Abstract
Introduction
Ambient air and bulk deposition samples were collected between June 2008 and June 2009. Eighty-three polychlorinated biphenyl (PCB) congeners were targeted in the samples.
Discussion
The average gas and particle PCB concentrations were found as 393 ± 278 and 70 ± 102 pg/m3, respectively, and 85% of the atmospheric PCBs were in the gas phase. Bulk deposition samples were collected by using a sampler made of stainless steel. The average PCB bulk deposition flux value was determined as 6,020 ± 4,350 pg/m2 day. The seasonal bulk deposition fluxes were not statistically different from each other, but the summer flux had higher values. Flux values differed depending on the precipitation levels. The average flux value in the rainy periods was 7,480 ± 4,080 pg/m2 day while the average flux value in dry periods was 5,550 ± 4,420 pg/m2 day. The obtained deposition values were lower than the reported values given for the urban and industrialized areas, yet close to the ones for the rural sites. The reported deposition values were also influenced by the type of the instruments used. The average dry deposition and total deposition velocity values calculated based on deposition and concentration values were found as 0.23 ± 0.21 and 0.13 ± 0.13 cm/s, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atmospheric deposition plays an important role for the toxic materials to reach surface waters and the other environments. Besides heavy metals such as As, Cd, Pb, and Hg, some of the organic materials are also counted as toxics. Because these materials have negative effects on human health and environmental risks, the investigation of the atmospheric deposition of toxic materials has been studied widely (Eisenreich and Strachan 1992; Rojas et al. 1993; Buehler and Hites 2002; Landis and Keeler 2002; Rolfhus et al. 2003).
Semi-volatile organic compounds (SVOCs) are found in the atmosphere in both particulate and gas forms. As a result of this global scale transport mechanism, SVOCs are carried long distances from the source, deposit in pristine sites, and cause pollution in those environments. Atmospheric transport modeling of the SVOCs and determination of the deposition characteristics become more of an issue (Simcik et al. 1997).
Photolytic and oxidative reactions as well as wet and dry deposition mechanisms are among the atmospheric removal mechanisms of the SVOCs including polychlorinated biphenyls (PCBs; Dickhut and Gustafson 1995; Hillery et al. 1998; Garban et al. 2002; Fang et al. 2004).
Atmospheric deposition occurs in two ways such as wet and dry deposition. Pollutants deposit either by dry deposition or by precipitation. Different measurement methods are used in the literature in order to determine wet and dry deposition amounts and fluxes of the SVOCs. Determination of the flux values are very important to build up global and regional model simulations of the air pollutants. More sensitive, easier, and more reliable measurements are needed to reach this goal. In the recent years, a number of studies have been carried out in various countries and sites in order to determine the mechanism of the atmospheric deposition (Arndt et al. 1998; Walker et al. 2000; Irwin et al. 2002; Sakata et al. 2006).
Bulk deposition is a sampling technique in which dry and/or wet depositions are collected at the same time. Different shaped samplers are used in collecting atmospheric bulk deposition samples. The sampling devices made of stainless steel are used as well as bottles made of borosilicate glass or dark color glasses (Grünhage et al. 1993; Manoli et al. 2000; Garban et al. 2002; Gocht et al. 2007; Motelay-Massei et al. 2007).
The aims of this study include (a) determination of the atmospheric concentrations and bulk deposition fluxes of the PCBs in a semi-rural area, (b) assessment of the temporal changes of bulk deposition fluxes, (c) comparison of the measured values with the previously measured data obtained from a water surface sampler (WSS) and a bulk deposition sampler, and (d) determination of the bulk deposition velocities of the PCBs.
2 Material and method
2.1 Sampling program
In order to determine the PCB concentrations and the bulk deposition fluxes, 70 airborne samples and 25 bulk deposition samples were collected from a sampling point (40°13′40.66″ N–28°52′35.11″ E) in the Uludag University Campus (UUC). The sampling was carried out between June 2008 and June 2009. The sampling site was assumed to be semi-rural region, located 1.5 km to the Bursa–İzmir highway and the UUC has over 40,000 students (Tasdemir and Günez 2006).
Ambient air samples were collected using a high-volume air sampler (HVAS; Thermo Andersen GPS 11 Model, USA) and bulk deposition sampler (BDS). The samplers were placed on a 1-m high platform placed on the roof a building with four floors. The sampling site is shown in Fig. 1. The meteorological data measured during the sampling period is summarized in Table 1.
