Abstract
Introduction
Field experiments at the Shenyang Experimental Station of Ecology were conducted to study the adsorption, accumulation, and remediation of heavy metals by poplar and larch grown in artificially contaminated soil.
Materials and methods
The soil was spiked with a combination of Cd, Cu, and Zn at concentrations of 1.5, 100, and 200 mg·kg−1, respectively.
Results
The results showed that the biomass of poplar (Populus canadensis Moench) was lower by 26.0% in the soil spiked with a mixture of Cd, Cu, and Zn, compared with the control. Concentrations of Cd in poplar leaf and Cu in poplar roots in the treated soil were 4.11 and 14.55 mg kg−1, respectively, which are much greater than in corresponding controls. The migration of heavy metals in woody plant body was in the order Cd > Zn > Cu. Poplar had higher metal concentrations in aboveground tissues and a higher biomass compared with larch of the same age and therefore is potentially more suitable for remediation. In the heavy metal-polluted soil of this study, phytoremediation by poplar may take 56 and 245 years for Cd and Cu, respectively, for meeting the soil standards of heavy metals, and the corresponding phytoremediation times by larch would take 211 and 438 years.
Conclusion
The research findings could be used as a basis to develop ecological engineering technologies for environmental control and remediation of pollution caused by heavy metals in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
With an increase in metal mining, smelting, and sewage sludge application to land, more and more toxic metal ions are polluting the soil (Jamali et al. 2007a, b). The heavy metals causing most concern include Cd, Pb, Zn, Cr, Cu, and Ni. When present in high concentrations in soil, these metals cause toxic effects on plants, reducing their growth (Shah and Dubey 1998; Agrawal and Sharma 2006). Bioaccumulation of such toxic metals in the plants may also pose a health risk to humans and animals (Wang et al. 2003).
The remediation of soil polluted by heavy metals may take a very long time, often hundreds of years (Alloway 1995). Generally, removal of excess heavy metals from contaminated sites is brought about by chemical, physicochemical, or biological approaches. The remediation by chemical and physical methods is not only expensive but also may cause the destruction of soil structure and reduce bioactivity and fertility in the soil (Dermont et al. 2008). The biological approach (phytoremediation) is environmental friendly, is cost effective and energetically inexpensive, and is highly acceptable to the public. Therefore, it has received significant worldwide attention during the last decade (Cunningham et al. 1995; Terry and Banuelos 1999; Vangronsveld and Cunningham 1998; Guerinot and Salt 2001; Pilon-Smits 2005).
Many plant species in the genera of Thlaspi, Alyxia, Astragalus, Phyllanthus, Ipomoea, Haumaniastrum, and Alyssum are the heavy metal hyperaccumulators that can be used for phytoremediation (Lasat 2002). Among them, Zn/Cd hyperaccumulator Thlaspi caerulescens has been widely studied as model plant (Milner and Kochian 2008; Liang et al. 2009). Alyssum hyperaccumulator species for Ni phytoextraction have been developed into a commercial phytomining technology (Chaney et al. 2007).
For the phytoremediation of heavy metals, more popular trees are Populus, Salix, and Birch worldwide (Zalesny and Bauer 2007a, b; Unterbrunner et al. 2007; Mertens et al. 2006). Salix dasyclados has similar accumulation capabilities and remediation effectiveness with hyperaccumulators of Arabidopsis halleri and T. caerulescens (Fischerova et al. 2006).
The woody plants have advantages for remediation of heavy metal-contaminated soil because they can produce a large biomass if fast-growing species were selected. They also have a deep root system for remediating deep soil/water depths.
Distribution depth of heavy metals in soil profile is varied meanly due to the soil characteristics. Normally, the exogenous heavy metals predominantly accumulated at depths of 0–40 or 0–60 cm at arable land in some European countries (Fernandez et al. 2008), if deep plowing can accelerate migration, Cd displacing on average to about 0.7 m (Ingwersen and Streck 2006). In China, the famous Cd pollution area occupies 1,825 ha in Shenyang; the anthropogenic Cd is predominantly in 0–40-cm layer (Li et al. 2009).
The deep roots of a plant are direct in contact with soil, facilitating heavy metal uptake and accumulation. Woody plants can therefore remediate polluted soil and prevent heavy metals being transferred into the food chain. Phytoremediation by trees can therefore reduce the pollution risks and protect soil-environmental quality. According some reports, conventional remediation procedures cost US$ 100,000–1,000,000 per hectare (Russel et al. 1991); however, the cost of phytoremediation is estimated to be considerably less at only US$ 60,000–300,000 per hectare (Wolfe and Biornstad 2002).
