Abstract
Introduction
To assess the status of polycyclic aromatic hydrocarbons (PAHs) contamination in sediments from the upper reach of Huaihe River, East China, 16 surface sediment samples were collected in March 2007 and analyzed for 16 USEPA priority PAHs.
Results and discussion
The results indicated that the total concentrations of 16 PAHs (∑PAHs) were 95.2–877.5 μg kg−1 dry weight (dw) with a mean value of 370.8 μg kg−1 dw for the main stream, 85.7–935.2 μg kg−1 dw with a mean concentration of 480.7 μg kg−1 dw for tributaries, and 144.8–303.2 μg kg−1 dw with an average concentration of 224.0 μg kg−1 dw for lakes. PAHs pollution was closely related to sewage input and industrial activities. Furthermore, the distribution of PAHs in sediments from the main stream indicated that the input of tributaries was an important factor for Huaihe River. In comparison to a worldwide survey of sedimentary PAHs concentrations, PAHs pollution in Huaihe River sediments was ranked as low to moderate. The dominant compounds in surface sediment samples were four-ring and five-ring PAH compounds.
Conclusion
Selected PAH ratios suggested that PAHs mainly came from the contamination of pyrogenic processes, such as coal and biomass combustion. Risk assessment indicated that PAHs in sedimentary environment in the upper reach of Huaihe River may cause mild toxic effects but would not cause immediate biological effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a class of diverse compounds that consist of two or more fused aromatic rings. These contaminants originated mainly from incomplete combustion or pyrolysis of organic materials and in connection with the worldwide use of oil, gas, coal, and wood in energy production and can be introduced into the environments through various sources (Zakaria et al. 2002; Doong and Lin 2004). Due to their carcinogenicity, mutagenicity, and toxicity (IARC 1987), some of PAHs are of environmental concern and included in the list of priority pollutants of the US Environmental Protection Agency (USEPA) and European Union. Therefore, behavior, transport, fate, and environmental risk of PAHs to ecological systems have been extensively studied (Fang et al. 2007; Liu et al. 2008; Tian et al. 2009; Barakat et al. 2011).
Due to their low water solubility and high hydrophobicity, once PAHs are introduced into the aquatic environment, they rapidly associate with suspended particles and subsequently deposit in sediments (Chiou et al. 1998). In such a way, sediments become a huge sink for PAHs. Sedimentary PAHs tend to accumulate to high concentrations and are a source of exposure of PAHs directly to benthic organisms and indirectly (e.g., via resuspension) to pelagic organisms. The analysis of sediment PAHs can serve as a useful index of the contamination level and the source of PAH input to the aquatic environment. Therefore, distributions of PAHs in sediments are the subject of intensive studies from the middle 1970s to the present (Fang et al. 2007; Liu et al. 2008; Barakat et al. 2011). These studies indicate that PAHs are distributed globally from inland lakes and urban rivers to the open ocean with a wide range of concentrations. Some of the most highly industrialized and urbanized locations have extremely high concentrations of PAHs of more than 10,000 μg g−1 (Marvin et al. 2000; Van Metre et al. 2000).
Huaihe River, one of the most important rivers in East China, flows 1,000 km from the Tongbai Mountain to Yangtze River and drains a populous area of 30,000 km2. The upper reach of Huaihe River Basin locates in Henan province and flows across extensive agricultural regions. In addition to being used for agriculture, Huaihe River is the main source of drinking water and industrial water in this basin. With the rapid growth of economy and urbanization, more and more wastes are discharged into the river. Recently, organic pollutants contamination of large rivers in China, especially persistent organic pollutants, has drawn great concern from the public and government (Fang et al. 2007; Liu et al. 2008; Yu et al. 2009; Li et al. 2010). So far, little information is available for PAHs contamination in the upper reach of Huaihe River. Investigation on PAHs levels in sediments from the upper reach of Huaihe River is essential in order to better understand its contribution to the middle and lower reaches’ pollution.
