 2008). Acetochlor has strong mobility and possibly poses an environmental risk including the contamination of arable soil and the surface water and groundwater (Ye 2003; Liu et al. 2005; Cai et al. 2007; Dictor et al. 2008). Furthermore, acetochlor is a suspected endocrine disruptor and regarded as a probable human carcinogen (Xiao et al. 2006a, b; Foley et al. 2008). Therefore, the environmental impairments caused by the repeated application of huge quantities of acetochlor have attracted much attention. However, although the mineralization of acetochlor and its impacts on microbial communities have been extensively studied (Taylor et al. 2005; Cai et al. 2007; Dictor et al. 2008; Li et al. 2008; Xu et al. 2008), its dissipation and detriment in the soil–plant system in situ have been scarcely reported.
Microbial biomarkers, as chemical and biological components of microorganisms, can be interpreted quantitatively and qualitatively in terms of microbial biomass and used to characterize soil microbial communities. They are also sensitive indicators of the environmental impact of acetochlor because microbial populations (Wu et al. 2009; Barriuso et al. 2010), diversity (Su et al. 2007; Li et al. 2008), and composition (Cai et al. 2007; Devare et al. 2007; Foley et al. 2008; Li et al. 2010) are all readily affected by its application. One of the most common viable biomarkers is phospholipid fatty acid (PLFA) since it exists in a relatively constant proportion in nearly all living microbes and decomposes quickly after the cytolysis in vitro (Vestal and White 1989; Sundh et al. 1995; Keinanen et al. 2002). The PLFAs of 18:2ω6,9 and 18:1ω9 are common in eukaryotic organisms such as saprotrophic fungi, while those of i15:0, a15:0, 15:0, i16:0, 16:1ω9, i17:0, cy17:0, and cy19:0 are commonly of bacterial origin (Frostegård and Bååth 1996; Ringelberg et al. 1997; Zelles 1999; Bååth 2003; Liang et al. 2012). Soil microbial biomass carbon (SMBC) determined by chloroform fumigation extraction (CHCl3 FE) has been widely used to estimate the amount of living soil microorganisms (Vance et al. 1987; Liphadzi et al. 2005). Although the quantities of soil microorganisms represented by the colony-forming units (CFUs) may underestimate the amounts of soil microbes, they have routinely been used to detect culturable bacterial and fungal numbers and their biological characteristics (Taylor et al. 2002; Li et al. 2005; Foley et al. 2008; Zabaloy et al. 2008). The combination of the above techniques can reveal the microbial community composition, carbon sequestration capability, as well as the countable quantity of soil microorganisms quickly responding to exogenous herbicide.
Rhizosphere is a hot microbial colonization spot and affects the nutrient transformation through complex biochemical processes (Barriuso et al. 2010). Compared to the bulk soil, the microbial groups in the rhizosphere soil are always significantly stimulated by rhizodeposits, thus promoting the mineralization of certain herbicides (Baudoin et al. 2001; Piutti et al. 2002; Puglisi et al. 2009). However, the exact differences of acetochlor dissipation dynamics and its influences on the soil microbial communities in the rhizosphere soil versus the bulk soil have rarely been reported.
Through a field experiment, this study was carried out to investigate the dissipation kinetics of acetochlor and its impact on the soil microbial communities in the rhizosphere and bulk soil. We hypothesized that (1) the acetochlor dissipation dynamics would be accelerated in situ as well as by the rhizospheric effect; (2) the responses of microbial communities to residual acetochlor in the rhizosphere soil might be different from those in the bulk soil; and (3) the residual acetochlor would detrimentally impact soil microbial communities in situ.
Materials and methods
Experimental design and soil sampling
A field experiment was carried out in the Chinese National Field Research Station of Shenyang Agro-ecosystems (41°31′ N, 123°34′ E). In this area, the mean annual temperature was 7–8 °C. Average precipitation was around 700 mm, and the monthly precipitation during sampling time was determined as 76.7 mm on average. Soil was an Alfisol (Typic Hapludoll) (Soil Survey Staff 2003), and its basic characteristics were listed in Table 1.
The acetochlor solution (50 %, v/v; Dalian Raiser Herbicides Co., Ltd.) was applied at the local field rate of 3,000 mL/ha, approximately equaling 0.85 mg acetochlor/kg dry soil. There were six plots, and each plot was 11 m long and 3.64 m wide. Three plots were selected randomly for the amendment of acetochlor, and the others were considered as the controls. The soil samples in these plots included: (1) the rhizosphere soil amended with acetochlor (ace-rhizosphere); (2) the rhizosphere soil without acetochlor (non-rhizosphere); (3) the bulk soil amended with acetochlor (ace-bulk); and (4) the bulk soil without acetochlor (non-bulk). The maize (Zea mays L.) was sowed on April 28, 2008, and the acetochlor solution was sprayed directly onto the soil surface on May 5, 2008. The rhizosphere and bulk soil samples were separately collected on days 0, 8, 10, 12, 15, 19, 24, 33, and 40 after the application of acetochlor.
The bulk soil samples were taken at 0–15 cm depth (cultivation layer) using a soil drill from five different sites along an S-shaped path in the same plot and then mixed thoroughly. The rhizosphere soil was collected by gently shaking off the soil that adhered to the roots and mixed well (Mijangos et al. 2009). Soil samples were passed through a 2-mm sieve. Portions of the soil samples were freeze-dried for later PLFA analysis. Another part of the soil samples was stored at 4 °C for SMBC and countable microorganism determination. The rest of the soil sample was air-dried for residual acetochlor measurement.