2.2 Sample collection
When there was no precipitation, HVAS was activated in the sampling site and air samples were collected. In order to provide the particulate and gas phases, 10.2 cm glass fiber filter (GFF) and two polyurethane foams (PUFs) with 5 cm length and 5.5 cm diameter were used in the sampler. The average air volume during the period was 165.78 ± 62.08 m3. Four samples were collected for each bulk deposition sample if there were no rain.
The bulk deposition samples were collected by using a BDS made up of stainless steel, with 60.5 cm diameter and 19 cm height. In order to prevent the negative effects of the turbulence, a leading edge with 20 cm width was placed around the BDS (Cindoruk et al. 2008). The schematic figure of the BDS is presented in Fig. 2. The BDS was exposed to under the atmosphere for 15 days, and bulk deposition samples were collected.
Schematic display of bulk deposition sampler (Esen et al. 2008)
2.3 Analytical procedure
All glass equipment used in the laboratory was rinsed with hot tap water, distilled water, methanol (MeOH), and acetone (ACE; Cindoruk et al. 2007; Cindoruk et al. 2008).
In order to remove the probable organic residues completely, GFFs used in the HVAS were loosely covered with aluminum foil, kept in the 450°C oven overnight, and then cooled and kept in the freezer until usage (Cindoruk et al. 2007, 2008; Cindoruk and Tasdemir 2007).
Before their first usage, the PUFs used in the HVAS were extracted with distilled water, MeOH, acetone/hexane (ACE/HEX 1:1), and dichloromethane (DCM) in Soxhlet extractor for 24 h and then dried under 60°C (Esen et al. 2006; Cindoruk and Tasdemir 2007). After the GFFs and cartridges reach to the room temperature, GFFs were kept in aluminum foil and PUF cartridges were kept in glass jars with Teflon covers until usage (Cindoruk and Tasdemir 2007).
After sampling, the PUF cartridges were extracted for 24 h in the Soxhlet extractor with 1:4 (v/v) DCM/petroleum ether (PE) mixture (Esen et al. 2006). On the other hand, the GFFs were extracted with the ultrasonic bath (Elmasonic, S 80 H Model, Germany) with 25 mL DCM/PE (1:4) mixture for 30 min. This procedure was repeated once more. Then, the bottle with the sample was rinsed with 5 mL same solvent mixture and added to the other solvent mixture.
BDS samples (if there was water on them) were filtered through XAD-2 resin, and then, the resin was extracted in the ultrasonic bath for 30 min twice with 100 mL ACE/HEX (1:1) mixture. The samples were filtered through sodium sulfate (Na2SO4) to remove any water exist. For the samples taken in dry periods, the surface of the BDS was rinsed with ACE/HEX (1:1) mixture. This procedure was repeated several times and the solvents were kept in Teflon-covered jars. Finally, the BDS surface was cleaned with tissue to remove the pollutants on it and the tissues were also kept for analysis.
After the samples were extracted, their volumes were reduced to 5 mL by using a rotary evaporator (Heidolp, Laborota 4001 Model, Germany) working under 22°C and 20 rpm. Then, 15 mL of HEX was added to the sample, and the volume was reduced to 5 mL again. After that, the volume was reduced to 2 mL by using pure nitrogen flow.
Two milliliters samples were passed through a column containing 3 g of silicic acid, 2 g of alumina, and 2 g of Na2SO4 to separate PCBs (Esen et al. 2006; Cindoruk et al. 2007). Twenty milliliters DCM and 20 mL PE were used to rinse the column, respectively, in order to clean column; after that, 2 mL sample and 25 mL PE were added to the column to collect the PCB fractions (Tasdemir et al. 2004, 2005). The sample volume was reduced to 5 mL with a rotary evaporator, and the volume was reduced to 2 mL again after 15 mL HEX addition. The samples including PCBs were acid-washed to remove the probable pollutants before the chromatographic analyses. Two milliliters sample was placed in glass centrifuge tube with Teflon cover. One milliliter of sulfuric acid (98% purity, Merck, Germany) was added, and the sample was centrifuged (Sigma, 1-15P Model, Germany) under 3,000 rpm for 2 min. After this, the supernatant including PCB was obtained. In order to collect any PCB residuals in acid, 0.5 mL HEX was poured into the glass tube and centrifuged again. Then, 0.5 mL HEX on the top of the tube was taken to the sample bottle (Cindoruk et al. 2008). After the processes of extraction, volume reduction, and fractionation, the samples were ready for the chromatographic analyses and they were put in 2 mL vials and kept in the −20°C freezer until being analyzed.
