Abstract
Background, aim and scope
This study is the first to investigate PBDE body burden with regard to the concurrent analyses of multiple human matrices, namely milk, placenta, and hair, collected from a group of childbearing-aged women at an electronic waste (e-waste) recycling site to determine the partitioning of PBDEs in these different human matrices and the possible health risks imposed to infants at the e-waste recycling site.
Methods and methods
Five sets of milk, placenta, and hair samples were collected from an e-waste site (Taizhou, Zhejiang Province) and a reference site (Lin’an city, Zhejiang Province; 245 km away from Taizhou) in China. The concentrations of total PBDEs in different human tissues were analyzed according to US EPA standard methods.
Results
PBDE body burdens of women from the e-waste site (milk 117 ± 191, 8.89–457 ng/g fat, placenta 19.5 ± 29.9, 1.28–72.1 ng/g fat, hair 110 ± 210, 8.47–486 ng/g dry wt.) showed significantly higher levels than those from the reference site (milk 2.06 ± 0.94, 1.0–3.56 ng/g fat, placenta 1.02 ± 0.36, 0.59–1.42 ng/g fat, hair 3.57 ± 2.03, 1.56–5.61 ng/g dry wt.) and were higher than those reported in other studies, due to e-waste recycling operations, especially open burning. On a dry-weight basis, the following trend was found for PBDE among the samples from Taizhou: hair≫milk>placenta. Among the donors, the body burden of an e-waste worker ranked second. Higher brominated BDEs (hepta-BDEs) contributed a significantly greater proportion to total PBDEs in hair of the Taizhou women (20%) than that in milk (2.9%) and in placenta (2.6%). The estimated intake of PBDEs of 6-month-old breastfed infants living at the e-waste site was 572 ± 839 ng/kg body wt/day, which was 57 times higher than that of infants from the reference site (10.1 ± 4.60 ng/kg body wt/day). Moreover, the maximum calculated value (2,240 ng/kg body wt/day) exceeded the chronic oral reference dose for penta-BDE (2,000 ng/kg/day) of US EPA.
Discussion
BDE-47 was the dominant congener accounting for 20–30% in all the individual samples, while higher-brominated congeners, for example, BDE-183 and BDE-190, contributed between 2% and 20%. The presence of hepta-BDE congeners (BDE-181, BDE-190) in hair of the women in Taizhou suggest that thermal degradation of Deca-BDE from the open burning of e-waste may have been their source because these congeners are not found in either Penta-BDE or Octa-BDE technical products. Of the three types of samples analyzed, it was also suspected that hair may be more favorable to higher-brominated compounds which might explain why the hair samples contained the highest total PBDE concentrations and the highest proportion of higher-brominated BDEs (hepta-BDEs).
Conclusion
This study provides evidence that primitive e-waste recycling in China leads to high PBDE body burdens in local residents and can potentially threaten the health of infants.
Recommendations and perspectives
Control measures should be imposed to minimize the level of pollutants resulting from e-waste processing operations to the environment and to humans. In-depth investigations on epidemiological studies of health impacts caused by e-waste recycling operations should be conducted. It is recommended that further measurements of PBDE levels in local food (e.g., fish, shellfish, dairy products, meat, fruits, and vegetables), dust, air, water, and human specimens be collected from a larger sample size at the e-waste processing site for the determination of human exposure pathways to PBDEs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
PBDEs are brominated flame retardants (BFR) which have the ability to chemically reduce and retard the development of fire. The three major commercial mixtures of PBDEs are Penta-, Octa-, and Deca-BDE which had been widely applied to furniture, textiles, electronic appliances (particularly computers and television sets), and building materials (Darnerud et al. 2001). Penta-BDE and Octa-BDE were banned in the EU in 2004, and in May 2009, they were added to the Stockholm Convention on Persistent Organic Pollutants for elimination (UNEP 2005, 2009a). DecaBDE is still being used worldwide; however principal manufacturers in the USA have recently announced an end to production, importation, and sales by 31 December 2012 (USEPA 2009b).
The global market demand in 2002 for PBDEs was estimated at 67,490 metric tons, of which 37% was used in Asia (Bromine Science and Environmental Forum 2003). Rapid development of the electronic industry has caused an increasing demand for BFRs at 8%/year in China (Xian et al. 2008) with an annual domestic production of BFRs at approximately 10,000 metric tons/year (Zou et al. 2007). BFR production factories in China are mainly located in Shandong and Jiangsu Provinces (Xian et al. 2008). There are also imports of BFR from three of the largest BFR manufacturers in the world (i.e., Great Lakes Chemical, Indianapolis, IN; Albemarle Chemical, Richmond, VA; and Dead Sea Chemical, Beer-Sheva, Israel) (Mai et al. 2005). Moreover, in the southern part of China (Pearl River Delta region), there are enormous numbers of electronic, furniture, and appliance manufacturing plants which consume significant amounts of PBDEs (Tanabe 2008). The input of PBDEs from riverine runoff from the Delta to the coastal ocean has been estimated to be 2,140 kg/year (Guan et al. 2007).
Approximately 20 to 50 million tonnes of e-waste are generated globally (UNEP 2005) with 70% sent to China for “recycling” (Xinhua Online 2007). The amount of PBDEs imported to China in the form of e-waste was estimated at 35,000 metric tons/year. Therefore, inflows of e-waste have been an important source of PBDEs contamination to the environment of China (Guan et al. 2007).