The objectives of the present work were to investigate the contamination level and distribution of PAHs, identify their possible sources in sediments samples from the upper reach of Huaihe River, and assess the potential risk of PAHs to the environment. The results provided a valuable reference data set for environmental managers.
2 Materials and methods
2.1 Sample collection
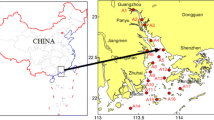
The sampling sites are shown in Fig. 1. A total of 16 sampling sites along the upper reach of Huaihe River and its tributaries and 2 lakes were selected as representative in this area. Sediment samples were collected using a grab sampler in March 2007 (representative of low river flow) and then put in stainless steel containers. The sediments were kept in the refrigerator at −20°C before analysis. All sediment samples were freeze-dried and then ground, homogenized, and stored in precleaned dark glass bottles at −18°C prior to analysis.
2.2 Chemical reagents
Standard PAHs (16 USEPA priority compounds, each at 200 ng mL−1) including naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (InP), dibenzo[a,h]anthracene (DBA), benzo(g,h,i)perylene (BgP) and deuterated PAHs standards containing naphthalene-D8, acenaphthene-D10, phenanthrene-D10, chrysene-D12, and perylene-D12 were obtained from Ultra Scientific Inc. (North Kingston, RI, USA). Internal standard (hexamethylbenzene) were acquired initially as a solid of 99% purity (Aldrich Chemical, Gillingham, Dorset, UK). The organic solvents, dichloromethane (DCM), n-hexane, acetone, and methanol, used for sample extraction and purification procedures were purchased from Dikma Co. (Beijing, China). All organic solvents were redistilled in a full-glass distilling appliance.
Anhydrous sodium sulfate (analytical grade; Guangzhou Chemicals Inc., Guangzhou, China), neutral silica gel (80–100 mesh), and alumina (100–200 mesh) (Wushi Chemicals Inc., Shanghai, China) were baked in a furnace oven at 650°C for 6 h and then kept in a sealed desiccator prior to use. Cu was obtained from Damao Chemicals Co. (Tianjin, China). Water was prepared from a Milli-Q system (Millipore, Bedford, MA, USA) with a specific resistivity of 18.2 MΩ cm. Glassware were cleaned in an ultrasonic cleaner (KQ-502B, Kunshan Ultrasonic Instruments, China) and rinsed with acetone first and then with n-hexane.
2.3 Sample extraction
The experimental procedure for PAHs analysis was based on the procedure of Zeng et al. (1999). Briefly, 15 g of freeze-dried, homogenized sediment was spiked with surrogate recovery standards and then Soxhlet-extracted for 48 h with 120 mL of DCM. Two grams of activated Cu was added for desulphurization. The extracts were concentrated to 1–2 mL by a rotary evaporator and subjected to solvent exchange using hexane. The concentrated extract was passed through a 1:2 alumina/silica gel glass column with 1 cm anhydrous sodium sulfate on the top for cleanup and fractionation. Elution was performed with 70 mL hexane/DCM (7:3, v/v). The eluting solutions containing PAHs were concentrated to 1–2 mL, subjected to solvent exchange using hexane, and then concentrated to 0.2 mL with a gentle stream of purified nitrogen. The internal standard (hexamethylbenzene, 200 mg L−1, 5 μL) was added to the sample prior to instrumental analysis.
2.4 Analysis methods
Quantification of PAHs was performed using a Hewlett-Packard 5890 gas chromatography and 5972 mass selective detector operated in the electron impact mode (70 eV). Separation was carried out using a HP-5 capillary column (30 m × 0.25 mm × 0.25 μm). Instrumental conditions were as follows: injector temperature, 280°C; ion source temperature, 180°C; temperature program: the column started initially at 60°C and held for 2 min, increased to 290°C at a rate of 3°C min−1, and held for 30 min. The carrier gas was helium at a constant flow rate of 1.5 mL min−1. Sample (1 μL) was injected in splitless mode. Mass range m/z 50–500 was used for quantitative determinations. Data acquisition and processing was controlled by HP Chemstation software. Chromatographic peaks of samples were identified by mass spectra and by comparison with the standards.