Gas chromatography analyses were done by using HP 7890A-μECD (Micro-Electron Capture Detector; Hewlett-Packard, USA) instrument. The oven temperature program used in the PCB analyses was 70°C (2 min), increasing with 25°C/min to 150°C, then 3°C/min to 200°C, then 8°C/min to 280°C, followed by 8 min of holding under 280°C, increasing with 10°C/min to 300°C, and holding for 2 min. The final program time was 41.87 min. The inlet temperature was kept for 250°C and the detector temperature was 320°C. The carrying gas was helium and the makeup gas was nitrogen. HP5-MS, 30 m × 0.32 mm × 0.25 μm, was used as a capillary column.
Eighty-three PCB congeners were targeted in the samples, and they were PCB#4/10, PCB#9/7, PCB#6, PCB#8/5, PCB#19, PCB#12/13, PCB#15/17, PCB#16/32, PCB#26, PCB#31, PCB#28, PCB#21, PCB#53, PCB#22, PCB#45, PCB#52, PCB#47, PCB#49/48, PCB#44, PCB#37/42, PCB#71/41/64, PCB#100, PCB#74, PCB#70/61, PCB#66/95, PCB#91, PCB#56/60, PCB#92, PCB#84, PCB#89/101, PCB#99, PCB#119, PCB#83, PCB#81/87, PCB#86, PCB#85, PCB#77/110, PCB#135/144, PCB#114/149, PCB#118, PCB#123, PCB#131, PCB#153, PCB#132/105, PCB#163/138, PCB#126, PCB#128, PCB#167, PCB#174, PCB#202/171/156, PCB#172, PCB#180, PCB#200, PCB#170/190, PCB#169, PCB#199, PCB#207, PCB#194, PCB#205, and PCB#206. For the calibration of the instrument, five standards were prepared with the concentrations between 0.05 and 25 ng/mL. After each 25 samples injection, the medium standard (18 ng/mL) was injected to check the stability. Instrument detection limit was determined as 0.1 pg for 1 μL injection.
2.4 Quality control/quality assurance
In order to prevent the probable organic contamination, only the materials made of glass, stainless steel, and Teflon were used in the sampling, extraction, and analysis.
To determine the losses during the extraction and analysis of the collected samples, surrogate standards were used. This standard had a concentration of 4 ng/mL and contained the congeners of PCB#14, PCB#65, and PCB#166. PCB#30 and PCB#204 congeners were used for the volume corrections. The recovery efficiencies obtained in PCB#14, PCB#65, and PCB#166 congeners for the HVAS and BDS samples were given in Table 2.
In order to determine the probable contamination generated during sampling, extraction, and analysis, blank samples were collected and analyzed. They were at the amount of 15% of the total number of samples. The ratios of the total PCB mass in the blank samples to the PCB mass in the samples were found as 5.9 ± 8.0% and 2.5 ± 3.5% for GFFs and PUF cartridges, respectively. The blank ratio was found as 1.74 ± 1.55% for the BDS. Limit of detection (LOD) values were calculated for each congener as blank average concentration plus three times standard deviation (average ± 3 σ; Yeo et al. 2003; Gambaro et al. 2004; Biterna and Voutsa 2005; Kim and Masunaga 2005). The obtained LOD values for individual PCB congeners ranged from 0 to 1.5 ng for the HVAS GFF, 0 to 1.4 ng for the HVAS PUF cartridge, and 0 to 1.6 ng for the BDS. Congener values under the LODs in the samples were neglected. All results in this study were blank corrected. The concentration and flux values were corrected with the field blanks in order to eliminate the background contamination and artifacts by subtracting the average blank amount from the sample values.
3 Results and discussion
3.1 Ambient air concentrations
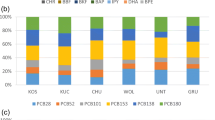
Atmospheric PCB concentration samples were collected by using a HVAS. The average gas and particulate PCB concentrations were 393 ± 278 and 70 ± 102 pg/m3, respectively. It was found that the 85% of the total PCB concentration was in the gas form. Figure 3 presents the average distribution of the gas and particulate concentrations of the PCB congeners. When the distribution of the PCB congeners is assessed, it is observed that congeners with the low molecular weights are dominant.