Acetochlor analysis
Extraction of acetochlor from soil
Exactly 20 g of air-dried soil were weighed and mixed with 10 mL distilled water and 50 mL acetone in a 250-mL flask with a stopper. Amyl phthalate was added as an internal standard for quantification (Feng et al. 2008). After 30 min of oscillation (200 rpm) and 5 min of ultrasonic wave extraction, the acetochlor solution was filtrated through a Buchner’s filter. The residue was then rinsed with 30 mL acetone into the receiving flask three times. The filtration solution was poured together with a 50-mL 8 % NaCl solution into a 250-mL separating funnel and then extracted using 30 mL petroleum ether three times. The organic phase was then loaded onto the anhydrous sodium sulfate layer and filtrated through it under dry conditions. The filtrated solution was rotary-evaporated at 45 °C, and the concentrated acetochlor sample was resolved with 30 mL mixed solvent (petroleum ether/acetic ether = 7:3, v/v). After being transferred onto a chromatographic column with 10 g neutral aluminum oxide stopped with a 2-cm thick layer of anhydrous sodium sulfate, the collection was rotary-evaporated to dryness and resolved in hexane to a final volume of 2 mL.
Gas chromatography determination of acetochlor
The acetochlor content was determined by a gas chromatograph (6890, Agilent Technologies Inc., USA) with an electron capture detector (ECD) and a fused silica capillary column (HP-5, 30 m × 0.32 mm × 0.25 μm). The temperature program consisted of the following four steps (Feng et al. 2008): (1) the initial temperature was set at 120 °C and held for 1 min; (2) the temperature was increased at 30 °C/min to 180 °C; (3) the temperature was increased at 3 °C/min to 205 °C and held for 5 min; and (4) finally, the temperature was increased at 20 °C/min to 270 °C and held for 2 min. Carrier gas (N2) flow rate was 1 mL/min. The injection volume was 1 μL with an injection temperature of 220 °C. The split ratio was 10:1. The ECD detection temperature was 300 °C. The acetochlor content was calculated using the following equation:
where C x was the acetochlor content, C i was the internal standard content, A i and A x were, respectively, the peak areas of internal standard and acetochlor, and R f was the response factor.
The dissipation dynamics of acetochlor was described by the model of Ace t = Ace0 × e (−k × t), where t was the sampling time (in days), k was the first-order dissipation rate constant, Ace0 and Ace t were, respectively, the acetochlor concentrations at the initial and sampling time t. The dissipation half-lives (DT50) (in days) were derived by DT50 = ln2 ÷ k (Ma et al. 2004; Dictor et al. 2008).
Soil microbial determination
Soil microbial biomass carbon
Soil microbial biomass was extracted by the CHCl3 FE method with 0.5 M K2SO4 solution and detected by TOC/TN analyzer (Multi N/C 3000, Analytikjena, Germany) (Vance et al. 1987). SMBC content was calculated using an extraction efficiency factor (K ec) of 0.38 (Ocio and Brookes 1990).
Microbial counts
Ten grams of fresh soil were mixed with 90 mL sterile water (dilution 10−1), and then 1 mL of the dilution 10−1 was poured into 9 mL sterile water (dilution 10−2). After a successive tenfold dilution series, 10−2 to 10−7 dilutions were prepared. Aliquots (0.1 mL) of 10−3 to 10−5 dilutions were spread on Martin agar medium for the fungal number counts. Similarly, aliquots (0.1 mL) of 10−5 to 10−7 dilutions were spread on beef protein agar medium for the bacterial number counts. The numbers of bacteria or fungi between 20 and 200 colonies occurring on one plate were counted using the plate counting method after 48 h of incubation at 28 °C (fungi) or 37 °C (bacteria).
PLFA analysis
The modified Bligh and Dyer (1959) method was used to extract PLFA. Nonadecanoic acid methyl ester (19:0; Sigma-Aldrich Chemical Co., USA) was added as an internal standard and the fatty acid methyl esters (FAMEs) were measured by gas chromatography (6890, Agilent Technologies Inc., USA) with a 30-m fused silica, HP-5 capillary column (30 m × 320 μm × 0.25 μm). The carrier gas (N2) flow rate was 0.8 mL/min, and the injection volume was 1 μL. The injection temperature was 250 °C, and the split ratio was 10:1. The flame ionization detector was set at 300 °C. The oven temperature program was as follows: (1) the initial temperature was 140 °C and held for 3 min; (2) the temperature was increased at 4 °C/min to 190 °C and held for 1 min; (3) the temperature was increased at 3 °C/min to 230 °C and held for 1 min; and (4) finally, the temperature was increased at 10 °C/min to 300 °C and held for 2 min. Peaks were quantified automatically with a computing integrator and identified using Supelco 37-Component FAME Mix and Bacterial Acid Methyl Esters Mix. The fatty acids C18:2ω6,9 and C18:1ω9c represented saprotrophic fungi (Vestal and White 1989; Bååth 2003; Liang et al. 2012), while the sum of iC15:0, aC15:0, C15:0, iC16:0, C16:1ω9c, iC17:0, cyC17:0, and C17:0 represented bacterial biomass (Frostegård and Bååth 1996; Priha et al. 1999).