E-waste recycling in China is conducted using primitive methods whereby workers burn piles of wires in open air for the recovery of metals, melt circuit boards over coal grills to extract valuable chips, and burn leftover useless plastics (Yu et al. 2006). Heating of these waste are expected to mobilize BFRs in electronic components into the environment (Deng et al. 2007). Soil and sediment could be seriously contaminated by e-waste processing. Our previous studies in Guiyu (a notable e-waste recycling site) revealed that soil at an open burning site (2,906–44,473 ng/g dry wt.) (Wong et al. 2007) and river sediment in Guiyu (4,434 to 16,088 ng/g dry wt.) (Luo et al. 2007) were heavily contaminated by PBDEs. Combusted residues and ash from the open burning of e-waste contained high levels of PBDEs (33,000–97,400 ng/g dry wt.) (Leung et al. 2007).
Recently, PBDEs have been widely detected in the environment of China: sediment (Mai et al. 2005), soil (Zou et al. 2007), air (Chen et al. 2008), water (Guan et al. 2007), birds (Chen et al. 2007), and fish (Xian et al. 2008). The extensive use of PBDEs in China over the last 20–30 years can be reflected by vertical profiles of PBDEs in sediment cores from the Pearl River Estuary (Mai et al. 2005). Levels of PBDEs in finless porpoises from the South China Sea were six times higher in 2001 than in 1990 (Ramu et al. 2006). Furthermore, PBDEs can be bioaccumulated in biota and thus may pose a potential threat to human health through dietary intake. House dust may also be another major source of exposure pathway for PBDEs (Jones-Otazo et al. 2005).
Although there have been few studies conducted on human PBDE body burdens in China (in particular at e-waste sites), PBDEs have been detected in several human matrices. These include maternal and fetal blood, and milk from the general population in South China (Bi et al. 2006), blood serum from e-waste workers in South China (Qu et al. 2007), and hair of residents living at an e-waste site in Southeast Zhejiang Province (Zhao et al. 2008); PBDE levels were found to be significantly higher than those from reference sites. Hair serves as a noninvasive matrix for the biomonitoring of pollutants (Smolders et al. 2009), and breast milk provides information on exposure levels of both the mother and her child (Esteban & Castaño 2009). To date, there are very limited studies of PBDE body burden with regard to the concurrent analyses of multiple human matrices.
The major objectives of this study were to (1) investigate the body burdens of PBDEs of local residents at an e-waste recycling site, compared with a reference site in eastern China, by analyzing three matrices (milk, placenta, hair); (2) elucidate the partitioning of PBDEs in these different human matrices; and to (3) determine the possible health risks imposed on infants at the e-waste recycling site.
2 Materials and methods
2.1 Description of sampling sites
Taizhou region (TZ) (28° N latitude and 122° E longitude) is located in Zhejiang Province, Eastern China. E-waste recycling activities are mainly carried out in Luqiao City situated in the southern part of Taizhou, with a total area of 274 km2 and a population of 400,000. Since the late 1970s, e-waste recycling activities in Taizhou involved the handling of e-waste generated in China (Taizhou Economic Committee, China 2007), especially imports of PCB-containing capacitors from other provinces. In the 1980s, dismantling capacitors became an important industry in Taizhou, where more than 1,300 U capacitors were stored (The World Bank 2005). The city began processing imported e-waste in the early 1990s, mainly from Japan, the USA, and western European countries (Ma et al. 2008), and mainly receives scrap metals, obsolete electric capacitors, household appliances, electric generators, and cable wires. It is the largest center for the dismantling of obsolete transformers and capacitors in China (Zhao et al. 2008), with 40,000 people working in the e-waste recycling sector. Most of the recycling operations involve open-air burning, acid leaching, and physical dismantling by hammer, chisel, screw driver, and bare hands. The reference site, Lin’an city (HZ) (30° N latitude and 118° E longitude) (Fig. 1), is situated inland in the northern part of Hangzhou prefecture, Zhejiang Province, about 245 km away from Taizhou.
2.2 Study population and data collection
The details of the study population are described in Chan et al. (2007) as the same two groups of women were studied. Briefly, milk, placenta, and hair samples were collected from women in 2005 by the officials of the local Centers for Disease Control and Prevention. Five women from Taizhou and Lin’an were randomly selected to participate in this study, and each agreed to donate the three types of samples.
Socio-demographic data and food consumption habits of the study populations were obtained via interviews during which the donors answered questions modified from the second round of the WHO PCDDs, PCDFs, and PCBs exposure study (Liem et al. 1996). The response rate was 100%. To our knowledge, this was the first total diet study to estimate the dietary intakes of PBDEs for people living at an e-waste site, in China. Dietary change over the course of pregnancy was ignored. Epidemiological data for adults living in Taizhou (from year 2004 to 2006) was also obtained from the local Centers for Disease Control and Prevention.
Of the five donors (TZ1–TZ5) from Taizhou, only one donor (TZ5) handled e-waste. Although the details of her work were not specified, she indicated in the questionnaire that she had direct dermal contact with e-waste and was exposed to e-waste via the inhalation pathway. Donor TZ5 had only one child resulting from a sole pregnancy, while TZ1 and TZ3 were primipara and TZ2 and TZ4 were multiparous mothers (two children each). All of the mothers except for TZ5 have had at least one miscarriage.
