Abstract
Background, aim
The aims of the NORMACAT project are: to develop tools and unbiased standardized methods to measure the performance and to validate the safety of new materials and systems integrating photocatalysis, to develop new photocatalytic media with higher efficiency and to give recommendations aimed at improving the tested materials and systems.
Method
To achieve this objective, it was necessary to design standardized test benches and protocols to assess photocatalytic efficiency of materials or systems used in the treatment of volatile organic compounds (VOCs) and odour under conditions close to applications. The tests are based on the validation of robust analytical methods at the parts per billion by volume level that not only follow the disappearance of the initial VOCs but also identify the secondary species and calculate the mineralization rates.
Results
The first results of inter-laboratory closed chamber tests, according to XP B44-013 AFNOR standard, are described. The photocatalytic degradation of mixtures of several defined pollutants under controlled conditions (temperature, relative humidity, initial concentration) was carried out in two independent laboratories with the same photocatalytic device and with various analytical procedures. Comparison of the degradation rate and of the mineralization efficiency allowed the determination of the clean air delivery rate in both cases. Formaldehyde was the only by-product detected during photocatalytic test under standardized experimental conditions. The concentration of transient formaldehyde varied according to the initial VOC concentration. Moreover the photocatalytic reaction rate of formaldehyde in mixture with other pollutants was analysed. It was concluded that formaldehyde concentration did not increase with time.
Conclusion—perspective
This type of experiment should allow the comparison of the performances of different photoreactors and of photocatalytic media under controlled and reproducible conditions against mixtures of pollutants including formaldehyde.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim and scope
Today, most people living in industrial regions, particularly during the colder months, spend 90% of their time in closed rooms with only short aeration periods. At the same time, heat-insulating measures decrease the fresh air supply and lead to an increasing accumulation of emitted substances, sometimes exceeding the values measured in outdoor air. Moreover, in the 1960s and 1970s, materials containing unhealthy substances were used uncritically, creating sources of long-term emission. Building product, furnishing and household commodities often emit volatile chemical compounds. These are so-called volatile organic compounds (VOC) or semi-volatile organic compounds. Solvents, residual monomers, plasticizers, fire-proofing agents, auxiliary agents for processing, preservatives (biocides), and reaction or decomposition products are responsible for the occurrence of these emissions. Well-known examples are formaldehyde (emissions from wood-based materials), pentachlorophenol and its water-soluble sodium salt (wood preservatives) and polychlorinated biphenyls (insulating materials). As an illustration, Fig. 1 presents different sources of VOCs which can be found in a typical residence. In addition, many health problems are caused by biological particles such as fungi, moulds, bacteria and other microorganisms. All the above-mentioned factors have led to numerous investigations on the deterioration of indoor hygiene. Nevertheless, the subject of “indoor air” is a relatively young branch of chemical and medical science.
Different types of VOC sources which can be found in a typical residence (from Kuske and Nicolas 2000)
For many indoor contaminants, odour and pungency can become a concern at very low concentration. Pungency is the measure of a chemical’s ability to cause irritation, stinging, tingling and burning in the nose and the eyes. Odour thresholds are lower than pungency thresholds for any chemical. However, combinations of various VOCs can reach odour and pungency levels below the threshold of individual components, suggesting the occurrence of additive or even synergistic effects.
The control of indoor air quality is thus becoming a major concern in modern buildings, due to their increased isolation for energy saving and to the use of materials containing volatile chemicals. Indoor air is a complex medium containing VOCs, pathogenic or non-pathogenic microorganisms and aerosols. A better control of this air pollution in enclosed environment may be achieved by controlling pollution sources, increasing air exchange rate and purifying the polluted air. The use of air purifiers can decrease indoor air contamination, but the long-term performance of an air purifier depends on its maintenance. Usually, these air purifiers are made up of different filters and collectors, which can remove most particulate materials including, biological and chemicals contaminants.
Several purification techniques may be used separately or in combination depending on the complexity of the mixture of pollutants in the air to be treated:
-
Filtration for aerosols and microorganisms but not for volatile molecules,
-
Air ionization, although very few scientific data on this process are available,
-
Adsorption which only transfers the pollutants to another (solid) phase,
-
Chemical destruction by cold plasma, ozonization or catalytic oxidation is not adapted to indoor air purification due to the possible formation of undesirable by-products (plasma, ozonization) or to the constraint of the process (i.e. high temperature for catalytic oxidation), and
-
Photocatalysis.