2.5 Quality control
All analytical operations were conducted according to a quality assurance/quality control procedure recommended by the USEPA (1994). The instruments were calibrated daily with calibration standards. Procedural blanks, standard reference samples (NIST 1941a), sample duplicates, and spiked samples with surrogate standards were used to monitor procedural performance and matrix effects. Average recoveries of standard reference material NIST 1941a were 92.74 ± 8.61% for six parallel samples. The mean surrogate recoveries for sediment samples were naphthalene-D8, 41.74 ± 7.57%; acenaphthene-D10, 61.29 ± 6.33%; phenanthrene-D10, 86.70 ± 9.21%; chrysene-D12, 92.45 ± 12.04%; and perylene-D12, 102.56 ± 10.90%. The average relative standard deviation of duplicates was <10%. The method detection limits were 0.44–0.95 μg kg−1 by dry weight (dw) for each PAH. Reported concentrations were corrected according to the recoveries of the surrogate standards.
2.6 Other analysis
Total organic carbon (TOC) contents of sediments were determined using a Liqui TOC (Elementar, Germany) analyzer. The dried and homogenized sediment samples were first acidified with 1.6%HCl to remove carbonates, then dried at 60°C and analyzed for TOC.
3 Results and discussion
3.1 Concentrations of PAHs in sediments
Surface sediments could reflect the current sediment contaminant status. The concentrations of PAHs in sediments from the upper reach of Huaihe River were summarized in Table 1. As shown in Table 1, most of the 16 USEPA priority PAHs were detected at all sampling sites. The total concentrations of 16 USEPA PAHs (∑PAHs) were 95.2–877.5 μg kg−1 dw with a mean value of 370.8 μg kg−1 dw for the main stream, 85.7–935.2 μg kg−1 dw with a mean concentration of 480.7 μg kg−1 dw for tributaries, and 144.8–303.2 μg kg−1 dw with an average concentration of 224.0 μg kg−1 dw for lakes. To better understand the magnitude of PAHs concentrations in sediments from the upper reach of Huaihe River, comparison of PAHs levels in this study with published sediments data was conducted. The results were listed in Table 2. As shown in Table 2, the content of ∑PAHs in sediments from the upper reach of Huaihe River was at a medium level in comparison to those of other Chinese studied rivers. Among the Chinese rivers, the level of ∑PAHs in sediments from the upper reach of Huaihe River was comparable to those in sediments from the middle and lower reaches of Huaihe River (He and Yan 2006), Qiantang River (Chen et al. 2007), Minjiang River (Zhang et al. 2003), Daliao River watershed (Guo et al. 2007), Aojiang River (Li et al. 2010), and Tonghui River (Zhang et al. 2004). However, it was much lower than those of Haihe River (Tianjin) (Jiang et al. 2007; Cheng et al. 2009), Pearl River (Mai et al. 2002), Tianjing River (Shi et al. 2005), Huangpu River (Liu et al. 2008), Yellow River (Sun et al. 2009; Yu et al. 2009), and Yangtze River (Feng et al. 2007). Compared to other rivers around the world, the level of ∑PAHs in sediments was also much lower (Headley et al. 2001; Koh et al. 2004; Ko et al. 2007; Sanctorum et al. 2011; Leite et al. 2011). This was probably due to the short contamination history of the upper reach of Huaihe River. The upper reach of Huaihe River basin had been a typical agricultural area for a long time until this region underwent rapid industrialization and urbanization two decades ago. Thus, less PAHs were absorbed into sediments from the upper reach of Huaihe River than those from more developed areas and the levels of PAHs in sediments from the upper reach of Huaihe River were lower than other rivers.