Experimental studies on the responses of trees to metal contamination in soil have been carried out (Vamerali et al. 2009; Komarek et al. 2007; Wisniewski and Dickinson 2003; Giachetti and Sebastiani 2006; Gonzalez-Oreja et al. 2008). Besides heavy metals, the fast-growing species like genera Salix (willows and osiers) and Populus have also emerged as the most efficient species for phytoremediation of boron, PCBs, and naphthalene in contaminated soils (Robinson et al. 2007; Liu and Schnoor 2008; Andersen et al. 2008).
Little work, however, has been done on the use of conifers such as larch for phytoremediation. Therefore in this study, larch was selected as the subject of a reconnaissance investigation. The objectives of this field study were therefore to investigate the potential abilities of poplar (Populus canadensis Moench) and larch (Larix olgensis Henry) for remediation of soil contaminated by mixtures of heavy metals (Cd, Cu, and Zn). The research is intended to provide a scientific basis for comparisons of these species for phytoremediation of heavy metal-polluted soil in environmental protection and agricultural practice.
2 Materials and methods
2.1 Experimental site
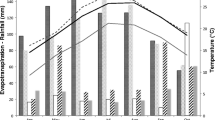
A field experiment was conducted at Shenyang Experimental Station of Ecology (41°31′ N, 123°41′ E, elevation 32 m above sea level), Chinese Academy of Sciences, 35 km south of Shenyang in northeastern China. The annual mean temperature is 7–8°C, annual precipitation is 700 mm, and the frost-free period is 147–164 days. It is located in a temperate zone with a subhumid continental climate. The experimental field is about 500 m2, there are 12 tree experimental plots, and each plot is 24 m2 (4 × 6 m).
2.2 Tree test species
The two tree species grown in the field experiment were poplar and larch, both common native tree species in the north of China. They are inexpensive and hardy, and they are not a food source for livestock so there is no risk of livestock poisoning. Two-year-old poplar and larch seedlings were selected; in order to keep experimental variables consistent within each species, poplar seedlings were selected to be between 3.0 and 3.2 m, and larch were selected between 0.6 and 0.8 m.
2.3 Soil properties
The soil type in the Shenyang Experimental Station of Ecology, Chinese Academy of Sciences, is a meadow brown soil (IFSS 1980). The physical and chemical properties are shown in Table 1.
2.4 Pollutant selection
The pollutants selected in the study were a mixture of Cd, Cu, and Zn contamination (hereafter referred to as Cd + Cu + Zn treatment). The experimental concentrations of these elements were based on the National Soil-Environmental Quality Standard of China (NSEQSC; Xia 1996). The concentrations of Cd, Cu, and Zn were five, two, and one times, respectively, of the maximum values within grade B soil of the NSEQSC (grade B applies to soil suitable for farm land, vegetable land, tea land, fruit land, and grazing land). The heavy metal compounds added to the soil were CdCl2·2.5H2O for Cd, CuSO4·5H2O for Cu, and ZnSO4·7H2O for Zn, all as analytical reagents. The pollutant concentrations for the metals are presented in Table 2. The chemical fractions of Cd, Cu, and Zn in soils are presented in Table 3.
2.5 Experimental design
The field plot trial was carried out with two tree species (poplar and larch). There were 12 plots (4 × 6 m), and each plot had four trees (spacing 2 × 3 m). In the early spring of 2006, mixtures of Cd, Cu, and Zn were spiked into the surface soil (0–15 cm) of six plots at the levels of 1.5, 100, and 200 mg kg−1, respectively. The pollutants were uniformly mixed with the soil and equilibrated for 2 weeks. The design therefore provided three replicates of each treatment. Two-year-old poplar and larch were transplanted in May of 2006.
2.6 Sample collection and sample analysis
Surface soil samples were collected from each plot by randomly taking five 15-cm deep soil cores (5-cm diameter) in each plot. These soil samples were dried at 105°C, ground to powder (to pass 100-mesh/in. screens), and stored in brown paper envelopes until analysis. At the end of the trial in autumn of 2006, the trees were harvested, washed with tap water, and rinsed twice with deionized water to remove any attached particles. Each tree was separated into root, trunk, leaves, and branches; 1-kg samples were taken for every fraction. Each tree tissue sample was placed in a brown paper envelope and dried to constant weight in ovens at 80°C before being subjected to size reduction by plant grinder. The ground samples were then air dried in preparation for analysis.
