Abstract
Introduction
Ciliated protozoa play important roles in aquatic ecosystems especially regarding their functions in micro-food web and have many advantages in environmental assessment compared with most other eukaryotic organisms. The aims of this study were focused on analyzing the application of an indicator based on taxonomic relatedness of ciliated protozoan assemblages for marine environmental assessment.
Materials and methods
The spatial taxonomic patterns and diversity measures in response to physical–chemical variables were studied based on data from samples collected during 1-year cycle in the semi-enclosed Jiaozhou Bay, northern China.
Results
The spatial patterns of ciliate communities were significantly correlated with the changes of environmental status. The taxonomic distinctness (Δ*) and the average taxonomic distinctness (Δ+) were significantly negatively correlated with the changes of nutrients (e.g., nitrate nitrogen and soluble active phosphate; P < 0.05). Pairwise indices of Δ+ and the variation in taxonomic distinctness (Λ+) showed a decreasing trend of departure from the expected taxonomic breadth in response to the eutrophication stress and anthropogenic impact.
Conclusion
The taxonomic relatedness (especially the pairwise Δ+ and Λ+) indices of ciliate communities are robust as an indicator with scientifically operational value in marine environmental assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In aquatic ecosystems, ciliated protozoa are a main component of the microplankton communities and play important roles in the microbial food web (Finlay and Esteban 1998). It is increasingly being recognized that ciliated protozoa have many advantages in assessment of marine environments. With their short life cycle and delicate external membranes, they may react more quickly to environmental changes than any other eukaryotic organisms (Coppellotti and Matarazzo 2000). Furthermore, many ciliates can often inhabit environments that are unfavorable to most metazoans and some can even tolerate extremes of environmental conditions to macrofauna (Coppellotti and Matarazzo 2000; Jiang et al. 2007). Thus, special attention is being focused on ciliated protozoa as a favorable bioindicator for monitoring water quality (Ismael and Dorgham 2003; Jiang et al. 2007; Xu et al. 2009).
Several investigations have demonstrated that the distribution of taxonomic relatedness is useful to evaluate marine biodiversity and environmental issues (Clarke and Warwick 1998; Warwick and Clarke 2001). So far the availability of the taxonomic relatedness indices for assessing environmental stress and anthropogenic impact has been demonstrated in several researches on macrofauna (Mouillot et al. 2005; Leonard et al. 2006; Somerfield et al. 2008; Prato et al. 2009). With regard to the relationships between these relatedness indices based on marine microbial data and environmental status, however, little understanding was well documented.
In the present study, the taxonomic relatedness of planktonic-ciliated protozoan communities in marine ecosystem was analyzed based on an annual data of Jiaozhou Bay, Qingdao, northern China, which is a semi-closed biotope with multi-pollution of environmental stress regarding the surrounding conditions. The aims of our study were: (1) to investigate the spatial variations in taxonomic relatedness of ciliated protozoan communities; (2) to reveal their relationships to environmental status in multi-polluted marine waters; and (3) to document the availability of taxonomic relatedness measures based on ciliate assemblages in marine environmental assessment.
2 Methods
2.1 Study sites and period
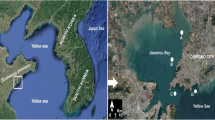
Jiaozhou Bay (35° 58′ N~36° 18′ N, 120° 04′ E~120° 23′ E) is a semi-enclosed basin in the western part of the Yellow Sea, with an area of 390 km2 and an average depth of about 7 m. It is surrounded by the city Qingdao and connects the South Yellow Sea via a narrow mouth. About ten small seasonal streams empty into the bay with varying water and sediment loads, most of which have become discharge canals of industrial and domestic waste discharge from Qingdao and important sources of external nutrients entering Jiaozhou Bay (Fig. 1). In recent decades, the deterioration of water quality has increasingly led serious concern of social and scientific communities (Liu et al. 2008).
Five sampling sites were selected from June 2007 to May 2008 (Fig. 1) according to their environmental status and type of pollutions based on the marine water quality standard of China. Site A was slightly stressed by pollutants (mainly nutrients) from inshore waters due to tidal circulation. Site B was selected as a severely stressed area polluted by organic pollutants, nutrients, and heavy metals (e.g., Pb, Zn) from domestic sewage and industrial discharge via two primary rivers. Site C was located at the where the sources of heavy organic pollution mainly come from mariculture activities and inshore water by tidal circulation. Site D was moderately stressed by both domestic organic and heavy metal pollutants (e.g., Cr, Cu) from two rivers. Site E was located at the mouth of the bay, which was the cleanest area (Fig. 1).