3.2 Spatial distributions of PAHs in sediments
Spatial differences of PAHs levels in sediment from the upper reach of Huaihe River were investigated. The Kolmogorov–Smirnov test was carried out to test the frequency distribution of PAHs data. The results indicated a normal distribution (p > 0.05) of all the analyzed variables. Furthermore, in order to evaluate the differences on PAHs levels among the main stream, tributaries, and lakes of the Huaihe River, repeated measures of the one-way ANOVA procedure were performed. The results indicated that there were no significant differences among these sediment samples (at the significance level of 0.05), which revealed that complicated sources and different factors both contributed to the PAHs contaminations in these sediments.
In the tributaries and lakes, the highest levels of PAHs were observed in T7 site located in Shiguan River. The upper reach of Shiguan River was in front of the local sewage output of the wastewater treatment plant, which may be responsible for the introduction of PAHs from domestic waste into Shiguan River. Other reports have previously considered sewage effluents as a potential source of PAHs (Villar et al. 2006). This observation pointed out the importance of PAHs sources from urban sewage discharge. The second highest PAHs-contaminated site was recorded at T4 that lies in the Huang River (867.7 μg kg−1). T4 was in the center of Huangchuan County and in close vicinity to urban and industrial sources. The industrial wastewaters containing PAHs were directly discharged into Huang River. In addition, particulate-associated PAHs emitted from factories not only caused serious air pollution but also may transport and deposit into the river sediments. Thirdly, there were roads on both sides of the river, which could be sources of PAHs from vehicular traffic. High concentrations of PAHs (521.2 μg kg−1) were also observed at site T3 (Lv River), located near a large-scale fertilizer plant where industrial wastewater could be discharged into Lv River directly or in directly.
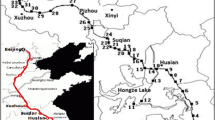
The spatial concentration profiles of PAHs in the main stream of the upper reach of Huaihe River were plotted in Fig. 2. As illustrated in Fig. 2, the spatial distribution of ∑PAHs was complicated due to the intricate river system. In general, the upper section (M1 to M3) was more contaminated by PAHs. Where the river flows through downtown, the concentration of ∑PAHs gradient increased from 216.9 μg kg−1 at site M1 to 877.5 μg kg−1 at site M3, which was the highest PAHs level in the main stream. Then, the concentration of ∑PAHs decreased to 95.2 μg kg−1 at site M4. In the lower section (M6 to M7), the concentration of ∑PAHs increased again and reached 428.3 μg kg−1 at site M7. In the upper section of this basin, there were many industries, such as a chemical plant, a coke-oven plant, and a large-scale coal-fired power plant. The results showed that these industries were likely responsible for the high level of PAHs in M3. However, a relatively low level of ∑PAH was observed at M4. This may be because site M4 was located in the proximity of low urbanized/rural lands. PAHs levels appeared to decrease as the distance from urban/industrial centers increased, which was reported in previous works (Laflamme and Hites 1978; Cantillo et al. 1997; Miles and Delfino 1999). In the lower section of this area, the second increase of ∑PAH concentration (M6 to M7) probably resulted from the inflow of Shiguan River. Site M7 is located downstream of the confluence of Huaihe River and Shiguan River (Fig. 2). Thus, it may be attributed mainly to PAHs inputs from Shiguan River, which had the highest PAHs levels in the studied area (Table 1). Moreover, the mixing of rivers caused the resuspension of surface sediments. More PAHs in water could thus be easily absorbed to the particles and then flocculate in the mixing zone, leading to the accumulation of PAHs in sediment.
The factors controlling the level of PAHs in the sediments were complex. It has been revealed that the physicochemical properties of the sediments could affect the distribution and concentration of PAHs. The distribution of PAHs in the sediments is influenced by the chemical composition of the sediments such as TOC. Sediments with high TOC were characterized by high contents of PAHs (Yang 2000). In order to evaluate the relationship between PAHs concentration and the TOC percentage in the present study, a correlation analysis was conducted. The linear regression did not show any significant correlation between the above-mentioned parameters (R 2 = 0.016, p = 0.641). Some reports have demonstrated similar results of no significant correlation for sediments from other areas (Ortega et al. 2010). These studies, including the present work, suggest that the distribution and concentration of PAHs in sediments was determined more by other factors such as site specificity and proximity to sources rather than by the type of the sediment found locally.