Statistical analysis
Acetochlor, SMBC, bacterial and fungal CFUs, and PLFA were presented as the means of three replicates under the same treatment. Principal component analysis (PCA) was performed by JMP 9 to show microbial community composition alteration between treatments over sampling time. A one-way analysis of variance (ANOVA) was conducted to assess the significant differences between different treatments, while a two-way ANOVA was used to indicate the significance of the applied acetochlor and rhizospheric effect with SPSS 16.0 (p < 0.05).
Results
Content of residual acetochlor in the rhizosphere and bulk soil
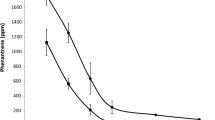
The temporal dynamics of acetochlor in the rhizosphere and bulk soil almost followed the same trend across the sampling time: rapidly declined in the early period (from day 0 to 8), and then remained relatively constant from the 10th to 40th day, ranging from 0.02–0.04 μg/g in the rhizosphere soil to 0.04–0.07 μg/g in the bulk soil (Fig. 1). As a result, on the 40th day, over 96.9 and 95.1 % of the acetochlor dissipated in the rhizosphere and bulk soil, respectively. The dissipation dynamics of acetochlor fitted well with the first-order kinetics (R 2 > 0.97), and the DT50 were 2.8 days in the rhizosphere soil and 3.4 days in the bulk soil, respectively (Fig. 1). The differences of residual acetochlor concentrations between the rhizosphere and bulk soil caused their ratios (Ace-R/B) to vary substantially as a function of time, which declined sharply from 0.78 to the minimum 0.46 within the first 15 days, and thereafter, rebounded to 0.63 on the 40th day (Fig. 1).
The dissipation of applied acetochlor in the rhizosphere and bulk soil. Bulk the bulk soil, Rhizosphere the rhizosphere soil, Ace-R/B the ratios of residual acetochlor concentrations between the rhizosphere and bulk soil, Predicted Bulk the acetochlor’s dissipation dynamics in the bulk soil derived by Ace t = Ace0 × e (−k × t), Predicted Rhizosphere the acetochlor’s dissipation dynamics in the rhizosphere soil derived by Ace t = Ace0 × e (−k × t)
Dynamics of soil microbial populations in the rhizosphere and bulk soil
As shown in Fig. 2, the SMBC changed outstandingly from 73.6–762.5 mg/kg in the rhizosphere soil to 22.0–158.0 mg/kg in the bulk soil, being 1.2–4.5 times greater in the rhizosphere regardless of the application of acetochlor. The SMBC content of ace-rhizosphere was significantly increased compared to non-rhizosphere (p < 0.05, except for the 24th day; Fig. 2), and the SMBC ratio between ace-rhizosphere and non-rhizosphere (ace-R/non-R) on the 33rd day was over 1.5 and much greater than the ratio of the bulk soil (ace-B/non-B) (Fig. 2).
The SMBC in the rhizosphere and bulk soil after acetochlor application. Ace-rhizosphere the rhizosphere soil with acetochlor, Non-rhizosphere the rhizosphere soil without acetochlor, Ace-bulk the bulk soil with acetochlor, Non-bulk the bulk soil without acetochlor, Ace-R/Non-R the ratios of SMBC between the rhizosphere soil with and without acetochlor, Ace-B/Non-B the ratios of SMBC between the bulk soil with and without acetochlor. SMBC ratios on the right y-axis indicate the ratios of SMBC in the treatments with acetochlor to their counterparts without acetochlor. Different letters on the top of columns indicate significant difference at the same sampling time (p < 0.05). Bars denote the standard errors (SE) of the means (n = 3)
Compared to the bulk soil, the culture-dependent microbial amounts in the rhizosphere soil not only were significantly higher but also inclined to present a much greater shift derived from the residual acetochlor (Fig. 3). The log values of fungal CFUs in the bulk soil ranged from 4.4 to 4.9 and were not significantly affected by the acetochlor amendment; in the rhizosphere soil, however, their log values varied between 5.6 and 6.9 and were evidently decreased to 0.11–0.23 log units by acetochlor (Fig. 3a). Furthermore, the fungal CFUs ratios of ace-rhizosphere to non-rhizosphere (ace-R/non-R) were between 0.59 and 0.79 and lower than those in the bulk soil (ace-B/non-B, 0.83–1.40) (Fig. 3a). As for the bacterial CFUs, however, completely reverse trends responding to the residual acetochlor were observed: on the 15th day, their log values were significantly decreased by 0.19 units in the rhizosphere soil and 0.59 units in the bulk soil, respectively (p < 0.05; Fig. 3b); thereafter, however, increased significantly under acetochlor amendments by 0.09–0.53 in the rhizosphere soil (p < 0.05; Fig. 3b).