2.3 Sample collection
Placenta samples were collected on the day of childbirth, and hair samples were collected 1 day after. Milk was sampled when the infant was 4 to 5 days old. Before the collection of specimens, donors completed a written informed consent. Placenta samples (portion) were individually placed in clean glass containers. Hair samples were collected near the scalp and from the nape of the neck with stainless steel scissors and stored in sealable polyethylene bags, while milk was manually expressed and stored in hexane-rinsed reagent bottles with Teflon-lined caps. All samples were frozen immediately after collection and stored at −20°C until chemical analyses.
2.4 Laboratory analysis
Chemical analyses were conducted by The State Key Laboratory for Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, China. Milk and placenta samples were freeze-dried and then homogenized. Hair was washed with commercial detergent (1%) for 5 min, followed by distilled water to remove on-surface impurities (Covaci et al. 2002). It was then oven-dried at 70°C. The samples (placenta, 5 g; milk, 3 g; hair, 2 g) were individually Soxhlet-extracted (3540C, US EPA 1996a) for 24 h with 250 mL of n-hexane/dichloromethane (1:1, v/v). Prior to the extraction, each sample was spiked with 13C-labeled BDE surrogate (US EPA 1614 LCS, EO-5100) containing the congeners BDE-3, BDE-15, BDE-28, BDE-47, BDE-99, BDE-100, BDE-118, BDE-123, and BDE-183 (purchased from Cambridge Isotope Laboratories Inc). The lipid contents of milk and placenta samples were gravimetrically determined from an aliquot of the extract.
The cleanup process involved two types of column chromatography. The first one consisted of, from top to the bottom, 5 g of anhydrous sodium sulfate, 10 g of 3% deactivated alumina, 5 g of anhydrous sodium sulfate, 2 g of deactivated silica gel (3.3% reagent water, w/w), 10 g of acidified silica gel (44% concentrated sulfuric acid, w/w), and finally, 4 g of deactivated silica gel (US EPA 1996b). The eluting solvent was 150 ml n-hexane/dichloromethane (9:1, v/v). The eluate was then concentrated and purified by a second column which consisted of 2 g of anhydrous sodium sulfate and 2 g of 10% AgNO3-silica gel (US EPA 1996c). It was eluted with 120 ml n-hexane followed by 60 ml of 50% n-hexane/dichloromethane (1:1, v/v). The eluate was evaporated to dryness under a gentle stream of nitrogen and redissolved in 10 µL of n-nonane containing 10 µL of internal standard (EO-5101; Cambridge Isotope Laboratories Inc.).
The detection and quantification were done using HRGC/HRMS based on US EPA Draft Method 1614 (US EPA 2003). The analyses were performed on Agilent 5890II gas chromatograph (Agilent Technologies, USA) coupled to a Finnigan MAT 95s mass spectrometer (Thermo Electron, USA) equipped with a CTC A200S autosampler (CTC, Switzerland) at a resolution of 10,000. A RTX-DIOXIN2 capillary column (60 m × 0.25 mm i.d. × 0.25 µm f.t., Restek Co.) was used for the compound separation. The temperature of the transfer line was set at 300°C. The oven temperature program was 100°C for 2 min followed by an increase to 240°C at 20°C/min. The temperature was then elevated to 280°C at 2.5°C/min and to 310°C at 10°C/min and held for 62 min. The compounds were identified by the mass spectrometer in the EI and multiple ion detection mode, by tracing two reference masses with the strongest peaks and the most intensive ions of the isotope cluster. The ion source was set at 280°C. The congeners targeted for determination were: di-BDE (BDE-7, 8, 10, 11, 12, 13, 15), tri-BDE (BDE-17, 25, 28, 30, 32, 33, 35, 37), tetra-BDE (BDE-47, 49, 66, 71, 75, 77), penta-BDE (BDE-85, 99, 100, 116, 118, 119, 126), hexa-BDE (BDE-138, 153, 154, 155, 166), and hepta-BDE (BDE-181, 183, 190). BDE-209, although an important congener because of its widespread use, was not analyzed due to analytical constraints.
2.5 Data expression
The body burdens were expressed as nanogram PBDEs per gram dry weight for hair and nanogram PBDEs per gram of fat for both milk and placenta. They were also expressed as nanogram PBDE per gram dry weight when comparisons were made among hair, milk, and placenta. Values below the detection limit were indicated as not detected.
2.6 Data analysis
The Statistical Package for Social Sciences (SPSS 11.0 for Windows; SPSS Inc., Chicago, IL, USA) was used for the quantitative data analysis. The differences among groups were assessed by chi-square tests, analysis of variance (ANOVA), t test, or Mann–Whitney U test. Spearman correlation coefficients were calculated to assess the relationship among the concentrations of different congeners as well as that between body burdens and characteristics at different sites. All p values were two-tailed, and an association with a p value <0.05 was considered statistically significant. Data are presented as mean ± SD.