3.3 Composition and sources identification of PAHs in sediments
According to the number of aromatic rings, the 16 PAHs were divided into five groups representing two-ring, three-ring, four-ring, five-ring, and six-ring PAHs. The composition and relative abundance of individual PAHs in surface sediments from the upper reach of Huaihe River was shown in Fig. 3. As illustrated in Fig. 3, four-ring PAHs (42.6% of total PAHs, on average) was most abundant and five-ring PAHs took second place, which accounted for 28.5% of total PAHs. Two-ring and three-ring PAHs occupied 9.4% and 15.8% of total PAHs on average, respectively, while six-ring PAHs (3.7%) had the least proportions of 3.7%. In general, high-molecular-weight (HMW) four-ring to six-ring PAHs (74.8%) were prevalent in the sediments from the upper reach of Huaihe River. Usually, HMW PAHs predominated in sediment samples. The higher concentration of HMW PAHs than that of low-molecular-weight (LMW) two-ring to three-ring PAHs has been commonly observed in sediments from river and marine environments (Mostafa et al. 2009; Berto et al. 2009).
PAHs congener distribution varied with the source as well as the composition and combustion temperature of the organic material. Molecular indices based on ratios of selected PAHs may be used to differentiate PAHs from pyrogenic and petrogenic origins. Pyrogenic sources, formed mainly via combustion processes, were enriched in HMW PAHs. Petrogenic sources, such as releasing of fuel oil or light refined petroleum products, were dominated by LMW PAHs. Thus, as illustrated in Table 3, the LMW/HMW ratio may be used to determine the petrogenic source and pyrogenic source (Soclo et al. 2000; Rocher et al. 2004; Wang et al. 2006). To estimate the origin of the PAHs in sediment samples from the upper reach of Huaihe River, the LMW/HMW ratios were determined and the results were listed in Table 3. As shown in Table 3, relatively high HMW/LMW ratios (1.1–8.2) were recorded at all sampling sites, which indicated that the origin was predominately pyrolytic. In addition, typical combustion origin (COMB) PAHs could be represented by the sum of the major combustion-specific compounds which were Fla, Pyr, BaA, Chr, BbF, BkF, BaP, InP, and BgP (Prahl and Carpenter 1983) The ratio of COMB/∑EPA PAHs was moderate to high (0.50–0.92) in sediment samples from the upper reach of Huaihe River, revealing that there was extensive combustion activities in the study area.
Several ratios of specific PAHs compounds including Ant/Ant + Phe, Fla/Fla + Pyr, BaA/BaA + Chr, and InP/InP + BgP have been developed for interpreting compositions and inferring possible sources (Soclo et al. 2000; Yunker et al. 2002), as shown in Table 3. In our study, Ant/Ant + Phe and BaA/BaA + Chr ranged from 0.29 to 0.63 and from 0.58 to 0.96, respectively, indicating that the main source of PAHs in sediments in this basin was pyrolysis (Table 3). This was in agreement with the pyrogenic index ratios discussed above. However, some Fla/Fla + Pyr and InP/InP + BgP ratios were less than 0.40 and 0.20, respectively, suggesting that the surface sediments were also contaminated by petrogenic PAHs. This is likely to be due to the usage of crankcase oil in urban areas (Takada et al. 1991). Normally, pyrolytic PAHs were mainly from coal, grass, and wood combustion and/or petroleum combustion. Plots of Ant/Ant + Phe vs. Fla/Fla + Pyr (Fig. 4a) indicated that biomass and coal combustion sources were more commonly found for PAHs in sediments from the upper reach of Huaihe River. The same results were also observed when using BaA/BaA + Chr and InP/InP + BgP ratios (Fig. 4b).