The measured gas and particulate phase concentrations are higher than rural area values yet lower than the urban area values (Mandalakis et al. 2002; Tasdemir et al. 2004). Some results of the various literature studies about atmospheric concentrations of PCBs are given in Table 3. The average gas and particulate PCB concentrations measured at the same site in the previous years were 328 ± 284 and 86 ± 128 pg/m3, respectively (Cindoruk and Tasdemir 2007). However, the previous study included 41 PCB congeners.
When the seasonal fluctuations of the PCB concentrations are assessed, it is seen that the highest PCB concentrations are observed in the summer. This is because of the higher evaporation rates from various surfaces (soil, water, plant surfaces) in the summer (Biterna and Voutsa 2005; Tasdemir et al. 2005). Moreover, different emission sources including primary emissions from buildings can be important air contaminants in urban sites. No statistical difference is determined among the other seasons. Total PCB concentrations depending on seasonal changes are shown in Fig. 4.
3.2 Bulk deposition fluxes
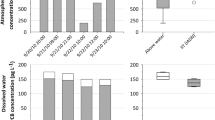
In this study, it was aimed to determine the bulk deposition fluxes of the PCBs employing a BDS in the UUC considered as a semi-rural site. The average bulk deposition flux of PCBs is 6,020 ± 4,348 pg/m2 day changing from 1,342 to 20,362 pg/m2 day. The average value determined in this study is lower than industrial/urban areas, but it is close to the ones reported from the rural sites. Temporal variation of bulk deposition flux values and PCB congener distributions from the samples are shown in Figs. 5 and 6.
Table 4 summarizes the bulk deposition flux values from various sites, and there are high oscillations among the values. The big differences depend on the sampling sites, meteorological conditions, atmospheric levels, sampler types, and targeted PCB numbers. Generally stainless steel samplers were used; moreover, Pyrex and glass were also employed as the sampler construction materials. There are some sampling artifacts for PCB bulk sampling in general. For example, specially volatile PCBs can evaporate from the collection surface during the long sampling periods. PCBs are also subject of photo-degradation. Moreover, deposited particle phase PCBs can be re-suspended due to wind effects. All these reasons cause underestimation of PCB fluxes. On the other hand, after precipitation, there will be some water volume on the collection surface. In this case, when the particles reach the water surface they do not bounce off to the atmosphere and dissolve in the water. Therefore, particle collection efficiency of the sampler increases.
Cindoruk (2007) employed a similar bulk deposition sampler, but targeted PCB congener number was 41 while in our study the targeted congener number was increased to 83. Moreover, the sampling sites were different between this study and the studies conducted by Cindoruk (2007) and Cindoruk et al. (2008). For example, in one of the studies achieved by Cindoruk et al. (2008), the samples were collected from the Bursa organized industrial district (BOID), and its average value was 15.4 ± 14.3 ng/m2 day. In the second study, the average bulk deposition value was 36.2 ± 21.4 ng/m2 day measured in an urban site, Gülbahce, where plastics and other wastes were partially combusted for heating (Cindoruk 2007). Therefore, the difference among the bulk deposition values was mainly due to the sampling site characteristics and sources affecting the atmospheric levels.
When the seasonal variation in the deposition fluxes of the PCBs are assessed, no statistical differences are observed among the seasonal averages but higher deposition fluxes are obtained in summer and winter seasons. In the summer, the atmospheric concentrations are high; however, in the winter, rainy periods are dominant. The seasonal changes of bulk deposition flux values are shown in Fig. 7.
The correlations between the bulk deposition fluxes of the PCBs and the ambient air temperature and wind speed were analyzed, but no statistically significant relationship was found among these three parameters (for the temperature–flux relationship r 2 < 0.01, p > 0.05; for the wind speed–flux relationship r 2 = 0.10, p > 0.05). This is likely due to the fact that wet and dry deposition of pollutants from the atmosphere is a complex process dependent on the wide spectra of parameters including height of clouds, type and intensity of precipitation, quality of surface, and many others.