2.1.1 Sampling, fixation, measurements, identification, and enumeration
A total of 24 samplings were carried out biweekly at three depths of 1 m (upper layer), 3 m (middle layer), and 6 m (under layer) from each sampling site during the study period considering the vertical difference in ecological patterns of the planktonic ciliate communities. For quantitative and qualitative studies, 1,000 ml water samples were fixed with Lugol’s solution to a final concentration of 2% (volume/volume). For enumeration of ciliates, a 0.1 ml aliquot of each concentrated sample was placed in a Perspex chamber and the ciliates were counted under a light microscope at a magnification ×400. A total of 0.5 ml concentrated sample were counted and yielded a standard error of <8% of the mean values of counts.
The measurements of concentrations of dissolved inorganic nitrogen (DIN, sum of NO3-N, NO2-N and NH4-N), soluble reactive phosphate (SRP), and chlorophyll a (Chl a) were carried out in the same scheme as Xu et al. (2008). Water temperature, salinity, pH and dissolved oxygen (DO) were recorded in situ with appropriate sensors at three depths.
Protargol staining method was performed according to Montagnes and Humphrey (1998). Species identification of ciliates was based on the published references to keys and guides such as Song et al. (2009). The taxonomic scheme used was according to Lynn (2008).
2.2 Data analysis of samples
Taxonomic diversity (Δ), taxonomic distinctness (Δ*), average taxonomic distinctness (Δ+) and variation in taxonomic distinctness (Λ+) of samples were computed following the equations:
where, x i (i = 1, 2, …, S) denotes the abundance of the ist species, N is the total number of individuals in the sample, ω ij is the “distinctness weight” given to the path length linking species i and j (with i < j, for sake of definiteness), and S is the number of species (Warwick and Clarke 1995).
The distinctness weights used in this study were according to Clarke and Warwick (1998), in context of the class of ciliates: ω = 1 (species in the same genus), 2 (same family but different genera), 3 (same order but different family), 4 (same class but different order), and 5 (same phylum but different class). The distinctness of two species connected at the highest taxonomic level is set equal to 100 (Clarke and Warwick 1998; Warwick and Clarke 2001). A regional master list of ciliates was compiled using the data from Jiaozhou Bay and the local areas of Yellow Sea near Qingdao, in which total 242 ciliate species (except for the sessile forms) was recorded by Song et al. (2009).
Multivariate analyses of spatial variations in ciliate communities were analyzed using the PRIMER v6.1 package (Clarke and Gorley 2006). Bray-Curtis and Sørensen similarity matrices were computed on log-transformed species-abundance and P/A (presence/absence) data, respectively. The separate clusters and multidimensional scaling (MDS) ordination of biotic and abiotic samples were assigned by the routine CLUSTER and MDS on Bray–Curtis similarities from log-transformed species-abundance data. Differences between groups of samples were tested by the submodule ANOSIM (Clarke and Gorley 2006). The spatial environmental status of the five sampling sites was summarized using the principal component analysis (PCA) based on log-transformed/normalized abiotic data, while the biota-environment correlation was tested using the routine RELATE (Clarke and Gorley 2006). Variances of qualitative taxonomic distinctness values (Δ+ and Λ+) with respect to the master list values were summarized by plotting 95% ellipse confidence limits using randomization tests based on the regional master species list by the submodule TAXDTEST (Leonard et al. 2006).
Univariate correlation analyses were carried out using the statistical program SPSS v16.0. Data were standardized by logarithmic transformation before analyzing (Xu et al. 2008).
3 Results
3.1 Physical–chemical parameters
The ranges of physical–chemical parameters at the five sampling sites for a 1-year cycle were summarized in Table 1. Among these variables, the temperature and pH showed minor differences at all sampling stations during the 1-year cycle (Table 1). Salinity ranged between 29.99 and 31.48 with lowest average values at site E and highest at site A. The concentration of DO was commonly higher than 8 mg l−1 at all five sites, with the minimum average value at site D but the maximum at site B. However, the high concentrations of Chl a occurred in site C and D, the lowest in site E. Furthermore, the concentrations of NO3-N, NOn-N (sum of NO3-N and NO2-N) and combination of NOn-N and SPR basically showed high values at site B and C but low in site E and A although DIN, SRP and sum of DIN and SRP presented maximum values at site D mainly due to the most primary contributor NH4-N (Table 1).