3.4 Ecological risks of PAHs in sediments
The levels of PAHs in sediments from the upper reach of Huaihe River were moderate in comparison with many other aquatic systems (Table 2). In order to evaluate whether sediments in this catchment would potentially cause toxic effects, PAHs levels in sediments were compared against effect-based sediment guideline values such as effect range low (ERL) and effect range median (ERM), which were developed by the US Natural Oceanic and Atmospheric Administration (Long et al. 1995). These two guideline values were useful in addressing sediment quality issues and provided qualitative guidelines on what needed to be done to effectively protect the aquatic environment. PAH concentrations higher than ERM suggest a probable toxic response in aquatic organisms, while those lower than ERL are considered to have no toxic effects. Levels of PAHs between ERM and ERL could have moderately adverse effects on aquatic organisms. The ERL and ERM values for some PAH compounds have been compared to PAH concentrations in sediments from the upper reach of Huaihe River (Table 4). The highest ∑PAHs content was found at T7 site (935.2 μg kg−1) and was much lower than the corresponding ERL value (4,022 μg kg−1). In terms of individual PAH, levels of most of the 16 PAHs in these sediment samples were lower than their respective ERL values (Table 4). Only Nap (T3), Ace (M2, M3, M7, T1, T3, T4, and T7), Flu (M2, M7, and T1), Ant (M3), and BaA (M3 and T4) were above their respective ERL values but lower than their ERM values. These results represented a range of possible effects within which toxic effects would occasionally occur.
4 Conclusions
This study has provided data on the distributions and sources of PAHs in surface sediments from the upper reach of Huaihe River. PAHs pollution was closely related to sewage input and industrial activities. In addition, the distribution of PAHs in sediments from the main stream indicated that the input of tributaries was an important factor for Huaihe River. Four-ring and five-ring PAHs were major species in sediment samples. Fingerprinting analysis indicated that PAHs in the sediment were mostly pyrogenic in origin, likely due to the combustion of coal and biomass, whereas petrogenic origin was found for PAHs in some sediments, probably due to the usage of crankcase oil in urban areas. Comparison of the concentration range with a worldwide survey of sedimentary PAHs concentrations ranked PAHs contamination in sediments as low to moderate pollution. This may be related to the short contamination history of the upper reach of Huaihe River. Adverse effects to benthic communities would occur occasionally at the levels of PAHs contamination observed from the studied area. Data on the PAHs found in this survey can be used as baseline reference concentration for future PAHs monitoring programs.
References
Barakat AO, Mostafa AR, Wade TL, Sweet ST, Sayed NBE (2011) Spatial distribution and temporal trends of polycyclic aromatic hydrocarbons (PAHs) in sediments from Lake Maryut, Alexandria, Egypt. Water Air Soil Pollut 218:63–80
Berto D, Ausili A, Sunseri G, Bellucci LG, Frignani M, Albertazzi S, Giani M (2009) Polycyclic aromatic hydrocarbons (PAHs) from diffuse sources in coastal sediments of a not industrialised Mediterranean island. Water Air Soil Pollut 200:199–209
Cantillo AY, Lauenstein GG, O’Conner TP (1997) Mollusc and sediment contaminant levels and trends in south Florida coastal waters. Mar Pollut Bull 34:511–521
Chen YY, Zhu LZ, Zhou RB (2007) Characterization and distribution of polycyclic aromatic hydrocarbon in surface water and sediment from Qiantang River, China. J Hazard Mater 141:148–155
Cheng YM, Zhu LY, Tian SY, Liang XW (2009) Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments from the Haihe River and the Bohai Sea. Acta Scientiae Circumstantiae 29(11):2420–2426 (in Chinese)