Transport of the particulates with the rain and the dissolution of the gas phase PCBs into the rain drops according to the Henry’s law were reported in the literature for SVOCs (Duinker and Bouchertall 1989; Poster and Baker 1996a, b; Offenberg and Baker 2002). In the scope of this study, the deposition flux values obtained in dry weather periods (5.55 ± 4.42 ng/m2 day) were lower than the values determined for the rainy periods (7.48 ± 4.08 ng/m2 day). Not only precipitation but also the accumulated rain water on the BDS was responsible for this result. The accumulated water on the BDS sampler captures particulates, and particulates containing PCBs do not bounce off when they hit the water surface (Taşdemir and Holsen 2005). Moreover, accumulated rain water attempts to get equilibrium with the gas phase PCBs in the air; thus, some sort of transportation takes place.
When the homolog distributions of the PCBs were analyzed in rainy and dry period samples, it was determined that 8-chlorinated biphenyls were dominant in dry periods; otherwise, in rainy periods, 6-chlorinated biphenyls (6-CBs) showed more dominant characters. PCB homolog distributions of PCBs in wet and dry period samples were shown in Fig. 8.
We tried to demonstrate the influence of the rain volumes on the flux values. Figure 9 presents the relationship between both of these variables. The correlation between the rainfall and the deposition fluxes was calculated, but no statistically significant relationship between these two variables was obtained (p > 0.050).
In order to show the effect of our sampler on the collected flux values, our data were compared with the values obtained by a WSS. The WSS, which has been described in detail elsewhere (Tasdemir et al. 2005; Cindoruk and Tasdemir 2007; Cindoruk et al. 2008), is briefly described here. Water entered the water surface from its center and overflowed from the weirs located along its sides. The retention time of the water on the surface was 2 to 3 min in order to minimize evaporative losses of deposited PCBs. The recycled water was passed through a filter and a XAD-2 resin column before completing a cycle. The filter collects the particle phase PCBs while the resin column, located after the filter, captures the dissolved PCBs. In a study by Cindoruk and Tasdemir (2007), the same site was sampled between the dates of June 2004 and May 2005. The WSS was used in that study and dry deposition flux was found as 46 ± 40 ng/m2 day and air–water gas exchange flux was determined to be 79 ± 41 ng/m2 day. Since the WSS study conducted when there was no precipitation, it would be fair to compare them with the BDS samples which were taken under dry atmospheric conditions. Deposition fluxes obtained with the BDS sampler were lower than the values obtained by the WSS. This was because particle phase pollutants collected on the WSS do not re-suspend to the atmosphere again. Moreover, gas phase pollutants dissolved in water are captured in the resin and do not volatilize to the atmosphere from the WSS either. Therefore, the WSSs were employed in the dry deposition of the gas and particle SVOCs in the literature (Seyfioglu and Odabasi 2006; Cindoruk and Tasdemir 2007). For the BDS, in the dry periods, settled particulate phase contaminants re-suspend to the atmosphere with the turbulence caused by winds and gas phase is not captured. On the other hand, in the rainy periods, the SVOCs dissolved in the accumulated water in the BDS may re-volatilize back to the atmosphere depending on the Henry’s law because BDS does not have a resin column to capture the dissolved phases.
3.3 Bulk deposition velocities
Atmospheric samples were collected with a HVAS during the sampling period for the determination of PCB concentration levels. Deposition velocity values were calculated by dividing the bulk deposition fluxes to the concentration values. Only dry deposition was observed for some of the samples. In this situation, the deposition velocity (v d = F p/C p) was calculated by dividing the flux value (F p) to the particle phase concentration (C p). On the other hand, both wet and dry depositions were observed for some samples. In these samples, bulk deposition velocity was calculated by dividing the bulk deposition flux to the total (gas + particle) concentration. Bulk deposition velocities were 0.23 ± 0.21 cm/s for dry period samples and 0.13 ± 0.13 cm/s for samples obtained in the rainy periods. Lower deposition velocities were calculated in the rainy period because not only particulate but also gas phase deposition occur simultaneously, and the gas phase deposition velocity was about ten times lower than particulate phase (Wethington and Hornbuckle 2005).
In the rainy period samples, higher deposition velocities were calculated for the 2-chlorinated biphenyls (2-CBs) and 3-chlorinated biphenyls (3-CBs). These values were 0.31 and 0.11 cm/s for 2-CBs and 3-CBs, respectively. This result points out that PCB congeners with low molecular weights dissolve in the rain. On the other hand, 4-chlorinated biphenyls (4-CBs) and 6-CBs have higher deposition velocities in the dry period samples and they were 0.12 and 0.10 cm/s, respectively.