Basic physicochemical soil analyses (particle size, CEC, etc.) were conducted according to the routine analytical methods of standard methods of China (GB/T17140-1997). Chemical fractions of heavy metals were analyzed by Tessier’s method (Tessier et al. 1979). The plant and soil samples were digested with a solution containing 87% HNO3 and 13% HClO4 (v/v) and diluted with 5% HNO3 for element analysis.
The concentrations of Cd, Cu, and Zn were determined by flame atomic absorption spectrometry using a Hitachi model 180-80 AAS spectrometer. Analytical quality control was verified using an environment standard substance for heavy metals from Environmental Monitoring Station of China (GBW08501). The experimental data were analyzed using the analysis of variance routine provided in SPSS version 11.5, and the P value was used to assess the statistical significance and quantitative differences.
3 Results and discussion
3.1 Effect of heavy metals in polluted soil on tree growth
Cu and Zn are essential elements for growth and development of plants, but Cd is not essential. In the concentrations treated, simultaneous pollution of these elements significantly influenced the development of the trees. The biomass of poplar root, trunk, branch, and leaf in trees subjected to the heavy metal soil contamination treatment was lower than the control (Table 4). As the heavy metal concentration in the soil was close to or exceeded the National Soil-Environmental Quality Standard, the poplar growth was inhibited by heavy metals in the soil. Poplar biomass with the Cd + Cu + Zn treatment was reduced by 26% compared with the control (significant difference P < 0.05). This is in accord with results of Huang et al. who had described that poplar biomass evidently declined in heavy metal-polluted soil (Huang et al. 1989).
The root and trunk biomass of larch in the Cd + Cu + Zn treatment was significantly increased compared with the control. However, the branch and leaf biomass of larch in the treatment was significantly lower than the control.
The weight of trunk, root, branch, and leaf of poplar occupied 64.5%, 15.8%, 15.5%, and 4.2%, respectively of the total weight in the Cd + Cu + Zn treatment trees, and the trunk, root, branch, and leaf weights of larch occupied, respectively, 39.3%, 23.0%, 27.1%, and 10.6% of the total weight in the Cd + Cu + Zn treatment trees. The aboveground tissue biomass (i.e., the sum of biomass for trunk, branch, and leaf) of poplar was therefore 84.2%, and the aboveground tissue biomass of larch was 77.0%. The aboveground biomass for plant plays an important role in remediation for contaminated soil of heavy metal. Since the aboveground biomass of poplar was more than the aboveground biomass of larch (84.2% > 77.0%) in the treatment soil, it would be more suitable for remediation of heavy metal-polluted soil since it is the aboveground biomass which will “harvest” more of the heavy metals.
3.2 Heavy metal adsorption and accumulation of woody plant
In general, the background contents of Cd, Cu, and Zn in plant tissues of larch are 0.019, 8.4, and 27.76 mg kg−1 in leaf and 0.067, 5.4, and 27.31 mg·kg−1 in branch, respectively (Liao 1989). However, the values depend on the plant species, the soil heavy metal concentrations, and the parts of the plant analyzed. In our study, the heavy metals were absorbed by the plant root from the soil and then redistributed in different tissues of the plant.
Metal concentrations in plant tissues of both poplar and larch grown in Cd-, Cu-, and Zn-treated soil were higher than controls, and concentrations in poplar were higher than in larch (Figs. 1, 2, and 3). Therefore the poplar is thus more suitable for phytoremediation in heavy metal-polluted soil.
The degree of migration of heavy metals in poplar and larch grown in heavy metal-polluted soils is different. The migration coefficients (calculated as the ratio of element concentration in relevant plant tissue and in soil) directly reflect translation of heavy metals from soil to plant tissues. For example, the leaf migration coefficients were calculated as the ratio of element concentration in leaf and corresponding element concentration in soil. The Cd concentration in poplar leaf was 4.11 mg kg−1, and the concentration in treated soil was 1.695 mg kg−1, so the migration coefficient is 4.11/1.695 = 2.45. The element migration coefficients in poplar and larch in the Cd + Cu + Zn treatment are in the order Cd > Zn > Cu (Fig. 4). The migration coefficients for all three elements are greater in poplar than those in larch, that is, the migration ability of heavy metals is more in poplar than in larch.