Photocatalytic oxidation (PCO), operating at room temperature and atmospheric pressure, is well suited for contaminated air with low pollutant concentration and low flow rate (Le Cloirec 1998). Since organic contaminants are usually present at very low concentrations, PCO, in combination with particle filters, is adapted to indoor air treatment, either as separate air purifier devices or included in air conditioning. PCO of airborne organic contaminants was shown to be very efficient and to mineralize (oxidation to H2O and CO2) number of them (Lewandoski and Ollis 2003; Blake 2001; Vorontsov 2008; Tompkins et al. 2005). However, most of these studies deal with high pollutants concentrations [10–100 parts per million in volume (ppmv)] with single or binary mixture of model molecules, out of the range usually encountered for indoor air [10–1,000 parts per billion in volume (ppbv)]. Few studies have been carried out in the parts per billion volume (ppbv) range, eventually coupled with odour characterization (Obee 1996; Jo et al. 2002; Hang and Lee 2002; Pichat et al. 2000). Moreover the comparison of the results obtained in different laboratories is often unreliable, since a lot of operating conditions (design of the reactor, irradiation source and light intensity, flow rate, pollutant nature and concentration, pure pollutant or mixture of them, relative humidity,…) influence the fate of the photocatalytic reactions. Moreover the intermediate products analysis and determination is not always complete, and one of the main reaction indicators, i.e. the extent of mineralization, issued from the determination of CO2 evolution during the reaction, is not always available.
1.1 Needs of standardization for photocatalytic reactors
As for photocatalytic materials, it is very important to design standard to assess the performances of commercial air purifier. Nowadays, a lot of advertising for photocatalytic air purifier may be found on the web. Those advertisings give incomplete information or sometimes totally wrong data. Usually these air purifiers are validated by the producer itself or by independent laboratories, often without any reliable information on how the evaluation was carried out. For instance, some materials are designed to be used under visible light, but they were tested under UVA irradiation. As most of these results are far from real use conditions, materials and devices need to be tested under defined and controlled conditions, closer to their actual working conditions, at low ppbv range.
By-product formation under real use conditions has to be compared, if available, to the concentration proposed as expositions limits. However, since the reported tests are most often carried out at concentration far away from indoor air concentration, by-product formation is expected.
Special attention has to be paid to formaldehyde formation. Actually, besides numerous formaldehyde sources in indoor environments, secondary formation of formaldehyde may also occur indoors as PCO by-product issued from other VOCs. Cancer International Research Center gave several reference values for formaldehyde:
-
10 μg/m3 or 8 ppbv: target reference value to reach before 10 years,
-
30 μg/m3 or 24 ppbv: reference value for air quality under which no regulating action is required,
-
50 μg/m3 or 40 ppbv: information value above which the formaldehyde sources have to be identified and suitable actions are required, and
-
100 μg/m3 or 80 ppbv: value for a quick regulating action in order to decrease before 1 month to 30 μg/m3 and World health Organization (WHO) short-term guideline recommendation.
The potential usefulness of photocatalysis for decreasing formaldehyde concentration in indoor air is thus a major concern.
To address the question of reliable and comparable tests for the evaluation of the efficiency of photocatalytic devices and materials devoted to indoor air purification, a standard was published by AFNOR (2009) and it will be proposed as a new item for the CEN TC 386. One of the aims of the NORMACAT project is to evaluate and make inter-laboratories comparison of photocatalytic devices and materials in several closed chambers defined in this standard. The analysis of the photocatalytic reaction rate and products, including CO2 to determine the mineralization yield, on a well-defined mixture of four VOCs (toluene, hexane, acetaldehyde, acetone) has to be followed with suitable analytical devices.
In the following, inter-laboratory experiments were carried out on the same photocatalytic device with two different closed chambers in two different laboratories with various analytical methods according to the XP B44-013 standard. As by-products formation during the photocatalytic reaction course is a major concern for indoor air applications, we also addressed formaldehyde formation and reaction under these conditions.
2 Materials and methods
2.1 Closed chamber
Similar closed chamber were used for the photocatalytic tests in the two laboratories IPREM and CERTECH (Fig. 2). A validation of the closed chambers was made before the photocatalytic tests in order to control the possible leaks, the release of VOCs from the material chamber and the stability of VOC concentration without photocatalytic device for 8 h (less than 10% variation). Chambers were equipped with an air homogenization system (external fan with a maximum flow 120 m3/h), relative humidity and temperature measurement system. Online connection of the analytical systems (VOC and CO/CO2 determination) to the chamber allowed automatic sampling (Table 1).