4 Conclusions
Bulk deposition fluxes and concentrations of the PCBs were measured between June 2008 and June 2009. The measured gas and particulate phase concentrations were higher than the values reported for the rural areas but lower than the values given for the urban areas. In order to have more reliable data set from the ambient air samples, the number of ambient air samples should be increased and some ambient air samples should be taken during the precipitation.
The flux values of the samples collected in the rainy periods were higher than the ones determined in the dry periods. On the other hand, higher deposition fluxes are observed in the summer season probably due to higher atmospheric concentrations.
Deposition velocities of the samples collected in the rainy period were lower than the values determined in the dry periods. When the homolog distributions of the PCBs were analyzed, it was determined that bulk deposition velocities of 2-CBs and 3-CBs in the rainy period samples and 4-CBs and 6-CBs in the dry period samples were dominant.
It should be also noted that site characteristics, atmospheric concentrations, and meteorological parameters have significant effects on the deposition mechanisms of the PCBs.
References
Alonso SG, Pastor SMP (2003) Occurrence of PCBs in ambient air and surface soil in an urban site of Madrid. Water Air Soil Pollut 146:283–295
Arndt RL, Carmichael GR, Roorda JM (1998) Seasonal source–receptor relationships in Asia. Atmos Environ 32:1397–1406
Biterna M, Voutsa D (2005) Polychlorinated biphenyls in ambient air of NW Greece and particulate emissions. Environ Int 31:671–677
Bruhn R, Lakaschus S, McLachlan MS (2003) Air/sea gas exchange of PCBs in the southern Baltic Sea. Atmos Environ 37:3445–3454
Buehler SS, Hites RA (2002) The Great Lakes integrated atmospheric deposition network. Environ Sci Technol September 1:354A–359A
Cindoruk SS (2007) Determination of the concentrations, dry deposition and air–water interface fluxes of PCBs. Ph.D. thesis, Science Institute, Uludag University, Turkey-Bursa
Cindoruk SS, Tasdemir Y (2007) Deposition of atmospheric particulate PCBs in suburban site of Turkey. Atmos Res 85:300–309
Cindoruk SS, Esen F, Tasdemir Y (2007) Concentration and gas/particle partitioning of polychlorinated biphenyls (PCBs) at an industrial site at Bursa, Turkey. Atmos Res 85:338–350
Cindoruk SS, Esen F, Vardar N, Tasdemir Y (2008) Measurement of atmospheric deposition of polychlorinated biphenyls and their dry deposition velocities in an urban/industrial site in Turkey. J Environ Sci Health A Tox/Hazard Subst Environ Eng 43:1252–1260
Dickhut RM, Gustafson KE (1995) Atmospheric washout of polycyclic aromatic hydrocarbons in the Southern Chesapeake Bay region. Environ Sci Technol 29(6):1518–1525
Duinker JC, Bouchertall F (1989) On the distribution of atmospheric polychlorinated biphenyl congeners between vapor phase, aerosols and rain. Environ Sci Technol 23:57–62
Eisenreich SJ, Strachan WMJ (1992) Estimating atmospheric deposition of toxic substances to Great Lakes. In: Proceedings from 1992 Workshop Sponsored by the Great Lakes Protection Fund and Environment, Windsor, Ontario, Canada
Esen F, Cindoruk SS, Tasdemir Y (2006) Ambient concentrations and gas/particle partitioning of polycyclic aromatic hydrocarbons in an urban site in Turkey. Environ Forensics 7:1–10
Esen F, Cindoruk SS, Tasdemir Y (2008) Bulk deposition of polycyclic aromatic hydrocarbons (PAHs) in an industrial site of Turkey. Environ Pollut 152:461–467
Fang GC, Chang KF, Lu C, Bai H (2004) Estimation of PAHs dry deposition and BaP toxic equivalency factors (TEFs) study at Urban, Industry Park and rural sampling sites in central Taiwan, Taichung. Chemosphere 55:787–796