3 Results and discussion
3.1 Difference in levels between TZ and HZ samples
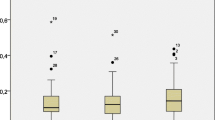
The average and range of PBDE concentrations in hair, milk, and placenta collected from the two sites are shown in Table 1. PBDE levels of TZ specimens were much higher than those of HZ samples, particularly in hair and milk (both p < 0.05). The Σ36PBDE concentrations of TZ milk (average 117 ± 191, range 8.89–457 ng/g fat), TZ placenta (19.5 ± 29.9, 1.28–72.1 ng/g fat), and TZ hair (110. ± 210, 8.47–486 ng/g dry wt.) exceeded the values of HZ milk (2.06 ± 0.94, 1.00–3.56 ng/g fat), HZ placenta (1.02 ± 0.36, 0.59–1.42 ng/g fat), and HZ hair (3.57 ± 2.03, 1.56–5.61 ng/g dry wt.) by over 57, 19, and 31 times, respectively. Among the donors, TZ3 had the highest total PBDE concentrations for all of the three matrices (hair 486 ng/g; milk 457 ng/g fat; placenta 72.0 ng/g fat), and TZ5 (e-waste worker) ranked second. The concentrations of TZ3 were 18.5-, 7.2-, and 4.7-fold higher than TZ5 for hair, milk and placenta, respectively (see Table 2), and the concentrations of TZ5 were 1.5-, 1.6-, and 2.4-fold higher than the next highest concentration for hair, milk, and placenta, respectively. Donor TZ3 and donor TZ5 lived in Taizhou for 2 and 5 years, respectively.
The present results confirmed that residents living at the e-waste recycling site had higher body burdens of PBDEs than the reference group, due to consistent exposure via intake of contaminated food and water, inhalation of contaminated air, and dermal contact with contaminated substances (e.g., soil, dust). Previous studies have shown that diet contributes about 95% of the total intake of PBDEs, with fish and shellfish contributing the greatest proportion (one third) (Schecter et al. 2004). Our parallel study of PCDD/Fs at the same site showed that the same group of people living at Taizhou consumed more food of animal origin including fish and shellfish than the women living at Lin’an (Chan et al. 2007). Donor TZ3, who had the highest body PBDE burden, indicated in our questionnaire that her seafood diet consisted of one serving of marine fish per week before and during her pregnancy, and six servings of shrimps and one serving of other types of seafood (such as clams, mussels, cuttlefish) per month before and during her pregnancy. Donor TZ5 did not consume any fish before or during her pregnancy and ate only one serving of clams, mussels, and other small shellfish per month. Fish collected from the rivers of Taizhou showed extremely high concentrations of PBDEs (up to 13,209 ng/g fat) (Chan 2008), revealing serious local food contamination. Therefore, the elevated body burden of PBDEs of donor TZ3 may be attributed to consumption of contaminated local fish. Our previous study indicated that the mean PBDE concentrations in the abdominal muscle of bighead carp from rivers at Guiyu, China (another e-waste recycling site) was 1,088 ng/g wet wt., which was the highest among published values (Luo et al. 2007). Higher intakes of food of animal origin (especially fish and shellfish) led to greater body burdens of PBDEs in TZ mothers (r > 0.88, p < 0.05).
PBDEs can evaporate from disposed e-waste into the air and can also leach into soil during warm weather or during heating/open burning of e-waste (Leung et al. 2007). PBDE concentrations in air (PM2.5 and TSP) collected from Guiyu (16.6 and 21.5 ng m−3, respectively) were at least 100 times higher than other published data (Deng et al. 2007). Elevated levels of PBDEs were also found in indoor air at an electronics recycling plant in Sweden; therefore, inhalation of particulate-bound PBDEs could be an important exposure pathway of PBDEs (Sjödin et al. 2001).
3.2 Congener profiles of PBDEs
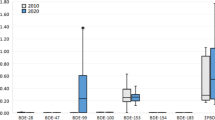
Of the di- to hepta-PBDE congeners analyzed in the present study, milk, placenta, and hair samples were dominated by di- to hexa-BDEs which accounted for more than 80% of the total concentrations. BDE-47 was the dominant congener accounting for 20–30% in all the individual samples, while higher-brominated congeners, for example, BDE-183 and BDE-190 contributed between 2% and 20%. Previous studies related to human body burdens also revealed BDE-47 as the major congener even though the global use and production of Penta-BDE have been greatly reduced since 2004 (She et al. 2007). On a congener basis, the concentrations of BDE-28, BDE-47, BDE-100, BDE-99, BDE-154, BDE-153, and BDE-183 were found to be highest in donor TZ3 whereby Σ7PBDE made up 77%, 68%, and 60% of the total PBDE concentration for hair, placenta, and milk, respectively (see Table 2). Concentrations of BDE-190 were also elevated in hair ranging from 0.132–2.69 ng/g dry wt. In donor TZ1, it accounted for 31.8% of the total PBDE concentration and may have stemmed from the debromination of Deca-BDE.