3.2 Taxonomic composition and spatial distribution
Taxonomic compositions crossing five taxonomic levels of ciliate communities in Jiaozhou Bay was summarized in Table 2. A total of 75 ciliate species from 27 genera, 17 families, nine orders, and four classes was recorded through examining the 360 samples collected biweekly from five sites during June 2007 and May 2008 (Table 2). From species to order level, the annual cumulative number of taxa showed maximum values at site C but minimum at site B (Table 2).
A dendrogram of the spatial species distribution was plotted using group-average clustering on Sørensen similarities for presence/absence data of each species within the protozoan communities from the five sampling sites in Jiaozhou Bay (Fig. 2). The cluster analysis resulted in the 75 ciliate species falling into five groups (I–V) at a 60% similarity level. Group I was composed of 52 common taxa with high occurrence, while the other four groups (II–V) represented the assemblages with low occurrence (Fig. 2). A total of 33 common species (e.g., Strombidinium sulcatum, Tintinnopsis parvula, Strombidinopsis loricata, Rimostrombidium orientale, Mesodinium pupula, and Leprotintinnus bottnicus) in group I appeared at all five sampling sites. Of the other five groups, almost all species (e.g., Eutintinnus apertus and Metastrombidium sonnifer) of group II occurred at both site A and E, whereas group III (e.g., Tintinnopsis ovalis and Strombidinopsis elongata) mainly at site C and E, group IV at site E, and group V at site D (Fig. 2).
3.2.1 Spatial variations in taxonomic relatedness of ciliate communities
Discriminating among a total of 360 samples from the five sampling sites was carried out by the routine ANOSIM on Bray–Curtis similarities from log-transformed data. It was shown that there were significant differences among the five sites (R = 0.109, P < 0.001) and between each pair of sites (P < 0.05) apart from sites B and C, between which there were no significant differences (R = 0.017, P = 0.17). However, no significant differences were found among the three depths at each site
The annual average values of taxonomic diversity indices of ciliate communities at five sites in Jiaozhou Bay were summarized in Table 3. All four parameters presented highest value at site A/E, moderate at site C/D, and the lowest at site B (Table 3). Additionally, it is notable that although the highest value of annual cumulative species number occurred in site C the biodiversity behaved in lower value (Tables 2 and 3)
Ellipses plots of 95% probability regions with a range sublist sizes (10, 20) for the pairwise (Δ+, Λ+) values of ciliate communities from each sampling site in Jiaozhou Bay were showed in Fig. 3. The Δ+−Λ+ pairs for each site were superimposed on the appropriate plot for their particular species list (Fig. 3). With regard to the sublist size of 10 species, the proportion of ciliate samples with lower values of pairs (Δ+, Λ+) from each site were outside the 95% probability contour in the order: site B (88.6%) > site C (80.6%) > site D (70.4%) > site E (56.7%) > site A (50.0%).
3.3 Linkage between biotic data and environmental status
The relationships among the five sampling sites based on average data for environmental variables and for ciliates of each sampling sites were summarized by clustering analyses and MDS ordination in Fig. 4. This multivariate approach revealed that the spatial patterns of the ciliate communities were consistent with those of the environmental variables. In both cases there were high similarities between the two most polluted sites (B and C) and somewhat lower similarities between the two least polluted sites (A and E). The moderately polluted site D was more similar to the former pair than the latter (Fig. 4). RELATE analysis revealed that there was a significant correlation between spatial variations in ciliate abundances and changes of environmental variables (R = 0.183; P = 0.001).
Cluster analysis and MDS ordination for spatial changes of environmental status a and b on Euclidean distance from log-transformed environmental data, and for spatial patterns of ciliated protozoan communities c and d on Bray–Curtis similarities for species-abundance data obtained from five sampling sites in Jiaozhou Bay during the period from June 2007 to May 2008
PCA ordination with vectors for both structural/taxonomic parameters of ciliate communities and physical–chemical variables was shown in Fig. 5. The two principal components, explaining 74.7% of the total spatial environmental variability, discriminated the five sites in a similar pattern that was shown by the clustering/MDS analyses (Fig. 5). The vectors for the physical–chemical variables (e.g., NH3-N, SRP, salinity and Chl a) pointed toward site D (upper left), while the other variables (e.g., NO3-N and DO) toward sites B and C (lower left). It should be noted that most structural parameters and all four taxonomic diversity indices pointed toward site A (upper right) with negative correlations with chemical parameters (Fig. 5). Pearson correlation analyses demonstrated that both relatedness indices Δ+ and Δ* significantly negatively correlated with the changes of NO3-N/NOn-N + SRP (P < 0.05; Table 4).