Gambaro A, Manodori L, Moret I, Capodaglio G, Cescon P (2004) Determination of polychlorobiphenyls and polycyclic hydrocarbons in the atmospheric aerosol of the Venice Lagoon. Anal Bioanal Chem 378:1806–1814
Garban B, Blanchoud H, Motelay-Massei A, Chevreuil M, Ollivon D (2002) Atmospheric bulk deposition of PAH’s onto France: trends from urban to remote sites. Atmos Environ 36:5395–5403
Gocht T, Klemm O, Grathwohl P (2007) Long-term atmospheric bulk deposition of polycyclic aromatic hydrocarbons (PAHs) in rural areas of Southern Germany. Atmos Environ 41:1315–1327
Gouin T, Thomas GO, Cousins I, Barber J, Mackay D, Jones KC (2002) Air–surface exchange of polybrominated diphenyl ethers and polychlorinated biphenyls. Environ Sci Technol 36:426–1434
Grünhage L, Dämmgen U, Hertstein U, Jäger HJ (1993) Response of grassland ecosystem to air pollutants: I—experimental concept and site of the Braunschweig Grassland investigation program. Environ Pollut 81:163–171
Harrad S, Mao H (2004) Atmospheric PCBs and organochlorine pesticides in Birmingham, UK: concentrations, sources, temporal and seasonal trends. Atmos Environ 38:1437–1445
Hillery BR, Simcik MF, Basu I, Hoff RM, Strachan WMJ, Burniston D, Chan CH, Brice KA, Sweet CW, Hites RA (1998) Atmospheric deposition of toxic pollutants to the Great Lakes as measured by the integrated atmospheric deposition network. Environ Sci Technol 32:2216–2221
Irwin JG, Campbell G, Vincent K (2002) Trends in sulphate and nitrate wet deposition over the United Kingdom: 1986–1999. Atmos Environ 36:2867–2879
Ishaq R, Näf C, Zebühr Y, Broman D, Järnberg U (2003) PCBs, PCNs, PCDD/Fs, PAHs and Cl-PAHs in air and water particulate samples—patterns and variations. Chemosphere 50:1131–1150
Jaward F, Barber JL, Booij K, Dachs J, Lohmann R, Jones KC (2004) Evidence for dynamic air–water coupling and cycling of persistent organic pollutants over the open Atlantic Ocean. Environ Sci Technol 38:2617–2625
Kim KS, Masunaga S (2005) Behavior and source characteristic of PCBs in urban ambient air of Yokohama, Japan. Environ Pollut 138:290–298
Kouimtzis TH, Samara C, Voutsa D, Balafoutis CH, Müller L (2002) PCDD/Fs and PCBs in airborne particulate matter of the greater Thessaloniki area. N Greece Chemosphere 47:193–205
Landis MS, Keeler GJ (2002) Atmospheric mercury deposition to Lake Michigan during the Lake Michigan mass balance study. Environ Sci Technol 36:4518–4524
Mandalakis M, Staphanou GE (2002) Study of atmospheric PCB concentrations over the eastern Mediterranean Sea. J Geo Res 107(D23):Art. No. 4716 DEC 13 2002
Mandalakis M, Tsapakis M, Tsoga A, Stefanou EG (2002) Gas–particle concentrations and distribution of aliphatic hydrocarbons, PAHs, PCBs and PCDD/Fs in the atmosphere of Athens (Greece). Atmos Environ 36:4023–4027
Manoli E, Samara C, Konstantinou I, Albanis T (2000) Polycyclic aromatic hydrocarbons in the bulk precipitation and surface waters of northern Greece. Chemosphere 41:1845–1855
Montone RC, Taniguchi S, Weber RR (2003) PCBs in the atmosphere of King George Island, Antarctica. Sci Total Environ 308:167–173
Montone RC, Taniguchi S, Boian C, Weber RR (2005) PCBs and chlorinated pesticides (DDTs, HCHs and HCB) in the atmosphere of the southwest Atlantic and Antarctic oceans. Mar Pollut Bull Baseline 50:778–786
Motelay-Massei A, Ollivon D, Garban B, Tiphange-Larcher K, Zimmerlin I, Chevreuil M (2007) PAHs in the atmospheric bulk deposition of the Seine river basin: source identification and apportionment by ratios, multivariate statistical techniques and scanning electron microscopy. Chemosphere 67:312–321
Offenberg JH, Baker JE (2002) Precipitation scavenging of polychlorinated biphenyls and polycyclic aromatic hydrocarbons along an urban to overwater transect. Environ Sci Technol 36:3763–3771
Ogura I, Masunaga S, Nakanishi J (2001) Atmospheric deposition of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and dioxin-like polychlorinated biphenyls in the Kanto Region, Japan. Chemosphere 44:1473–1487