2.2 Primary VOC analysis
In IPREM, gas chromatography systems with flame ionization (GC-FID) and photoionization (GC-PID) detectors (Airmotec) were connected online with the closed chamber in order to sample and analyse primary VOC in the ppbv to several parts per million volume (ppmv) range. In CERTECH, the chemicals evolution is followed using online gas chromatography with a photoionization detector (device GC-PID from EPA). This equipment allows the analysis of components from sub-ppbv to ppmv level without any pre-concentration device. VOC analysis can be also done by soft chemical ionization coupled with a mass spectrometer (V&F).
2.3 CO/CO2 analysis
In IPREM, a gas chromatography system with a methanizer and flame ionization detector (GC-methanizer-FID; Airmotec) was connected online with the closed chamber in order to sample and analyse CO and CO2 in the range 1–1,000 ppmv. In CERTECH, CO2 was analysed online by micro-GC in the range 2 ppmv to several % and CO by photoacoustic method (Innova).
2.4 Formaldehyde sampling and analysis
In IPREM, the LpDNPH S10 Cartridge (Supelco) containing high purity silica coated with 2,4-dinitrophenylhydrazine (DNPH) was used for sampling air. A sampling pump draws air through the device at flow rates 1 L min−1 for 15 to 20 min. Following sample collection, the cartridges were desorbed in reverse direction of the air flow with 5 mL acetonitrile. Twenty microliters of eluate was injected into HPLC system (Knauer). A formaldehyde curve of formaldehyde–DNPH was used (from commercial standard Sigma Aldrich) for the quantification of the content of formaldehyde in air sample. During the photocatalytic test, the sample volume should always be kept under 5% of the chamber volume, i.e. 60 L maximum of sampled air. In CERTECH, formaldehyde is either analysed by HPLC (Waters) through DNPH Cartridge or monitored continuously online by soft chemical ionization coupled with a mass spectrometer (V&F).
2.5 Photocatalytic device for comparative inter-laboratory tests
The benchmark system developed by CERTECH was an aluminium box 76 × 13 × 13 cm with inner wall coated with commercial TiO2. A fan (flow 30 m3 h−1) circulates the air through the system, according to AFNOR standard XP B44-013, defined for the evaluation of photocatalytic prototypes and commercial devices with maximal flow 1000 m3 h−1. In our study, a gas flow rate of 30 m3 h−1 corresponds to a 0.49 m s−1 gas velocity on the photocatalytic media, usually found in air treatment. In the middle of the device, a lamp (UV-C Philips TUV PL-L 36W/4P) was enclosed.
2.6 Procedure used for photocatalytic tests
The protocol used for photocatalytic test followed the AFNOR XP B44-13 standard. The photocatalytic device was put in the airtight chamber. Before the test, the chamber was first flushed with clean dry air and the photocatalytic device switched on during 12 h to condition the photocatalytic media. In the second step, the chamber was flushed with clean moist air until the defined relative humidity (50 ± 5%) was obtained. A control of this blank sample (temperature, relative humidity, VOC and CO2 data) warranted the quality of the chamber/device, with no unexpected pollutant in the baseline.
The photocatalytic device was then switched off and the mixture of four (or five) pollutants was added from pure liquid commercial solution (Sigma Aldrich or Acros) with a microsyringe through a septum into the chamber. Generally the four pollutants proposed in the AFNOR XP B44-13 standard (acetaldehyde, acetone, heptane, toluene) were used. Eventually a fifth one (o-xylene) was added to this mixture. Various concentrations were tested (from 250 to 1000 ppbv each compound). Formaldehyde was also added as an additional VOC in several tests made in CERTECH to study its behaviour in a COV mixture under photocatalytic conditions. An external fan was switched on during pollutants homogenization. After homogenization, the external fan was switched off and the photocatalytic device was started. The test was conducted until more than 90% VOC disappeared. Continuous VOC, CO2 analysis, temperature and relative humidity evolution were followed before pollutants addition and during the complete photocatalytic test. Switching on the UV light had no significant influence on the temperature (less than 2°C increase), and no COV desorption was noticed. Similar results were obtained in CERTECH and IPREM with slightly different temperatures (22°C in CERTECH and 28°C in IPREM).