The bacterial and fungal CFUs in the rhizosphere and bulk soil after acetochlor application. Ace-rhizosphere the rhizosphere soil with acetochlor, Non-rhizosphere the rhizosphere soil without acetochlor, Ace-bulk the bulk soil with acetochlor, Non-bulk the bulk soil without acetochlor, Ace-R/Non-R the ratios of CFUs between the rhizosphere soil with and without acetochlor, Ace-B/Non-B the ratios of CFUs between the bulk soil with and without acetochlor. The CFUs ratios on the right y-axes indicate the ratios of CFUs in the treatments with acetochlor to their counterparts without acetochlor. Different letters on the top of columns indicate significant difference at the same sampling time (p < 0.05). Bars denote the SE of the means (n = 3). a and b represent the fungal and bacterial CFUs, respectively
Saprotrophic fungal PLFAs varied from 0.9 to 4.1 nmol/g and were 1.4–2.7 times higher in the rhizosphere soil than the bulk soil on the 19th and 24th day, regardless of acetochlor (p < 0.05; Fig. 4a); the effect of acetochlor on saprotrophic fungal PLFAs was not observed (p > 0.05; Fig. 4a). Bacterial PLFAs ranged from 6.5 to 23.6 nmol/g (Fig. 4b) and were also significantly higher in the rhizosphere soil on the 19th and 24th day (p < 0.05, Fig. 4b). Furthermore, obvious stimuli of acetochlor on bacterial PLFAs and their ratios between the treatments with or without acetochlor (ace-R/non-R) were only observed in the rhizosphere soil on the 15th day (p < 0.05; Fig. 4b).
The PLFAs in the rhizosphere and bulk soil after acetochlor application. Ace-rhizosphere the rhizosphere soil with acetochlor, Non-rhizosphere the rhizosphere soil without acetochlor, Ace-bulk the bulk soil with acetochlor, Non-bulk the bulk soil without acetochlor, Ace-R/Non-R the ratios of PLFAs between the rhizosphere soil with and without acetochlor, Ace-B/Non-B the ratios of PLFAs between the bulk soil with and without acetochlor. The PLFA ratios on the right y-axes indicate the ratios of PLFAs content in the treatments with acetochlor to their counterparts without acetochlor. Different letters on the top of columns indicate significant difference at the same sampling time (p < 0.05). Bars denote the SE of the means (n = 3). a and b represent the saprotrophic fungal and bacterial PLFAs, respectively
Shifts of soil microbial community composition in the rhizosphere and bulk soil
The differentiation of microbial community composition derived from rhizospheric effect and acetochlor application over time could be identified by PCA of PLFAs (Fig. 5a), where PC 1 and PC 2 separately accounted for 57.6 and 22.5 % of the total variance. PLFAs profiles evidently changed along PC 1 (the rhizosphere soil to the right axis and the bulk soil to the left axis) and slightly varied along PC 2 due to the residual acetochlor, which could be distinguished on the 15th day as the treatments with acetochlor evidently separated from their counterparts without acetochlor along PC 2 (Fig. 5a). A two-way ANOVA on PC 1 and PC 2 was performed using a 2 × 2 general linear model with the aforementioned PCs. PC 1 values were significantly affected by the rhizospheric effect (F = 24.205, p < 0.001), while PC 2 showed no significant variance caused by acetochlor amendment (F = 1.420, p = 0.241). The PLFAs loading values of PC 1 >0.85 ordered as aC15:0 > iC16:0 > cy17:0 > C18:1ω9t > C14:0 > C18:0 > iC15:0 (Fig. 5b), suggesting that bacterial groups were markedly stimulated in the rhizosphere soil. A two-way ANOVA showed that the ratios of fungal/bacterial CFUs and cy17:0/C16:1ω9c were significantly higher in the treatments with acetochlor than their counterparts without acetochlor on the 15th day, while this trend reversed completely on the 33rd day (p < 0.05; Fig. 6b, c).
PCA scores (a) and loadings (b) of rhizosphere and bulk soil with or without acetochlor at different times. Ace-Rhizosphere the rhizosphere soil with acetochlor, Non-Rhizosphere the rhizosphere soil without acetochlor, Ace-Bulk the bulk soil with acetochlor, Non-Bulk the bulk soil without acetochlor. The numbers following the different treatments (in a) indicate the period of time after acetochlor application
Fungi/bacteria and cy17:0/C16:1ω9c ratios in different treatments across sampling time. Ace-bulk the bulk soil with acetochlor, Non-bulk the bulk soil without acetochlor, Ace-rhizosphere the rhizosphere soil with acetochlor, Non-rhizosphere the rhizosphere without acetochlor. Bars denote the SE of the means (n = 3). a, b, and c represent the ratios of saprotrophic fungal-to-bacterial PLFAs, fungal-to-bacterial CFUs, and cy17:0-to-C16:1ω9c, respectively
Discussion
Understanding the dissipation dynamics of acetochlor in the rhizosphere and bulk soil will not only clearly clarify its persistence in situ, but also allow for the evaluation of its impact on the soil microbial communities in the plant–soil system.
The DT50 of acetochlor in the rhizosphere and bulk soil were estimated at around 3 days in this study, and identical acetochlor DT50 (=3 days) in the field were also derived by Ma et al. (2004), which fairly matching the lower limit of 1–23 days recorded in the previous laboratory incubation studies (Mills et al. 2001; Taylor et al. 2005; Cai et al. 2007). The current short DT50 might firstly be ascribed to its low initial field application rate (0.85 mg/kg), as lower concentrations always led to faster degradation (Ma et al. 2004; Cai et al. 2007). Secondly, this rapid dissipation of acetochlor might be partly due to the non-desorbable residual acetochlor formation, which had been revealed to account for nearly two thirds of its initially adsorbed ones in a brown forest soil with clay alluviation (Luvisol) (1.16 % organic matter) (Nemeth-Konda et al. 2002), and their formation rates were closely correlated to organic matter content (Taylor et al. 2005). The abiotic reactions between acetochlor and soil chemical molecules could also play an important role in the dissipation of acetochlor (Lengyel and Földényi 2003; Ye 2003; Liu et al. 2005; Cai et al. 2007).