3.3 The potential for heavy metal-polluted soil remediation by poplar and larch
The degree of absorption and accumulation of heavy metal depends on the tree species and which metal is the soil pollutant. Thus, suitable trees can play an important role in the biological purification of corresponding heavy metals in polluted soil.
Based on (1) soil Cd, Cu, and Zn concentrations 1.695, 120.1, and 263.4 mg kg−1, (2) the aboveground tissue biomass of poplar and larch, (3) the Cd, Cu, and Zn concentrations of different poplar and larch tissues, (4) the growth rates of poplar and larch, and (5) a target of Environmental Quality Standard Grade B Soil, the remediation times of Cd- and Cu-contaminated soils were calculated (Table 5).
Poplar could remove about 56.2 g ha−1 Cd (calculated as the sum of aboveground tissue biomasses multiplied by their respective Cd concentrations, multiplied by the number of trees per hectare), 196 g ha−1 Cu, and 1,170 g ha−1 Zn from soil containing 1.695 mg kg−1 Cd, 120.1 mg kg−1 Cu, and 263.4 mg kg−1 Zn.
According to these uptake rates, it would take 56 years (calculated as (1,695 mgkg−1 − 0.3 mgkg−1) × 1 ha soil weight (2,250,000 kg) ÷ 56,200 mgha−1 = 56 years) to reduce the soil Cd burden down to 0.3 mg·kg−1; similarly, it would take 245 years to reduce the soil Cu burden down to 50 mg·kg−1. Because the Zn concentration in the treated soil is the same as the Environmental Quality Standard of Grade B soil (200 mg·kg−1), this remediation time need not be calculated. The larch could remove 14.9 g ha−1 Cd, 109.5 g ha−1 Cu, and 464.7 g ha−1 Zn from the same treated soil; according to these migration rates, it would take 211 years to reduce the soil Cd burden down to 0.3 mg·kg−1and 438 years to reduce the soil Cu burden down to 50 mg·kg−1. However, the estimates of remediation times in this study are somewhat speculative and should only be used as preliminary data for planning of future studies. It would be necessary for much longer period experiments in further studies to assess how the migration of the metals varies with the age of the trees and the concentration of the metals in the woody tissues. It would also be necessary to assess the long-term effects of the heavy metals on the growth rate of the trees.
However, our data does imply that the effect of remediation of Cd-polluted soil by poplar is better than larch. The times estimated here are comparable to those of Alloway (1995), who suggested that the disappearance (remediation) time of heavy metals in polluted soil is often hundreds of years when plants are used for the remediation.
Therefore, the remediation of polluted soil to environmental quality standards needs a long time in relation to human land management practices. The calculations of remediation time presented here are based on the assumption that the migration rate remains constant. It would be necessary to reassess the ability of the trees to remediate soils which were more highly contaminated.
There are some techniques which could be essential for improving the remediation time. Multiple species plantings must be considered in the remediation practice because a heavy metal usually co-occur with others in pollution lands, and a hyperaccumulator plant is only effective for one or two metals.
Cloning all genes needed and expressing them in high-biomass plants are a promising strategy (Kramer and Chardonnens 2001; Rylott and Bruce 2009). A number of transgenic Populus and Salix have been generated and tested for the improvement of dendroremediation of heavy metal in soil (Zalesny and Bauer 2007a, b; Eapen and D'Souza 2005). As an example, transgenic phytoextraction plants have been achieved for Hg (Chaney et al. 2007).
Agronomic management should be improved in practice. The metal concentrations in willow biomass compartments decreased with stand age. Therefore, the most efficient removal of Cd would require the combined harvest of stems and leaves, and willow is grown as a short rotation coppice crop for phytoremediation (Mertens et al. 2006).
Increase the bioavailable fraction of heavy metal in soil. Chelant-enhanced phytoextraction with trees can improve the remediation, in which ethylenediaminetetraacetic acid or ethylenediaminedisuccinic acid and others have been recommended (Komarek et al. 2007). The developing combined methods may eventually prove to be effective phytoremediation strategies on a practical scale.
The greatest advantage of using trees for cleaning the heavy metal-polluted soil is the utilization the inherent traits of trees, including high biomass, extensive root systems, and ability to withstand environmental stress. However, the fate of trees after phytoremediation is also of concern because the trees eventually contain higher contents of heavy metals than normal ones. Considering the economic benefit, the tree phytoremediation technology should combine with wood production of forest industry. The harvested trees could be used for different commercial purposes such as for timber or energy production. Another concern is that leaf falling onto the ground may make contaminants partially re-enter the roots and/or soil; therefore, the annual environmental management is important to avoid or minimize the leaf-bound heavy metals recycling into soil in autumn. Thus, phytoremediation by trees seems to be a promising technology because of low cost and high efficiency; hence, it has a large applicable market prospect in the future.