In Taizhou, the e-waste process stem largely from products manufactured prior to 2004; therefore, Penta- and Octa-BDE are expected to be relevant PBDEs at the site. People were directly exposed to sources of lower-brominated groups, i.e., commercial Penta-BDE, which may leach out from e-waste into the environment when heated (Leung et al. 2007). Our previous study (Deng et al. 2007) at Guiyu, China also indicated that BDE-47 contributed the greatest proportion (30%) to the total concentration (BDE-209 excluded) in air. Lower brominated BDEs have longer half-lives (years) and could be formed via the debromination of higher-brominated groups (Thomsen et al. 2001). They are also more bioavailable, leading to preferable bioaccumulation in biota when compared to highly brominated congeners. Dietary intake is an important exposure route, especially for BDE-47 as it is the major congener accumulated in biota (Sjödin et al. 1999), and exposure may occur through the consumption of PBDE-contaminated fish (Sjödin et al. 2003). As mentioned previously, it is possible that the higher intake of food of animal origin (especially fish and shellfish) led to greater body burdens in TZ mothers (r > 0.88, p < 0.05). This relationship was in accordance with the results obtained by Jakobsson et al. (2002) regarding levels of PBDEs in serum of computer technicians. Fish caught from rivers and fish ponds in Taizhou were dominated by BDE-47 (more than 50%) (Chan 2008). This result also agreed with our early study analyzing PBDE levels in fish from a river in Guiyu (Luo et al. 2007).
The concentrations of higher-brominated congeners (hepta-BDEs) in Taizhou donor specimens were 3–11 times higher than those in the reference group. Hepta-BDEs (i.e., BDE-183, which is characteristic of technical Octa-BDE) were found in all samples and may have implications for inhalation exposure as this congener was the most abundant congener in the air of a Swedish electronics dismantling plant (Sjödin et al. 1999).
Although BDE-209 was not analyzed in the present study, it is known that e-waste processed in Taizhou mostly originate from European countries, Japan, USA, and Russia where there is increasing use of the Deca-BDE technical mixture due to the ban on the use of Penta-BDE (Nakao et al. 2005). Future studies should include the investigation of the full range of BDE congeners. Some of the hepta-BDE congeners (BDE-181, BDE-190) detected in the hair of the women in Taizhou may have resulted from the thermal degradation of Deca-BDE during the open burning of e-waste because these congeners are not found in Penta-BDE and Octa-BDE technical products. Deca-BDE is the predominant BFR used in China, amounting to 30,000 metric tons in 2005 (Zou et al. 2007). Other studies have confirmed the presence of BDE-209 in the human body, e.g., serum (Qu et al. 2007) and hair samples (Zhao et al. 2008) from e-waste workers in China and placenta and milk samples from the general population of Spain (Gomara et al. 2007)
3.3 Correlations among the three types of human specimens
Studies on the relationships between concentrations of PBDEs in human hair and internal tissues are, to our knowledge, not available. Significant positive correlations were found only among the three types of TZ samples, regarding concentrations of total PBDEs and lower-BDEs (di- to hexa-BDEs) (r = 0.998 and p < 0.0001 for hair and milk; r = 0.995 and p < 0.0001 for hair and placenta; r = 0.999 and p < 0.0001 for milk and placenta). No statistical correlations were found among different types of HZ samples (p > 0.05). In addition, there were no significant correlations in the concentrations of the higher-brominated group (i.e., hepta-BDEs) among the human samples. In terms of nanogram per gram dry weight, hepta-BDEs contributed a significantly greater proportion to total PBDEs in TZ hair (20%) than that in TZ milk (2.9%) and in TZ placenta (2.6%). The highest hepta-BDE concentration was found in TZ hair (6.69 ng/g dry wt.; donor TZ2; ANOVA, p = 0.001), followed by TZ milk (0.083 ng/g dry wt.; donor TZ3), and TZ placenta (0.092 ng/g dry wt.; donor TZ3). Hair may be a good bioindicator of human body burdens in highly contaminated sites as exemplified by our earlier study using the same sets of samples, which showed that significant positive correlations were observed in TZ samples and not in HZ samples, with regard to PCDD/Fs concentrations (Chan et al. 2007).
The congener profiles for milk and placenta from TZ were similar, with BDE-47 (22% in milk, 30% in placenta), BDE-153 (16% in milk, 17% in placenta), and BDE-28 (10% in milk, 11% in placenta) being the predominant congeners. Regarding TZ hair, although BDE-47 was the dominant congener (23%), higher-brominated congeners (BDE-99—14%, BDE-183—9%, BDE-190—9%) tended to dominate the congener profiles (Table 1).
The total concentrations in TZ hair were significantly higher than TZ milk and TZ placenta compared on a nanogram per gram dry weight basis. This trend (hair≫milk>placenta) and the homolog profile pattern (TZ hair showed a different profile from the others) were also observed for the same set of samples regarding PCDD/Fs levels (Chan et al. 2007). The partitioning and accumulation patterns of PBDEs in the human body are not clearly known. However, studies have shown that hair can be used for reflecting the atmospheric concentrations of DDTs, PCBs, and PCDD/Fs (Nakao et al. 2005). These compounds penetrate into hair via atmospheric particle deposition and then adsorb into hair fat (Schramm et al. 1992). Due to the structural similarities of PBDEs to PCBs, this may also have occurred for PBDE in hair. Systemic absorption may be another pathway attributing to the high levels of PBDEs in hair compared with in milk or placenta. Previous studies showed that air at e-waste processing sites consisted of higher-brominated BDEs (Sjödin et al. 1999, 2001). Compared with other types of human tissues, it is suspected that hair may be more favorable to higher-brominated compounds. At the same time, it is more difficult for higher-brominated BDEs to pass through placenta than for lower-brominated BDEs (Bi et al. 2006). These might explain why the hair samples contained the highest total PBDE concentrations and the highest proportion of higher-brominated BDEs (hepta-BDEs). Further investigations should be carried out to elucidate the partitioning of PBDEs in different human tissues.