3 Results and discussion
3.1 Inter-laboratory comparison
The first tests were carried out in the two laboratories (IPREM–CERTECH) using similar experimental conditions: same photocatalytic device, each VOC at about 1 ppmv concentration, relative humidity 45–55% and temperature 22–28°C.
The results may be compared by following the pollutants concentrations against time and by calculating the clean air delivery rate (CADR) for individual and total VOC. CADR represents the “effective” clean airflow rate delivered by the air cleaner (Chen et al. 2006). The analysis is based on the well-mixed single-zone model. Assuming that the air is well mixed in the chamber and that the contaminant removal mechanisms other than air cleaning (e.g. surface deposition effect and chamber leakage effect) are the same with and without operating air cleaner, the reaction can be characterized by a first-order rate constant k n . The mass conservation of contaminant in the “pull-down” test can be written as:
where
- V :
-
Volume of the testing chamber (in m3)
- k n :
-
Contaminant concentration decay rate without air cleaner operating (chamber effects; h−1)
- k e :
-
Total contaminant concentration decay rate with air cleaner operating (h−1)
- C 0 :
-
Initial contaminant concentration inside the chamber (in mg m−3 for each VOC)
- t :
-
Time (in hours)
- C :
-
Contaminant concentration inside the chamber at time t (in mg m−3 for each VOC)
- CADR:
-
Clean air delivery rate (in m−3 h−1)
To characterize the chamber effects on the air cleaner test results for VOC (mainly a possible sink effect), an empty chamber test was conducted first. In the two studies (IPREM and CERTECH), the chamber sink effect and the chamber leakage rate were considered negligible, which implied k n = 0. Then, Eq. 1 was simplified as follows:
CADR can then be determined by linear regression of ln(C/C 0) versus t from the measured concentration decay curve. Total contaminant concentration (C 0 and C) was converted in milligrams of carbon/cubic metre before plotting the linear regression.
The evolution of VOC (Fig. 3a, b) and CO2 (Fig. 4a, b) concentration with time was compared in the two laboratories. Similar VOC decrease was obtained in both studies (Fig. 3a, b), with the following order: o-xylene > acetaldehyde > toluene > acetone > heptanes. Moreover, CO2 production was observed (Fig. 4a, b). Since the amount of produced CO2 was in good agreement with theoretical CO2, an efficient mineralization was achieved. From these experimental results, CADR estimation was made for each compounds and total VOC (Table 2).
Comparison of theoretical and experimental CO2 concentration during photocatalytic test in a IPREM and b CERTECH. The increase of CO2 concentration was followed online during photocatalytic test until total disappearance of each VOC. Theoretical CO2 concentration was calculated from the experimental VOC concentration considering that all VOC were entirely converted into CO2
IPREM and CERTECH results are in good agreement for every compound and total VOC CADR. The most significant variations were obtained for the fastest degraded compounds (acetaldehyde and o-xylene). For these two compounds, the CADR was determined at IPREM with only few data, accounting for the highest uncertainty of these values. To summarize, this inter-laboratory comparison was convincing and allowed the validation of the two bench tests with different analytical equipments.
3.2 Formaldehyde detection and reactivity during the photocatalytic test as a function of initial VOC concentration
3.2.1 Formaldehyde detection during photocatalytic test by DNPH–HPLC analysis (IPREM)
In addition to the previously described online VOC and CO2 analysis during the photocatalytic tests, formaldehyde detection (the only by-product detected) was performed with DNPH cartridge sampling. In this experiment, a mixture of four VOCs (acetaldehyde, acetone, n-heptane, toluene) was introduced in the chamber. Formaldehyde determination at various VOC concentrations is reported in Table 3. Formaldehyde produced by photocatalysis not only adsorbed on the surface of catalyst, but can also desorb and enter into gas phase where it was quantified for short irradiation time and for three VOC concentrations (sampling between 5 and 25 min after switching on the photocatalytic device). Its amount decreased with the VOC concentration, and a satisfactory linear correlation of formaldehyde concentration with total VOC concentrations was obtained (Fig. 5). Moreover, it may be concluded that this by-product was mineralized for longer irradiation time.