4 Conclusions
Metal concentrations in plant tissues of poplar and larch grown in Cd-, Cu-, and Zn-treated soil were higher than controls, and the heavy metal transferring capacities by both of poplar and larch is in the order Cd > Zn > Cu. The migration coefficients for these metals in poplar are greater than in larch; the concentrations and amount of Cd, Cu, and Zn in the aboveground tissue of poplar are greater than in larch. Therefore, the poplar is thus more suitable for phytoremediation in heavy metal-polluted soil. However, even poplar for phytoremediation needs long periods; in the heavy metal-polluted soil of this study, it may take 56 and 245 years for Cd and Cu, respectively. If phytoremediation by larch was used, it could even take 211 and 438 years for Cd and Cu, respectively. Therefore, the remediation of heavy metal-contaminated soil by this method requires that the land is excluded from food production for a long period. This field experiment provides a valuable basis for further studies on the real (rather than controlled experimental) polluted areas and then subsequent development of ecological engineering technologies for environmental control and remediation of pollution caused by heavy metals in soils.
References
Agrawal V, Sharma K (2006) Phytotoxic effects of Cu, Zn, Cd and Pb on in vitro regeneration and concomitant protein changes in Holarrhena antidysentrica. Biol Plant 50:307–310
Alloway BJ (1995) Heavy metals in soils. Blackie Academic and Professional, Glasgow
Andersen RG, Booth EC, Marr LC, Widdowson MA, Novak JT (2008) Volatilization and biodegradation of naphthalene in the vadose zone impacted by phytoremediation. Environ Sci Technol 42(7):2575–2581
Chaney RL, Angle JS, Broadhurst CL, Peters CA, Tappero RV, Sparks DL (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J Environ Qual 36(5):1429–1443
Cunningham SD, Berti WR, Huang JW (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152(1):1–31
Eapen S, D'Souza SF (2005) Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol Adv 23(2):97–114
Fernandez C, Monna F, Labanowski J, Loubet M, van Oort F (2008) Anthropogenic lead distribution in soils under arable land and permanent grassland estimated by Pb isotopic compositions. Environ Pollut 156(3):1083–1091
Fischerova Z, Tlustos P, Jirina S, Kornelie (2006) A comparison of phytoremediation capability of selected plant species for given trace elements. Environ Pollut 144(1):93–100
Giachetti G, Sebastiani L (2006) Metal accumulation in poplar plant grown with industrial wastes. Chemosphere 64:446–454
Gonzalez-Oreja JA, Rozas MA, Alkorta I, Garbisu C (2008) Dendroremediation of heavy metal polluted soils. Rev Environ Health 23(3):223–234
Guerinot ML, Salt DE (2001) Fortified foods and phytoremediation: two sides of the same coin. Plant Physiol 125:164–167
Huang HY, Jiang DM, Zhang CX (1989) Study on absorbing and accumulation cadmium from polluted soil and tolerance to it in woody plants, China. Environ Sci 9:323–330
Ingwersen J, Streck T (2006) Modeling the environmental fate of cadmium in a large wastewater irrigation area. J Environ Qual 35(5):1702–1714
Institute of Forestry and Soil Science, Chinese Academy of Sciences (1980) The soils in Northeast of China. Scientific Press, Beijing, pp 54–55
Jamali MK, Kazi TG, Arain MB, Afridi HI, Jalbani N, Memon AUR, Ansari R, Shah A (2007a) The feasibility of using an industrial sewage sludge produce in Pakistan as agricultural fertilizer used for cultivation of Sorghum bicolor L. Arch Agron Soil Sci 53(6):659–671
Jamali MK, Kazi TG, Arain MB, Afridi HI, Jalbani N, Memon AR (2007b) Heavy metal contents of vegetables grown in soil, irrigated with mixtures of wastewater and sewage sludge in Pakistan, using ultrasonic-assisted pseudo-digestion. J Agron Crop Sci 193:218–228
Komarek M, Tlustos P, Szakova J, Chrastny V, Ettler V (2007) The use of maize and poplar in chelant-enhanced phytoextraction of lead from contaminated agricultural soils. Chemosphere 67:640–651
Kramer U, Chardonnens AN (2001) The use of transgenic plants in the bioremediation of soils contaminated with trace elements. Appl Microbiol Biot 55(6):661–672