4 Discussion
Multivariate analyses are more sensitive than univariate analyses in detecting changes in community structures. They are also extremely useful for analyzing differences between communities on spatial and temporal scales, and for illustrating how these communities vary along gradients of environmental stress (Jiang et al. 2007; Xu et al. 2008, 2009; Tan et al. 2010).
In our study, both MDS ordination and clustering/PCA analyses revealed a clear spatial pattern of environmental status. For example, the less polluted sites (A and E) were separated from the more polluted sites (B, C, and D). Otherwise, MDS ordination and clustering/RELATE analyses demonstrated that the spatial variations in ciliate community structures were significantly correlated with certain environmental variables. These findings suggest that the spatial patterns of ciliate communities accurately reflect the water conditions and have the potential for use in assessing marine environmental status.
The traditional biodiversity parameters (e.g., species richness, diversity and evenness) are more widely used to summarize the structural patterns of a biotic community (Ismael and Dorgham 2003; Mouillot et al. 2005; Xu et al. 2008; Prato et al. 2009; Xu et al. 2009; Tan et al. 2010). However, these measures present a number of problems for quantifying environmental changes due to their sample size or sampling effort dependence (Warwick and Clarke 2001; Leonard et al. 2006; Prato et al. 2009). Notably, they are difficult to be linked with environmental data since their relationship remains controversial and not always monotonic, especially problematic when comparing data collected by sampling methods that are not standardized in terms of effort or sample size (Prato et al. 2009). Furthermore, there is no statistical framework for testing whether these parameters are higher or lower than expected for a given location or region (Clarke and Warwick 1998; Prato et al. 2009). Thus, the traditional diversity indices are largely biased and restricted in assessment of environmental traits (Mouillot et al. 2005; Xu et al. 2008). Many previous researches on metazoan fauna have demonstrated that the taxonomic diversity/distinctness measures showed less sensitivity to natural habitat type but more to anthropogenic environmental variables, in particular the taxonomic distinctness indices (Δ+ and Λ+) as the useful indicator of anthropogenic disturbances in coastal marine waters, in comparison to traditional biodiversity indices (Leonard et al. 2006; Somerfield et al. 2008; Prato et al. 2009).
Based on our data, both univariate and multivariate analyses demonstrated that the taxonomic diversity/distinctness (Δ* and Δ+) indices of ciliate communities represented a clear significantly negative correlation with the combination NO3-N/NOn-N and SRP (P < 0.05). Furthermore, the ellipse plotting analyses suggested that the paired taxonomic distinctness measures (Δ+ and Λ+) showed a clear decreasing trend of departure from the expected taxonomic breadth in response to anthropogenic impact and eutrophication stress. For example, the more the site was stressed, the more data sets (Δ+ and Λ+) were fallen below the simulated mean value or outside the 95% confidence limits; the lower the values of pairs were, the more the site was departed from the expected contour. Thus, results support the proposal that the taxonomic diversity/distinctness indices of ciliate communities are robust as an indicator available for assessing marine environmental status.
It should be noted that although the spatial patterns of ciliated protozoan communities were significantly different among the five sampling sites, no differences were detected among the three depths at which samples were collected. This finding suggests that the vertical variations are negligible, and thus the cost in time and resources could be further reduced by sampling at a single depth. This is also an advantage in detecting transient variations in environmental status using planktonic ciliated protozoa.
In summary, our study demonstrated that the spatial patterns of ciliate communities were significantly correlated with the changes of environmental status and that the taxonomic distinctness (Δ*) and the average taxonomic distinctness (Δ+) were significantly negatively correlated with the environmental stress. These findings suggest that the taxonomic diversity indices of ciliated protozoan communities may be served as a useful tool with scientifically operational value in assessment of marine environments. However, it should be noted that further studies on a range of marine environments and over long time periods are needed in order to verify this conclusion.