Compared to the laboratory incubation experiments, the measured acetochlor loss in the field was not only due to the abiotic mineralization and transformation and nonreversible sorption (Taylor et al. 2005), but also due to its leaching and runoff. The residual acetochlor had been detected at 60–70 cm depth of a Luvisol 7 days after being directly sprayed onto the soil surface (Baran et al. 2004). As the tested soil in this study had a relatively high compaction with porosity (50.74 %) and bulk density (1.31 g/cm3) (Table 1) and the precipitation amount during sampling time was also low (76.7 mm/month), therefore, the leaching and runoff processes would be limited. However, the present tested soil had relatively high organic matter content (19.94 g/kg) and proportion of clay (24.73 %) (Table 1), which probably causing the sprayed acetochlor to be easily absorbed in the top soil and, therefore, quickly degraded, as the acetochlor in the surface soil was prone to undergo highly chemical and biological loss and volatilization under relatively high temperature, as well as the solar radiation-correlated photodegradation (Jablonkai 2000; Ma et al. 2004; Dictor et al. 2008; Hiller et al. 2009). Although acetochlor concentrations in the rhizosphere and bulk soil declined in concert, their residual amounts in soil were extremely low and any tiny alterations in the concentrations between the rhizosphere and bulk soil could lead to their ratios varying greatly as a function of time (Fig. 1). Here, we supposed that the original concentrations of acetochlor in the rhizosphere and bulk soil were the same, and the differences in their residual concentrations were mainly caused by the rhizospheric effects, the changes of microbial structure and function (Yoshitomi and Shann 2001), and the correlated specific metabolic pathways (Baudoin et al. 2001; Marchand et al. 2002). Therefore, our first hypothesis was proved to be true.
Soil microbial communities were considered to be sensitive to the changes of soil physicochemical characteristics (Barriuso et al. 2010); however, since the residual acetochlor concentrations in the rhizosphere and bulk soil from the 15th to 40th day altered slightly (Fig. 1), we supposed that the distinguishing features of the microbial communities derived from residual acetochlor could be observed in this period of time. Thus, the soil microbial characteristics between days 15 and 33 were determined to assess whether there existed possible deleterious effect of residual acetochlor on the soil microbial communities in the maize field or not.
The residual acetochlor did not significantly affect microbial populations in the bulk soil for most cases (Figs. 2, 3, and 4), which had been also observed by Piutti et al. (2002) and Janniche et al. (2010). Dictor et al. (2008) also stated no evident relation between bacterial CFUs and the mineralization of acetochlor. However, in the rhizosphere soil, the significant inhibition of acetochlor on bacterial CFUs could be verified on the 15th day (Fig. 3b) or on fungal CFUs for the most part (Fig. 3a), while conversely in other cases, the residual acetochlor did significantly increase SMBC and culturable bacterial populations (Figs. 2 and 3b). In a sense, the influence of acetochlor on the microbial communities was exaggerated by the rhizospheric effect (Figs. 2, 3, and 4), which was also validated by PCA of PLFAs on day 15 (Fig. 5a). One of the most important reasons might be ascribed to the highly available carbohydrates in the rhizosphere soil, which could increase microbial community function diversity and activate the herbicide mineralizers (Kandeler et al. 2002; Piutti et al. 2002; Zhang et al. 2003; Singh et al. 2004; Puglisi et al. 2009). In the bulk soil, however, the nutrient deficiency made most microbial communities stay in the dormant status and less active to the low concentrations of residual acetochlor. Furthermore, it was believed that acetochlor was toxic to bacteria in a transient way; a considerable number of bacterial species might recover soon upon the residual herbicide (Virág et al. 2007; Barriuso et al. 2010), probably owing to the available metabolites released by the herbicide across time, greater bacterial tolerance, and recovery of aboriginal resistant species of communities who take advantage of the nutrients released from killed microbes (Foley et al. 2008; Zabaloy et al. 2008; Mijangos et al. 2009). In contrast to bacteria, the toxicities of acetochlor were observed to significantly reduce the soil fungal CFUs, total fungal biomass indicated by ergosterol, and fungal PLFAs in the microcosm experiment (Li et al. 2005; Feng et al. 2008). The above results implied that the highly heterotrophic communities and the increased functional evenness of soil bacteria arising from the complex rhizodeposits were probably more susceptible to be stimulated by the available carbon of residual acetochlor and its degradation metabolites (Cai et al. 2007; Foley et al. 2008; Mijangos et al. 2009), while the fungi might be less resistant to the toxicity of acetochlor and, therefore, depressed by its uptake, evidenced by a decrease in numbers of colony-forming fungi. Consequently, our second hypothesis was also confirmed.