Chiou CT, McGroddy SE, Kile DE (1998) Partition characteristics of polycyclic aromatic hydrocarbons on soils and sediments. Environ Sci Technol 32:264–269
Doong R, Lin YT (2004) Characterization and distribution of polycyclic aromatic hydrocarbon contaminations in surface sediment and water from Gao-ping River, Taiwan. Water Res 38:1733–1744
Fang MD, Hsieh PC, Ko FC, Baker JE, Lee CL (2007) Sources and distribution of polycyclic aromatic hydrocarbons in the sediments of Kaoping River and submarine canyon system, Taiwan. Mar Pollut Bull 54:1179–1189
Feng CL, Xia XH, Shen ZY, Zhou Z (2007) Distribution and sources of polycyclic aromatic hydrocarbons in Wuhan section of the Yangtze River, China. Environ Monit Assess 133(1–3):447–458
Guo W, He MC, Yang ZF, Lin CY, Quan XC, Wang HZ (2007) Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere 68:93–104
He Y, Yan JP (2006) Distribution and ecological risk assessment of PAHs in sediments from Huaihe River. Eco Environ 15(5):949–953 (in Chinese)
Headley JV, Akre C, Conly FM, Peru KM, Dickson LC (2001) Preliminary characterization and source assessment of PAHs in tributary sediment of the Athabasca River, Canada. Environ Forensics 2:335–345
IARC (1987) IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. In: Overall evaluation of carcinogenicity: an updating of IAPC monographs, vols. 1–42, suppl. 7. International Agency for Research on Cancer, Lyon
Jiang B, Zheng HL, Huang GQ, Ding H, Li XG, Suo HT, Li R (2007) Characterization and distribution of polycyclic aromatic hydrocarbon in sediments of Haihe River, Tianjin, China. J Environ Sci-China 19(3):306–311
Ko FC, Baker J, Fang MD, Lee CL (2007) Composition and distribution of polycyclic aromatic hydrocarbons in the surface sediments from the Susquehanna River. Chemosphere 66(2):277–285
Koh CH, Khim JS, Kannan K, Villeneuve DL, Senthilkumar K, Giesy JP (2004) Polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs) and 2,3,7,8-TCDD equivalents (TEQs) in sediment from the Hyeongsan River, Korea. Environ Pollut 132:489–501
Laflamme RE, Hites RA (1978) Tetra- and pentacyclic, naturally occurring, aromatic hydrocarbons in recent sediments. Geochim Cosmochim Acta 43:1687–1691
Leite NF, Peralta-Zamora P, Grassi MT (2011) Distribution and origin of polycyclic aromatic hydrocarbons in surface sediments from an urban river basin at the Metropolitan Region of Curitiba, Brazil. J Environ Sci-China 23(6):904–911
Li JW, Shang X, Zhao ZX, Tanguay RL, Dong QX (2010) Polycyclic aromatic hydrocarbons in water, sediment, soil, and plants of the Aojiang River waterway in Wenzhou, China. J Hazard Mater 173:75–81
Liu Y, Chen L, Zhao JF, Huang QH, Zhu ZL, Gao HW (2008) Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments of rivers and an estuary in Shanghai, China. Environ Pollut 154:298–305
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects with ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97
Mai BX, Fu JM, Sheng GY, Kang YH, Lin Z, Zhang G, Min YS, Zeng EY (2002) Chlorinated and polycyclic aromatic hydrocarbons in riverine and estuarine sediments from Pearl River Delta, China. Environ Pollut 117:457–474
Marvin CH, McCarry BE, Villella J, Allan LM, Bryant DW (2000) Chemical and biological profiles of sediments as indicators of sources of genotoxic contamination in Hamilton Harbour. Part I: analysis of polycyclic aromatic hydrocarbons and thia-arene compounds. Chemosphere 41:979–988
Miles CJ, Delfino J (1999) Priority pollutant polycyclic aromatic hydrocarbons in Florida sediments. B Environ Contam Tox 63:226–234
Mostafa AR, Wade TL, Sweet SW, Al-Alimi AKA, Barakat AO (2009) Distribution and characteristics of polycyclic aromatic hydrocarbons (PAHs) in sediments of Hadhramout coastal area, Gulf of Aden, Yemen. J Mar Syst 78:1–8
Ortega AN, Tauler R, Lacorte S, Barcelo D (2010) Occurrence and transport of PAHs, pesticides and alkylphenols in sediment samples along the Ebro River Basin. J Hydrol 383:5–17