The ratios of concentrations for all three human matrices were also investigated for seven PBDE congeners (Table 3). For milk/placenta, the ratios were similar among the donors for BDE-28 and BDE-47 (ranging from 3.40 to 6.52); however, there were large variations for BDE-100, BDE-154, and BDE-183 (ranging from 3.82 to 36.5). The relatively smaller difference in concentrations with regard for BDE-28 and BDE-47 may be due to the longer half-lives of these congeners compared with the penta- and hexa-BDE congeners (Thomsen et al. 2001). Therefore, BDE-28 and BDE-47 are more stable in the human body. It is recommended that further investigations be conducted to determine whether the concentrations of BDE-28 and BDE-47 in milk could serve as a bioindicator for concentrations in the placenta (i.e., for BDE-28 and for BDE-47, the concentrations in milk was between 3.6 and 6.5 and 4–6 times higher than in placenta, respectively, for the women in TZ). The milk/placenta ratios for BDE-28, BDE-47, and BDE-100 were also, respectively, similar. For the hair/milk and hair/placenta ratios, the calculations were conducted using the concentration of hair (ng/g, dry wt), milk (ng/g, fat), and placenta (ng/g, fat). The hair/milk ratios varied from 0.20 to 2.68 for the lower-brominated congeners (BDE-28, BDE-47, BDE-100, BDE-99) and were 1.3–10.2 for hair/placenta (BDE-28, BDE-47). The hair/milk ratio for BDE-183 and the hair/placenta ratios for BDE-100, BDE-154, and BDE-183 for donor TZ2 were considerably much higher compared with those of the other donors of Taizhou indicating a difference in the partitioning of PBDEs in the human matrices.
3.4 Comparison with international and national PBDE levels
There is a large database for human milk while studies dealing with placenta and hair are rather scarce. Thus, only comparisons of PBDE concentrations in human milk from other studies (largely from the general population) have been made, as shown in Table 4. The total concentrations in milk of the individuals from Taizhou were the highest among all the investigations; slightly higher than North America (Canada and USA) which has half of the annual global consumption of PBDEs (de Wit 2002) and more than 90% of the global application of Penta-BDE (She et al. 2007). European and Asian areas showed similar levels, ranging from 0.96 to 7.2 ng/g fat. These levels were much lower (at least by 16-fold) than the concentrations from Taizhou. In general, BDE-47 was the most abundant congener in human milk. BDE-47 was also reported to be abundant in placenta samples (Main et al. 2007; Strandam et al. 2000).
When compared with other regions (Guangzhou, Shandong, and Taiwan) in China (Bi et al. 2006; Chao et al. 2007; Jin et al. 2009), the Taizhou residents had higher body burdens in milk due to e-waste recycling activities. The values for Guangzhou and Taiwan were similar to that of the reference site, reflecting body burdens in the general Chinese population who are not exposed to uncontrolled e-waste processing operations. However, the average body burdens of BDE-100, BDE-154, and BDE-153 of residents living in the south coast area of Laizhou Bay, Shandong Province, China, where there are several deca-BDE and TBBPA production plants, were higher (Jin et al. 2009). In comparison to a study by Zhao et al. (2008) with regard to PBDE levels in hair of 44 workers working in primitive recycling operations in disassembly sites of four towns near Luqiao, the concentration ranges of BDE-28, BDE-47, BDE-154, and BDE-153 in our study were higher with the exception of BDE-183.
3.5 Health risk assessment for infants
Infants are exposed to PBDEs via prenatal and postnatal contamination. In this study, comparatively high levels of PBDEs were found in placenta, confirming that PBDEs can cross the placenta (Gomara et al. 2007). The estimated daily intakes (EDI) of PBDEs by infants via breastfeeding were determined, as follows:
EDI was calculated based on the assumption that an infant ingests 700 ml milk per day, and the weight of an infant is 5 kg (Hooper et al. 1997).
The EDI was dominated by penta-BDE. TZ infants consumed 572 ± 839 ng/kg body wt/day, with a maximum of 2,240 ng/kg body wt/day. The average value was 57 times higher than that of HZ infants (10.1 ± 4.60 ng/kg body wt/day, with the highest being 17.4 ng/kg body wt/day) and that reported by Meng et al. (2007) (6.87 and 7.37 ng/kg body wt/day) who calculated the values for infants of the general population in Guangzhou, South China. Furthermore, it exceeded the chronic oral reference doses for penta-BDE (2000 ng/kg/day) of the US EPA (Jones-Otazo et al. 2005). However, this reference value was based on toxicological effects toward the liver, excluding developmental and reproductive effects on embryo, fetus, infant, and child which are the most sensitive groups. Lower brominated congeners (e.g., penta-BDEs) are more carcinogenic and mutagenic than the higher ones (Costa and Giordano 2007; Darnerud et al. 2001). The recently proposed reference dose value for BDE-47 by US EPA, based on developmental neurotoxicity, was 100 ng/kg/day. The maximum values of EDI for BDE-47 and BDE-99 by TZ infants were 534 and 224 ng/kg body wt/day, respectively, in which the value for BDE-47 greatly exceeded the US EPA reference dose. A study conducted in Taiwan investigating the general population showed that elevated PBDE levels in human milk correlated with decreased birth outcome, particularly with regard to birth weight and length, chest circumference, and Quetelet's (body mass) index of infants (Chao et al. 2007). The EDI of PBDEs for a breastfed infant in Taiwan was 20.6 ng/kg/day, which was far below the estimated exposure levels in Taizhou.