Because of the above varied responses of the different microbial groups, pronounced shifts in soil microbial communities were observed over time (Figs. 5 and 6). The significant effects of acetochlor on the soil microbial communities were clearly observed on the 15th day rather than in the later days through the PCA of PLFAs (Fig. 5). The same tendency had also been validated by fungi/bacteria and cy17:0/C16:1ω9c ratios between the 15th and the 19th (or 24th) day (Fig. 6a, c). Furthermore, the effects of residual acetochlor on fungi/bacteria and cy17:0/C16:1ω9c ratios even completely reversed on day 33 compared to day 15 (Fig. 6b, c). The above results suggested that the impacts of residual acetochlor on viable microbial groups might decay with time. The temporal alterations of soil microbial responses to the herbicide application were revealed by previous studies (de Vries et al. 2006; Weaver et al. 2007; Mijangos et al. 2009) and might be attributed to different responses of microbial groups to the applied acetochlor: bacteria always quickly participated in and, therefore, affected by the metabolism of soluble compounds in the early stage; however, fungi more efficiently degraded resistant complexes in the later decomposition phase; in addition, there probably existed different resistant capabilities to acetochlor between fungi and bacteria as mentioned before (Bell et al. 2003; Feng et al. 2008; Foley et al. 2008; Zabaloy et al. 2008; Mijangos et al. 2009; Martins et al. 2011). The cy17:0/C16:1ω9c ratio was always considered as a stress indicator, which increased under environmental stress (Esperschütz et al. 2009; Helgason et al. 2010; Liang et al. 2012). The concordant higher cy17:0/C16:1ω9c and fungal/bacterial ratios under acetochlor amendments were only observed at day 15 for most cases (Fig. 6), which probably suggested that a significant stress of residual acetochlor on all microbial communities, especially for bacterial groups, might merely happen at its early stage of application. Thereafter, both fungal/bacterial ratios and bacterial stress indicator of cy17:0/C16:1ω9c ratios decreased in the acetochlor treatments in the later days. In a nutshell, our third hypothesis could not be totally supported in this study.
The contradictory alterations of PLFAs, CFUs, and SMBC were found in this study (Figs. 2, 3, and 4), and such difference was also observed in a poorly developed inceptisol: culturable bacterial groups were stimulated, but PLFAs were decreased by glyphosate application (Ratcliff et al. 2006). The above divergences between different biomarkers might be explained as follows: Bacteria could be acclimated to infertile nutrition status and preferred to feed on labile exogenous carbon substrates quickly, while fungi fed on much more complex compounds like lignin; culturable opportunists or r-strategists quickly responded and might be activated by root exudates as well as the added herbicides (Kandeler et al. 2002). Though the counted culturable colonies only represented a small proportion (<5 %) of the total biomass and could not differentiate between active and dormant groups (Lovell et al. 1995; Buyer and Kaufman 1996), bacterial CFUs and SMBC content successfully revealed the occurrence of fast-growing colonies which might be selectively stimulated by the energy and nutrients of root exudates as well as the residual acetochlor (Janssen et al. 2002; Marx et al. 2007; Zhang et al. 2003), which was not observed for culturable fungal groups. In spite of it being a popular and universal indicator of living soil microbial biomass, PLFA might not be a sensitive indicator for the above opportunistic microbial groups and, therefore, failed to detect the effects of acetochlor on microbial communities in the rhizosphere soil (Ratcliff et al. 2006). Furthermore, PLFA measurement might be interfered by humic acid-derived fatty acids and 5–10 % of total PLFA was estimated to be associated with nonliving biomass (Bååth and Anderson 2003).
Conclusions
In this field experiment, the acetochlor dissipated slightly faster in the rhizosphere soil than bulk soil with DT50 around 3 days, and over 95 % of acetochlor applied at field rate had dissipated in 40 days. The high quality of root exudates in the rhizosphere soil might be beneficial for better survival of soil microbial communities compared to the bulk soil, consequently stimulating the mineralizing processes of acetochlor. Microbial communities in the rhizosphere and bulk soil responded to the acetochlor in different ways. In the rhizosphere soil, the effects of acetochlor on the soil microbial groups were exaggerated. SMBC and bacterial populations were inclined to be activated by the residual acetochlor, while fungal growth exhibited a reverse trend. The microbial composition variation along sampling time showed that the impairment of acetochlor was only observed in the early stage of its application. Thereafter, its detrimental impacts on viable bacterial communities were attenuated or even could not be detected.
References
Bååth E (2003) The use of neutral lipid fatty acids to indicate the physiological conditions of soil fungi. Microb Ecol 45:373–383
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Baran N, Mouvet C, Dagnac T, Jeannot R (2004) Infiltration of acetochlor and two of its metabolites in two contrasting soils. J Environ Qual 33:241–249