References
Clarke KR, Gorley RN (2006) User manual/turorial. PRIMER-E Ltd, Plymouth
Clarke KR, Warwick RM (1998) A taxonomic distinctness index and its statistical properties. J Appl Ecol 35:523–531
Coppellotti O, Matarazzo P (2000) Ciliate colonization of artificial substrates in the Lagoon of Venice. J Mar Biol Assoc UK 80:419–427
Finlay BJ, Esteban GF (1998) Freshwater protozoa: biodiversity and ecological function. Biol Conserv 7:1163–1186
Gong J, Song W, Warren A (2005) Periphytic ciliate colonization: annual cycle and responses to environmental conditions. Aquat Microb Ecol 39:159–170
Ismael AA, Dorgham MM (2003) Ecological indices as a tool for assessing pollution in El-Dekhaila Harbour (Alexandria, Egypt). Oceanologia 45:121–131
Jiang J, Wu S, Shen Y (2007) Effects of seasonal succession and water pollution on the protozoan community structure in an eutrophic lake. Chemosphere 66:523–532
Leonard DRP, Clarke KR, Somerfield PJ, Warwick RM (2006) The application of an indicator based on taxonomic distinctness for UK marine biodiversity assessment. J Environ Manage 78:52–62
Liu D, Sun J, Zhang J, Liu G (2008) Response of the diatom flora in Jiaozhou Bay, China to environmental changes during the last century. Mar Micropaleontol 66:279–290
Lynn DH (2008) The ciliated protozoa. Characterization, classification and guide to the literature, 3rd edn. Springer, New York
Montagnes DJS, Humphrey E (1998) A decription of occurrence and morphology of a new species of red-water forming Strombidium (Spirotrichea, Oligotrichia). J Eukaryot Microbiol 45:502–506
Mouillot D, Gaillard S, Aliaume C, Verlaque M, Belsher T (2005) Ability of taxonomic diversity indices to discriminate coastal lagoon environment based on macrophyte communities. Ecol Indic 5:1–17
Prato S, Morgana JG, La Valle P, Finoia MG, Lattanzi L (2009) Application of biotic and taxonomic distinctness indices in assessing the ecological quality status of two coastal lakes: Gaprolace and Foglino Lakes (Central Italy). Ecol Indic 9:568–583
Somerfield PJ, Clarke KR, Warwick RM, Dulvy NK (2008) Average functional distinctness as a measure of the composition of assemblages. ICES J Mar Sci 65:1462–1468
Song W, Warren A, Hu X (2009) Free-living ciliates in the Bohai and Yellow Seas. Science Press, Beijing, In both Chinese and English
Tan X, Shi X, Liu G, Xu H, Nie P (2010) An approach to analyzing taxonomic patterns of protozoan communities for monitoring water quality in Songhua River, northeast China. Hydrobiologia 638:193–201
Warwick RM, Clarke KR (1995) New “biodiversity” measures reveal a decrease in taxonomic distinctness with increasing stress. Mar Ecol Prog Ser 129:301–305
Warwick RM, Clarke KR (2001) Practical measures of marine biodiversity based on relatedness. Oceanogr Mar Biol 39:207–231
Xu H, Min GS, Choi JK, Jung JH, Park MH (2009) An approach to analyses of periphytic ciliate colonization for monitoring water quality using a modified artificial substrate in Korean coastal waters. Mar Pollut Bull 58:278–1285
Xu H, Song W, Warren A, Al-Rasheid KAS, Al-Farraj SA, Gong J, Hu X (2008) Planktonic protist communities in a semi-enclosed mariculture pond: structural varation and correlation with environmental conditions. J Mar Biol Assoc UK 88:1353–1362
Xu K, Choi JK, Yang EJ, Lee KC, Lei Y (2002) Biomonitoring of coastal pollution status using protozoan communities with a modified PFU method. Mar Pollut Bull 44:877–886
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 40976075, 30700069), and a grant from the Center of Excellence in Biodiversity, King Saud University. Special thanks are due to Mr. X. Fan, Ms. J. Jiang and X. Chen, Laboratory of Protozoology, Institute of Evolution and Marine Biodiversity, Ocean University of China, China, for their help with sampling and sample processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Braunbeck
Rights and permissions
About this article
Cite this article
Xu, H., Jiang, Y., Al-Rasheid, K.A.S. et al. Application of an indicator based on taxonomic relatedness of ciliated protozoan assemblages for marine environmental assessment. Environ Sci Pollut Res 18, 1213–1221 (2011). https://doi.org/10.1007/s11356-011-0476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0476-6