The epidemiological data for adults obtained from the Centers for Disease Control and Prevention in Taizhou showed an obvious increase in morbidity from 2004 to 2006 due to incidences of malignant tumor and trauma, disorders in digestive, cardiovascular, respiratory, endocrine systems, gynecological, and surgical disease, and ophthalmologic and otolaryngological disorder (p < 0.05) from year 2004 to 2006 (p < 0.05). Moreover, the rate of incidence of cardiovascular disease was significantly higher than all others (79‱, p < 0.001), followed by respiratory (53‱) and digestive system (48‱) disorders. The causes of these diseases in Taizhou are not clearly known; however, they may be attributed to chronic exposure to a wide range of pollutants (such as PBDEs, PCDD/Fs, PAHs, heavy metals) (Wong et al. 2007) released into the environment during e-waste disassembly and recycling activities which have been occurring in Taizhou for the past 30 years. Adults are adversely affected by the pollution; however, fetuses and infants are the most vulnerable population because they can suffer neurological and developmental problems due to their early developmental stage. The high EDI implies a potential health risks for breastfed infants and warrants immediate action to prevent further releases of PBDEs and other pollutants into the environment.
4 Conclusion
This is the first study to determine PBDEs levels in milk, placenta, and hair collected from mothers at an intense e-waste recycling site and a reference site in China. Although a limited number of samples was analyzed, the results clearly indicated that e-waste recycling operations released significant levels of PBDEs to the environment resulting in increased body burdens of PBDEs in women of childbearing-age residing at the e-waste processing area. The PBDE body burdens in these women ranked among the highest in the world. Dietary intake and indoor dust were suspected to be the most important exposure routes to PBDEs. High levels in the body of the residents raise concerns on the physiological effects and potential health risks of PBDEs, particularly in relation to children. Control measures should be imposed in order to minimize the level of pollutants resulting from e-waste processing operations, to the environment and to humans. In-depth investigations on epidemiological studies of health impacts caused by e-waste recycling operations should be conducted. Further measurements of PBDE levels in local food (e.g., fish, shellfish, dairy products, meat, fruits, and vegetables), dust, air, water, and human specimens collected from a larger sample size at the e-waste processing sites should also be carried out for the determination of human exposure pathways to PBDEs.
References
Bi X, Qu W, Sheng G, Zhang W, Mai B, Chen D (2006) Polybrominated diphenyl ethers in South China maternal and fetal blood and breast milk. Environ Pollut 144:1024–1030
Bromine Science and Environmental Forum (2003) Total Market Demand. http://www.bsef.com
Chan JKY (2008) Dietary exposure, human body loadings, and health risk assessment of persistent organic pollutants at two major electronic waste recycling sites in China. PhD Thesis, Hong Kong Baptist University.
Chan JKY, Xing GH, Xu Y, Liang Y, Chen LX, Wu SC, Leung CKM, Wong MH (2007) Body loadings and health risk assessment of polychlorinated dibenzo-p-dioxins and dibenzofurans at an intensive electronic waste recycling site in China. Environ Sci Technol 41:7668–7674
Chao HR, Wang SL, Lee WJ, Wang YF, Päpke O (2007) Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Int 33:239–245
Chen D, Mai B, Song J, Sun Q, Luo Y, Luo X, Zeng EY, Hale RC (2007) Polybrominated diphenyl ethers in birds of prey from Northern China. Environ Sci Technol 41:1828–1833
Chen L, Mai B, Xu Z, Peng X, Han J, Ran Y, Sheng G, Fu J (2008) In- and outdoor sources of polybrominated diphenyl ethers and their human inhalation exposure in Guangzhou, China. Atmos Environ 42:78–86
Costa LG, Giordano G (2007) Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28:1047–1067
Covaci A, Tutudaki M, Tsatsakis AM, Schepens P (2002) Hair analysis: another approach for the assessment of human exposure to selected persistent organochlorine pollutants. Chemosphere 46:413–418
Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M (2001) Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect 109:49–68
Deng WJ, Zheng JS, Bi XH, Fu JM, Wong MH (2007) Distribution of PBDEs in air particles from an electronic waste recycling site compared with Guangzhou and Hong Kong, South China. Environ Int 33:1063–1069
de Wit CA (2002) An overview of brominated flame retardants in the environment. Chemosphere 46:583–624
Eslami B, Koizumi A, Ohta S, Inoue K, Aozasa O, Harada K, Yoshinaga T, Date C, Fujii S, Fujimine Y, Hachiya N, Hirosawa I, Koda S, Kusaka Y, Murata K, Nakatsuka H, Omae K, Saito N, Shimbo S, Takenaka K, Takeshita T, Todoriki H, Wada Y, Watanabe T, Ikeda M (2006) Large-scale evaluation of the current level of polybrominated diphenyl ethers (PBDEs) in breast milk from 13 regions of Japan. Chemosphere 63:554–561