Barriuso J, Marín S, Mellado RP (2010) Effect of the herbicide glyphosate on glyphosate-tolerant maize rhizobacterial communities: a comparison with pre-emergency applied herbicide consisting of a combination of acetochlor and terbuthylazine. Environ Microbiol 12:1021–1030
Baudoin E, Benizri E, Guckert A (2001) Metabolic fingerprint of microbial communities from distinct maize rhizosphere compartments. Eur J Soil Biol 37:85–93
Bell JM, Smith JL, Bailey VL, Bolton H (2003) Priming effect and C storage in semi-arid no-till spring crop rotations. Biol Fert Soils 37:237–244
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917
Buyer JS, Kaufman DD (1996) Microbial diversity in the rhizosphere of corn grown under conventional and low-input systems. Appl Soil Ecol 5:21–27
Cai XY, Sheng GY, Liu WP (2007) Degradation and detoxification of acetochlor in soils treated by organic and thiosulfate amendments. Chemosphere 66:286–292
de Vries FT, Hoffland E, van Eekeren N, Brussaard L, Bloem J (2006) Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol Biochem 38:2092–2103
Devare M, Londoño RLM, Thies JE (2007) Neither transgenic Bt maize (MON863) nor tefluthrin insecticide adversely affect soil microbial activity or biomass: a 3-year field analysis. Soil Biol Biochem 39:2038–2047
Dictor MC, Baran N, Gautier A, Mouvet C (2008) Acetochlor mineralization and fate of its two major metabolites in two soils under laboratory conditions. Chemosphere 71:663–670
Esperschütz WJ, Buegger F, Winkler JB, Munch JC, Schloter M, Gattinger A (2009) Microbial response to exudates in the rhizosphere of young beech trees (Fagus sylvatica L.) after dormancy. Soil Biol Biochem 41:1976–1985
Feng HM, He HB, Bai Z, Wu YY, Guo BD, Zhang M, Zhang XD (2008) Microbial degradation of acetochlor in mollisol and the effectsof acetochlor on the characteristics of soil phospholipid fatty acids. Ying Yong Sheng Tai Xue Bao 19:1585–1590 (in Chinese)
Foley ME, Sigler V, Gruden CL (2008) A multiphasic characterization of the impact of the herbicide acetochlor on freshwater bacterial communities. ISME J 2:56–66
Frostegård Å, Bååth E (1996) The use of phospholipids fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soils 22:59–65
Helgason BL, Walley FL, Germida JJ (2010) Long-term no-till management affects microbial biomass but not community composition in Canadian prairie agroecosytems. Soil Biol Biochem 42:2192–2202
Hiller E, Čerňanský S, Krascsenits Z, Milička J (2009) Effect of soil and sediment composition on acetochlor sorption and desorption. Environ Sci Pollut Res 16:546–554
Jablonkai I (2000) Microbial and photolytic degradation of the herbicide acetochlor. Intern J Environ Anal Chem 78:1–8
Janniche GS, Lindberg E, Mouvet C, Albrechtsen HJ (2010) Mineralization of isoproturon, mecoprop and acetochlor in a deep unsaturated limestone and sandy aquifer. Chemosphere 81:823–831
Janssen PH, Yates PS, Grinton BE, Taylor PM, Sait M (2002) Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl Environ Microbiol 68:2391–2396
Kandeler E, Marschner P, Tscherko D, Gahoonia TS, Nielsen NE (2002) Microbial community composition and functional diversity in the rhizosphere of maize. Plant Soil 238:301–312
Keinanen MM, Korhonen LK, Lehtola MJ (2002) The microbial community structure of drinking water biofilms can be affected by phosphorus availability. Appl Environ Microb 68:434–439
Lengyel Z, Földényi R (2003) Acetochlor as a soil pollutant. Environ Sci Pollut Res 10:13–18
Li XY, Zhang HW, Zhou QX, Su ZC, Zhang CG (2005) Effects of acetochlor and methamidophos on fungal communities in black soils. Pedosphere 15:646–652
Li XY, Zhang HW, Wu MN, Su ZC, Zhang CG (2008) Impact of acetochlor on ammonia-oxidizing bacteria in microcosm soils. J Environ Sci-China 20:1126–1131
Li XY, Su ZC, Li X, Zhang CG, Zhang HW (2010) Assessing the effects of acetochlor on soil fungal communities by DGGE and clone library analysis. Ecotoxicology 19:1111–1116
Liang C, Jesus ED, Duncan DS, Jackson RD, Tiedje JM, Balser TC (2012) Soil microbial communities under model biofuel cropping systems in southern Wisconsin, USA: impact of crop species and soil properties. Appl Soil Ecol 54:24–31
Liphadzi KB, Al-Khatib K, Bensch CN, Stahlman PW, Dille JA, Todd T, Rice CW, Horak MJ (2005) Soil microbial and nematode communities as affected by glyphosate and tillage practices in a glyphosate-resistant cropping system. Weed Sci 53:536–545
Liu SY, Chen YP, Yu HQ, Zhang SJ (2005) Kinetics and mechanisms of radiation-induced degradation of acetochlor. Chemosphere 59:3–19
Lovell RD, Jarvis SC, Bardgett RD (1995) Soil microbial biomass and activity in long-term grassland: effects of management changes. Soil Biol Biochem 27:969–975
Ma QL, Rahman A, Holland PT, James TK, McNaughton DE (2004) Field dissipation of acetochlor in two New Zealand soils at two application rates. J Environ Qual 33:930–938
Marchand AL, Piutti S, Lagacherie B, Soulas G (2002) Atrazine mineralization in bulk soil and maize rhizosphere. Biol Fertil Soils 35:288–292
Martins PF, Carvalho G, Gratão PL, Dourado MN, Pileggi M, Araújo WL, Azevedo RA (2011) Effects of the herbicides acetochlor and metolachlor on antioxidant enzymes in soil bacteria. Process Biochem 46:1186–1195
Marx M, Buegger F, Gattinger A, Zsolnay Á, Munch JC (2007) Determination of the fate of 13C labelled maize and wheat exudates in an agricultural soil during a short-term incubation. Eur J Soil Sci 58:1175–1185