3.2.2 Formaldehyde photocatalytic reactivity when introduced in mixture with other VOC, monitored by continuous CI-GC-MS analysis (CERTECH)
Formaldehyde formation as a by-product is a critical issue as it was shown in the previous experiments that it was the sole by-product observed during the initial irradiation time. In the following, we addressed the issue of formaldehyde behaviour when introduced at various concentrations in the test chamber, together with the other VOCs. All VOCs, including formaldehyde, were followed online by soft chemical ionization coupled with a mass spectrometer (SI-MS, V&F).
In a first experiment, a mixture of acetone, n-heptane, toluene, o-xylene and formaldehyde was used. Formaldehyde in aqueous solution was introduced in the chamber at same concentration as the other chemicals. One hundred minutes was necessary to reach a formaldehyde stationary concentration around 250 ppbv (Fig. 6). Once the equilibrium was reached, the reactor was switched ON and all the chemicals were removed almost at the same rate. In this case, no secondary formaldehyde production was observed during the VOCs mixture degradation. The final concentration was well below the WHO short-term guideline recommendation 0.1 mg/m3 (about 80 ppbv).
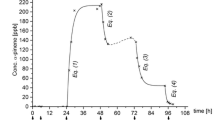
Two other tests were carried out using a mixture containing acetone, heptane, toluene and xylene at much higher concentration (1500–2500), and various concentration of additional formaldehyde or acetaldehyde. In the first case (Fig. 7), initial formaldehyde and acetaldehyde concentration were below 50 ppbv, while in the second case (Fig. 8), formaldehyde and acetaldehyde concentration were below 200 ppbv.
As observed previously, when formaldehyde was absent (Fig. 5) or at low concentration (<50 ppbv) at the beginning of the experiment (Fig. 7), its concentration first increased to 20–150 ppbv (depending on the conditions) when the device was switched ON, but decreased quickly with time to reach a level well below the 80-ppbv WHO recommendation for short exposure. Its production was thus quite limited and only detected over 50 min irradiation. Acetaldehyde transient formation was also observed (<100 ppbv, Fig. 7) and followed the same trend as formaldehyde.
In the second case (Fig. 8), where the initial formaldehyde concentration was 200 ppbv, ten times lower than the other pollutant, no formation of secondary formaldehyde was observed. The decrease of its concentration started immediately after the reactor was switched ON, just like when its initial concentration was of the same order of magnitude as that of the other VOCs (Fig. 6).
These experiments indicate that we must be very careful with the conclusion regarding the production of by-products such as formaldehyde. Depending on the starting mixture, possible transient formation of formaldehyde may actually be observed when starting with low or no initial content of formaldehyde. But when already present in the starting mixture at the same level of concentration as other VOCs, its secondary by-production is no longer observed and only a decrease of its concentration due to PCO is evidenced.
These experiments should be carried out with other photocatalytic devices and could actually discriminate set-up which will increase formaldehyde concentration in the chamber and other ones which will clean up the air and reduce formaldehyde concentration well below the WHO recommendation. For a more complete evaluation of photocatalytic devices, we could thus recommend to add in the standard VOC mixture formaldehyde at a slightly higher concentration than the WHO recommendation (80 ppbv).
4 Conclusion
The AFNOR XP B44-013 standard sets some parameters to assess the performances in closed chamber tests of photocatalytic air purifiers, such as the volume of the test chamber, the gas mixture to be tested, the temperature and the relative humidity. The analytical equipments to be used are not fixed, but should be sensitive enough to follow online the evolution of pollutant and of CO2 at ppbv–ppmv level, respectively.
Inter-laboratory experiments proved the validity of the XP-B44-13 standard to determine the efficiency of air-cleaning devices based on photocatalytic mineralization in closed chamber tests, for initial VOCs concentration in the 250–1000-ppbv range usually encountered for indoor air. From the disappearance rate of a mixture of standard VOCs, the CADR of the devices, based on first-order kinetics, may be measured under various conditions, while the CO2 emission rate is compared to the expected theoretical CO2 emission for complete mineralization. This study carried out in two independent laboratories proved that the standard is robust and reliable, since the same CADR was determined in both laboratories using the same photoreactor but different types of analytical tools.
It was also demonstrated in this inter-laboratory comparison with different test chambers and analytical procedures that no gaseous by-product except formaldehyde was detected during the photocatalytic process. This result is consistent with the formation of heavy by-products such as benzaldehyde, benzoic acid, ethanoic acid, in the first reaction step, adsorbed on the photocatalytic media and quickly oxidized to formaldehyde. However, in all the cases, formaldehyde is only a transient species detected at low concentration at the beginning of the test and further oxidized to carbon dioxide.