Lasat MM (2002) Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual 31:109–120
Li P, Wang X, Allinson G, Li X, Xiong X (2009) Risk assessment of heavy metals in soil previously irrigated with industrial wastewater in Shenyang, China. J Hazard Mater 161(1):516–521
Liang HM, Lin TH, Chiou JM, Yeh KC (2009) Model evaluation of the phytoextraction potential of heavy metal hyperaccumulators and non-hyperaccumulators. Environ Pollut 157(6):1945–1952
Liao ZJ (1989) Pollution and transformation of trace have metals in environment. Scientific Press, Beijing
Liu J, Schnoor JL (2008) Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in whole hybrid poplar plants after hydroponic exposure. Chemosphere 73:1608–1616
Mertens J, Vervaeke P, Meers E, Tack FM (2006) Seasonal changes of metals in willow (Salix sp.) stands for phytoremediation on dredged sediment. Environ Sci Technol 40(6):1962–1968
Milner MJ, Kochian LV (2008) Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot 102(1):3–13
Pilon-Smits EAH (2005) Phytoremediation. Ann Rev Plant Biol 56:15–39
Robinson BH, Green SR, Chancerel B, Mills TM, Clothier BE (2007) Poplar for phytomanagement of boron contaminated sites. Environ Pollut 150(2):225–233
Russel M, Colglazier EW, English MR (1991) Hazardous waste remediation: the task ahead. Waste management Research and Education Institute, University of Tennessee, Knoxville
Rylott EL, Bruce NC (2009) Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends Biotechnol 27(2):73–81
Shah K, Dubey RS (1998) A 18 kDa cadmium inducible protein complex: its isolation and characterization from rice (Oryza sativa L.) seedlings. J Plant Physiol 152:448–454
Terry N, Banuelos G (1999) Phytoremediation of contaminated soil and water. Lewis, Boca Raton
Tessier A, Campbell WG, Baisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–850
Unterbrunner R, Puschenreiter M, Sommer P, Wieshammer G, Tlustos P, Zupan M, Wenzel WW (2007) Heavy metal accumulation in trees growing on contaminated fast growing biomass plants such as Salix species are promising for use in phytoremediation of contaminated land. Sites in Central Europe. Environ Pollut 148(1):107–114
Vamerali T, Bandiera M, Coletto L, Zanetti F, Dickinson NM, Mosca G (2009) Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes (Torviscosa, Italy). Environ Pollut 157(3):887–894
Vangronsveld J, Cunningham SD (1998) Metal-contaminated soils: in-situ inactivation and phytorestoration. Springer, Berlin
Wang QR, Cui YS, Liu XM, Dong YT, Christie P (2003) Soil contamination and uptake of heavy metals at polluted sites in China. J Environ Sci Health 38:823–838
Wisniewski L, Dickinson NM (2003) Toxicity of copper to Quercus robur (English Oak) seedlings from a copper-rich soil. Environ Exp Bot 50:99–107
Wolfe AK, Biornstad DJ (2002) Why would anyone object? An exploration of social aspects of phytoremediation acceptability. Crit Rev Plant Sci 21:429–438
Xia JQ (1996) Environmental quality standard for soil in China. China Environmental Science Press, Beijing
Zalesny RS Jr, Bauer EO (2007a) Evaluation of Populus and Salix continuously irrigated with landfill leachate I. Genotype-specific elemental phytoremediation. Int J Phytoremediation 9(4):281–306
Zalesny RS Jr, Bauer EO (2007b) Evaluation of Populus and Salix continuously irrigated with landfill leachate II. Genotype-specific elemental phytoremediation. Int J Phytoremediation 9(4):307–323
Acknowledgments
The research presented here was supported by the project “Pollution control for industry effluent liquid and sediment discharge” of the Chinese Academy of Sciences and in part supported by the foundation for key laboratory of the Pollution Ecology and Environmental Engineering, Institute of Applied Ecology, Chinese Academy of Sciences. Many thanks to Dr. Tom McRae in Deakin University, Australia for the English proofreading and editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Wang, X., Jia, Y. Study on adsorption and remediation of heavy metals by poplar and larch in contaminated soil. Environ Sci Pollut Res 17, 1331–1338 (2010). https://doi.org/10.1007/s11356-010-0313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0313-3