Mijangos I, Becerril JM, Albizu I, Epelde L, Garbisu C (2009) Effects of glyphosate on rhizosphere soil microbial communities under two different plant compositions by cultivation-dependent and -independent methodologies. Soil Biol Biochem 41:505–513
Mills MS, Hill IR, Newcombe AC, Simmons ND, Vaughan PC, Verity AA (2001) Quantification of acetochlor degradation in the unsaturated zone using two novel in situ field techniques: comparisons with laboratory-generated data and implications for groundwater risk assessments. Pest Manag Sci 57:351–359
Nemeth-Konda L, Füleky G, Morovjan G, Csokan P (2002) Sorption behaviour of acetochlor, atrazine, carbendazim, diazinon, imidacloprid and isoproturon on Hungarian agricultural soil. Chemosphere 48:545–552
Ocio JA, Brookes PC (1990) An evaluation of methods for measuring the microbial biomass in soils following recent additions of wheat straw, and the characterization of the biomass that develops. Soil Biol Biochem 22:685–694
Piutti S, Hallet S, Rousseaux S, Philippot L, Soulas G, Martin-laurent F (2002) Accelerated mineralisation of atrazine in maize rhizosphere soil. Biol Fert Soils 36:434–441
Priha O, Grayston SJ, Pennanen T, Smolander A (1999) Microbial activities related to C and N cycling and microbial community structure in the rhizospheres of Pinus sylvestris, Picea abies and Betula pendula seedlings in an organic and mineral soil. FEMS Microbiol Ecol 30:187–199
Puglisi E, Fragoulis G, Ricciuti P, Cappa F, Spaccini R, Piccolo A, Trevisan M, Crecchio C (2009) Effects of a humic acid and its size-fractions on the bacterial community of soil rhizosphere under maize (Zea mays L.). Chemosphere 77:829–837
Ratcliff AW, Busse MD, Shestak CJ (2006) Changes in microbial community structure following herbicide (glyphosate) additions to forest soils. Appl Soil Ecol 34:114–124
Ringelberg DW, Sutton S, White DC (1997) Biomass, bioactivity and biodiversity: microbial ecology of the deep subsurface: analysis of ester-linked phospholipids fatty acids. FEMS Microbiol Rev 20:371–377
Singh N, Megharaj M, Kookana RS, Naidu R, Sethunathan N (2004) Atrazine and simazine degradation in Pennisetum rhizosphere. Chemosphere 56:257–263
Soil Survey Staff (2003) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys (Agriculture Handbook No. 436). Washington, D.C.: U.S. Government Printing Office
Su ZC, Zhang HW, Li XY, Zhang Q, Zhang CG (2007) Toxic effects of acetochlor, methamidophos and their combination on nifH gene in soil. J Environ Sci-China 19:864–873
Sundh I, Borga P, Milsson M (1995) Estimation of cell numbers of methanotrophic bacteria in boreal peatlands based on analysis of specific phospholipids fatty acids. FEMS Microbiol Ecol 18:103–112
Taylor JP, Wilson B, Mills MS, Burns RG (2002) Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol Biochem 34:387–401
Taylor JP, Mills MS, Burns RG (2005) Dissipation of acetochlor and its distribution in surface and sub-surface soil fractions during laboratory incubations. Pest Manag Sci 61:539–548
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vestal JR, White DC (1989) Lipid analysis in microbial ecology. Bioscience 39:535–541
Virág D, Naár Z, Kiss A (2007) Microbial toxicity of pesticide derivatives produced with UV-photodegradation. B Environ Contam Tox 79:356–359
Weaver MA, Krutz LJ, Zablotowicz RM, Reddy KN (2007) Effects of glyphosate on soil microbial communities and its mineralization in a Mississippi soil. Pest Manag Sci 63:388–393
Wu MN, Zhang XL, Zhang HW, Zhang Y, Li XY, Zhou QX, Zhang CG (2009) Soil pseudomonas community structure and its antagonism towards Rhizoctonia solani under the stress of acetochlor. B Environ Contam Tox 83:313–317
Xiao NW, Jing BB, Ge F, Liu XH (2006a) The fate of herbicide acetochlor and its toxicity to Eisenia fetida under laboratory conditions. Chemosphere 62:1366–1373
Xiao NW, Song Y, Ge F, Liu XH, Ou-Yang ZY (2006b) Biomarkers responses of the earthworm Eisenia fetida to acetochlor exposure in OECD soil. Chemosphere 65:907–912
Xu J, Yang M, Dai J, Cao H, Pan C, Qiu X, Xu M (2008) Degradation of acetochlor by four microbial communities. Bioresource Technol 99:7797–7802
Ye C (2003) Environmental behavior of the herbicide acetochlor in soil. B Environ Contam Tox 71:919–923
Yoshitomi KJ, Shann JR (2001) Corn (Zea mays L.) root exudates and their impact on 14C-pyrene mineralization. Soil Biol Biochem 33:1769–1776
Zabaloy MC, Garland JL, Gómez MA (2008) An integrated approach to evaluate the impacts of the herbicides glyphosate, 2,4-D and metsulfuron-methyl on soil microbial communities in the Pampas region, Argentina. Appl Soil Ecol 40:1–12
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol and Fert Soils 29:111–129
Zhang HW, Zhang Q, Zhou QX, Zhang CG (2003) Binary-joint effects of acetochlor, methamidophos, and copper on soil microbial population. B Environ Contam Tox 71:746–754
Acknowledgments
The authors would like to acknowledge the financial support for this study as research projects of the National Natural Science Foundation of China (nos. 41130524 and 41071161). We thank two anonymous reviewers for their great effort in improving the original manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Bai, Z., Xu, HJ., He, HB. et al. Alterations of microbial populations and composition in the rhizosphere and bulk soil as affected by residual acetochlor. Environ Sci Pollut Res 20, 369–379 (2013). https://doi.org/10.1007/s11356-012-1061-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1061-3