It was concluded that transient formaldehyde concentration was dependant on initial VOC concentration. Moreover, continuous monitoring by SI-MS of formaldehyde, introduced at the beginning of the experiments in mixture with other VOCs, evidenced that formaldehyde concentration with time was dependant on the ratio (initial HCHO)/VOCs and that formaldehyde was quickly removed. Secondary production of formaldehyde was only observed at null or weak initial formaldehyde concentration relative to other VOCs. In any case, detected formaldehyde concentration was always below the WHO recommendation (80 ppbv) under our conditions. It is thus recommended that in future evaluation of photocatalytic devices, formaldehyde should be added in the gas mixture to be studied. Further experiments are forecasted to assess the efficiency of various commercial devices, as well as the influence of the ageing of the photocatalytic medium and of the lamp.
References
AFNOR (2009) Méthode d’essais et d’analyse pour la mesure d’efficacité de systèmes photocatalytiques pour l’élimination des composés organiques volatiles/odeurs dans l’air intérieur en recirculation : test en enceinte confinée. Norme expérimentale XP B44-13, décembre 2009
Blake DL (2001) Bibliography of work on the heterogeneous photocatalytic removal of hazardous compounds from water and air. National Renewable Energy Laboratory NREL/TP-510-31319
Chen W, Gao Z, Zhang JS, Kosar D, Walker CE, Novosel D (2006) Reduced energy use through reduced indoor contamination in residential buildings, National Center for Energy Management and Buildings Technologies, Final Report NCEMBT 061106
Hang C, Lee SC (2002) Removal of indoor air ppb level volatile organic compounds (VOCs) and NOx by heterogeneous photocatalysis. Better Air Quality in Asian and Pacific Rim Cities (BAQ 2002), 16–18 Dec 2002, Hong Kong SAR
Jo W-K, Park J-H, Chum H-D (2002) Photocatalytic destruction of VOCs for in-vehicle air cleaning. J Photochem Photobiol A Chem 148:109–119
Kuske M, Nicolas J (2000) Les pollutions dans l’air intérieur des bâtiments. Diagnostic. Incidence sur la santé. Fondation Universitaire Luxembourgeoise. http://orbi.ulg.ac.be/retrieve/8288/27620/Les_pollutions_dans_l’air_à_l.pdf. Accessed 10 April 2011
Le Cloirec P (1998) Les composés organiques volatiles dans l’environnement Tec&Doc. Lavoisier
Lewandoski M, Ollis D (2003) Photocatalytic oxidation of gas-phase aromatic contaminants. In Ramamurthy V, Schnaze KS (eds) Molecular and supramolecular photochemistry, vol. 10. Semi-conductor photochemistry and photophysics. pp 249–282
Obee TH (1996) Photooxidation of sub-parts-per-million toluene and formaldehyde levels on titania using a glass-plate reactor. Environ Sci Technol 30:3578–3584
Pichat P, Disdier I, Hoang-Van C, Mas D, Goutailler G, Gaysse C (2000) Purification/deodorization of indoor air and gaseous effluents by TiO2 photocatalysis. Catal Today 63:363–369
Tompkins D, Lawnicki B, Zeltner W, Anderson M (2005) Evaluation of photocatalysis for gas-phase air cleaning part 1: process, technical, and sizing considerations. ASHRAE Transactions, volume 111, Part 2 4791 (RP-1134)
Vorontsov AV (2008) Photocatalytic transformations of organic sulfur compounds and H2S Russian. Chem Rev 77:909–926
Acknowledgement
The authors acknowledge the Ministère de l’économie et de l’emploi, Direction Générale Compétitivité Industrie et Services (DGCIS), Région Rhône-Alpes, Conseil Général de l’Isère, Conseil Général de l’Ain for funding this study in the NORMACAT project, labelled by the Axelera cluster.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor. Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kartheuser, B., Costarramone, N., Pigot, T. et al. NORMACAT project: normalized closed chamber tests for evaluation of photocatalytic VOC treatment in indoor air and formaldehyde determination. Environ Sci Pollut Res 19, 3763–3771 (2012). https://doi.org/10.1007/s11356-012-0797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0797-0