Prahl FG, Carpenter R (1983) Polycyclic aromatic hydrocarbon (PAH) phases associations in Washington coastal sediments. Geochim Cosmochim Acta 47:1013–1023
Rocher V, Azimi S, Moilleron R, Chebbo G (2004) Hydrocarbons and heavy metals in the different sewer deposits in the Le Marais’ catchment (Paris, France): stocks, distributions and origins. Sci Total Environ 323:107–122
Sanctorum H, Elskens M, Leermakers M, Gao Y, Charriau A, Billon G, Goscinny S, Cooman WD, Baeyens W (2011) Sources of PCDD/Fs, non-ortho PCBs and PAHs in sediments of high and low impacted transboundary rivers (Belgium–France). Chemosphere (in press)
Shi Z, Tao S, Pan B, Fan W, He XC, Zuo Q, Wu SP, Li BG, Cao J, Liu WX, Xu FL, Wang XJ, Shen WR, Wong PK (2005) Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ Pollut 134:97–111
Soclo HH, Garrigues P, Ewald M (2000) Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Mar Pollut Bull 40:387–396
Sun JH, Wang GL, Chai Y, Zhang G, Li J, Feng JL (2009) Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan Reach of the Yellow River, Middle China. Ecotoxicol Environ Saf 72:1614–1624
Takada H, Onda T, Harada M, Ogura N (1991) Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in street dust from the Tokyo metropolitan area. Sci Total Environ 107:45–69
Tian FL, Chen JW, Qiao XL, Wang Z, Yang P, Wang D, Ge LK (2009) Sources and seasonal variation of atmospheric polycyclic aromatic hydrocarbons in Dalian, China: factor analysis with non-negative constraints combined with local source fingerprints. Atmos Environ 43:2747–2753
US Environmental Protection Agency (USEPA) (1994) Test methods for evaluating solid waste, physical/chemical methods SW846, revision 2. Office of Solid Waste and Emergency Response, Washington, DC
Van Metre PC, Mahler BJ, Furlong ET (2000) Urban sprawl leaves it’s PAH signature. Environ Sci Technol 34:4064–4070
Villar P, Callejón M, Alonso E, Jiménez JC, Guiraúm A (2006) Temporal evolution of polycyclic aromatic hydrocarbons (PAHs) in sludge from wastewater treatment plants: comparison between PAHs and heavy metals. Chemosphere 64:535–541
Wang XC, Sun S, Ma HQ, Liu Y (2006) Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Jiaozhou Bay, Qingdao, China. Mar Pollut Bull 52:129–138
Yang GP (2000) Polycyclic aromatic hydrocarbons in the sediments of the South China Sea. Environ Pollut 108:163–171
Yu Y, Xu J, Wang P, Sun HW, Dai S (2009) Sediment-porewater partition of polycyclic aromatic hydrocarbons (PAHs) from Lanzhou Reach of Yellow River, China. J Hazard Mater 165:494–500
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zakaria MP, Takada H, Tsutsumi S, Ohno K, Yamada J, Kouno E, Kumata H (2002) Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environ Sci Technol 36:1907–1918
Zeng EY, Yu CC, Tran K (1999) In situ measurements of chlorinated hydrocarbons in the water column off the Palos Verdes Peninsula, California. Environ Sci Technol 33:392–398
Zhang ZL, Hong HS, Zhou JL, Huang J, Yu G (2003) Fate and assessment of persistent organic pollutants in water and sediment from Minjiang River Estuary, Southeast China. Chemosphere 52:1423–1430
Zhang ZL, Huang J, Yu G, Hong HS (2004) Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing, China. Environ Pollut 130:249–261
Acknowledgements
The research was supported by the Key Scientific and Technological Project of Henan province, People’s Republic of China (grant no. 082102350023) and Basic and Front-line Technological Research Project of Henan Province, People’s Republic of China (grant nos. 2009A610007, 092300410091, and 102300410196).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Feng, J., Zhai, M., Sun, J. et al. Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in sediment from the upper reach of Huaihe River, East China. Environ Sci Pollut Res 19, 1097–1106 (2012). https://doi.org/10.1007/s11356-011-0620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0620-3