Park JS, Wade TL, Sweet ST (2002) Atmospheric deposition of PAHs, PCBs and organochlorine pesticides to Corpus Christi Bay, Texas. Atmos Environ 36:1707–1720
Poster DL, Baker JE (1996a) Influence of submicron particles on hydrophobic organic contaminants in precipitation. 1. Concentrations and distributions of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in rainwater. Environ Sci Technol 30:341–348
Poster DL, Baker JE (1996b) Influence of submicron particles on hydrophobic organic contaminants in precipitation. 2. Scavenging of polycyclic aromatic hydrocarbons by rain. Environ Sci Technol 30:349–354
Rojas CM, Injuk J, Van Grieken RE (1993) Dry and wet deposition fluxes of Cd, Cu, Pb and Zn into the Southern Bight of the North Sea. Atmos Environ 27A:251–259
Rossini P, Guerzoni S, Molinaroli E, Rampazzo G, De Lazzari A, Zancanaro A (2005) Atmospheric bulk deposition to the lagoon of Venice. Part I. Fluxes of metals, nutrients and organic contaminants. Environ Int 31:959–974
Rolfhus KR, Sakamoto HE, Cleckner LB, Stor RW, Baiarz CL, Back RC, Manolopoulos H, Hurley JP (2003) Distribution and fluxes of total and methylmercury in Lake Superior. Environ Sci Technol 37:865–872
Sakata M, Marumoto K, Narukawa M, Asakura K (2006) Regional variations in wet and dry deposition fluxes of trace elements in Japan. Atmos Environ 40:521–531
Seyfioglu R, Odabasi M (2006) Investigation of air–water exchange of formaldehyde using the water surface sampler: flux enhancement due to chemical reaction. Atmos Environ 40:3503–3512
Simcik MF, Zhang H, Eisenreich SJ, Franz TP (1997) Urban contamination of the Chicago Coastal Lake Michigan atmosphere by PCBs and PAHs during AEOLOS. Environ Sci Technol 31:2141–2147
Sundqvist KL, Wingfors H, Lunden EB, Wiber GK (2004) Air–sea gas exchanges of HCHs and PCBs and enantiomers of α-HCH in the Kattegat Sea region. Environ Pollut 128:73–83
Taşdemir, Y, Holsen, TM (2005) Measurement of particle phase dry deposition fluxes of polychlorinated bipheyls (PCBs) with a water surface sampler. Atmos Environ 39:1845–1854
Tasdemir Y, Günez Y (2006) Dry deposition of sulfur containing species to the water surface sampler at two sites. Water Air Soil Pollut 105(1–4):223–240
Tasdemir Y, Vardar N, Odabasi M, Holsen TM (2004) Concentrations and gas/particle partitioning of PCBs in Chicago. Environ Pollut 131:35–44
Tasdemir Y, Odabasi M, Holsen TM (2005) Measurement of the vapor phase deposition of polychlorinated biphenyls (PCBs) using a water surface sampler. Atmos Environ 39:885–897
Yeo HG, Choi M, Chun MY, Sunwoo Y (2003) Gas/particle concentrations and partitioning of PCBs in the atmosphere of Korea. Atmos Environ 37:3561–3570
Walker JT, Aneja VP, Dickey DA (2000) Atmospheric transport and wet deposition of ammonium in Nort Carolina. Atmos Environ 34:3407–3418
Wethington DM, Hornbuckle K (2005) Milwaukee, WI, as a source of atmospheric PCBs to Lake Michigan. Environ Sci Technol 39:57–63
Acknowledgments
This study is supported by The Scientific and Technological Research Council of Turkey (TUBITAK) with the project number 107Y165. We also would like to thank Dr. S. Sıddık CINDORUK (Uludag University Environmental Engineering Department) for his help in GC-ECD analyses and Manolya GUNINDI for her contributions in the tiresome sampling and laboratory studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Euripides Stephanou
Rights and permissions
About this article
Cite this article
Birgül, A., Tasdemir, Y. Seasonal atmospheric deposition variations of polychlorinated biphenyls (PCBs) and comparison of some deposition sampling techniques. Environ Sci Pollut Res 18, 396–406 (2011). https://doi.org/10.1007/s11356-010-0383-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0383-2