Esteban M, Castaño A (2009) non-invasive matrices in human biomonitoring: A review. Envriron Int 35:438–449
Fängström B, Strid A, Grandjean P, Weihe P, Bergman Å (2005) A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ Health: Global Access Sci Source 4:12–20
Gomara B, Herrero L, Ramos JJ, Mateo JR, Fernandez MA, Garcia JF, Gonzalez MJ (2007) Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol 41:6961–6968
Guan YF, Wang JZ, Ni HG, Luo XJ, Mai BX, Zeng EY (2007) Riverine inputs of polybrominated diphenyl ethers from the Pearl River Delta (China) to the coastal ocean. Environ Sci Technol 41:6007–6013
Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman Å, Norén K (2003) Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect 111:1235–1241
Hooper K, Petreas MX, She JW, Visita P, Winkler J, McKinney M, Mok M, Sy F, Garcha J, Gill M, Stephens RD, Seminova G, Sharmanov T, Chuvakova T (1997) Analysis of breast milk to assess exposure to chlorinated contaminants in Kazakhstan: PCBs and organochlorine pesticides in southern Kazakstan. Environ Health Perspect 105:1250–1254
Ingelido AM, Ballard T, Dellatte E, Domenico AD, Ferri F, Fulgenzi AR, Herrmann T, Iacovella N, Miniero R, Päpke O, Porpora MG, Felip ED (2007) Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in milk from Italian women living in Rome and Venice. Chemosphere 67:301–306
Jakobsson K, Thuresson K, Rylander L, Sjödin A, Hagmar L, Bergman Å (2002) Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere 46:709–716
Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, Schepens P (2006) Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. Sci Total Environ 372:20–31
Jin J, Wang Y, Yang CQ, Ju JC, Liu WZ, Cui J, Tang XY (2009) Polybrominated diphenyl ethers in the serum and breast milk of the resident population from production area, China. Environ Int 35:1048–1052
Johnson-Restrepo B, Addink R, Wong C, Arcaro K, Kannan K (2007) Polybrominated diphenyl ethers and organochlorine pesticides in human breast milk from Massachusetts, USA. J Environ Monit 9:1205–1212
Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B (2005) Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol 39:5121–5130
Kalantzi OI, Martin FL, Thomas GO, Alcock RE, Tang HR, Drury SC, Carmichael PL, Nicholson JK, Jones KC (2004) Different levels of polybrominated diphenyl ethers (PBDEs) and chlorinated compounds in breast milk from two UK regions. Environ Health Persp 112:1085–1091
Kazda R, Hajšlová J, Poustka J, Čajka T (2004) Determination of polybrominated diphenyl ethers in human milk samples in the Czech Republic: Comparative study of negative chemical ionisation mass spectrometry and time-of-flight high-resolution mass spectrometry. Anal Chim Acta 520:237–243
Leung AOW, Luksemburg WJ, Wong AS, Wong MH (2007) Spatial distribution of polybrominated diphenyl ethers and polychlorinated dibenzo-p-dioxins and dibenzofurans in soil and combusted residue at Guiyu, an electronic waste recycling site in Southeast China. Environ Sci Technol 41:2730–2737
Liem AKD, Ahlborg UG, Back H, Haschke F, Nygren M, Younes M, Ynanheikke E (1996) Levels of PCBs, PCDDs and PCDFs in Human Milk: Second Round of WHO-Coordinated Exposure Study. WHO European Centre for Environment and Health, Copenhagen, Environmental Health in Europe No. 3
Luo Q, Cai ZW, Wong MH (2007) Polybrominated diphenyl ethers in fish and sediment from river polluted by electronic waste. Sci Total Environ 383:115–127
Ma J, Kannan K, Cheng JP, Horii Y, Wu Q, Wang WH (2008) Concentrations, profiles, and estimated human exposures for polychlorinated dibenzo-p-dioxins and dibenzofurans from electronic waste recycling facilities and a chemical industrial complex in eastern China. Environ Sci Technol 42:8252–8259
Mai B, Chen S, Luo X, Chen L, Yang Q, Sheng G, Peng P, Fu J, Zeng EY (2005) Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ Sci Technol 39:3521–3527
Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkaek NE, Toppari J (2007) Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect 115:1519–1526
Meng XZ, Zeng EY, Yu LP, Guo Y, Mai BX (2007) Assessment of human exposure to polybrominated diphenyl ethers in China via fish consumption and inhalation. Environ Sci Technol 41:4882–4887
Nakao T, Aozasa O, Ohta S, Miyata H (2005) Survey of human exposure to PCDDs, PCDFs, and coplanar PCBs using hair as an indicator. Arch Environ Contam Toxicol 17:124–130
Polder A, Gabrielsen GW, Odland JØ, Savinova TN, Tkachev A, Løken KB, Skaare JU (2008) Spatial and temporal changes of chlorinated pesticides, PCBs, dioxins (PCDDs/PCDFs) and brominated flame retardants in human breast milk from Northern Russia. Sci Total Environ 391:41–54
Qu W, Bi X, Sheng G, Lu S, Fu J, Yuan J, Li L (2007) Exposure to polybrominated diphenyl ethers among workers at an electronic waste dismantling region in Guangdong, China. Environ Int 33:1029–1034
Ramu K, Kajiwara N, Lam PKS, Jefferson TA, Zhou K, Tanabe S (2006) Temporal variation and biomagnification of organohalogen compounds in finless porpoises (Neophocaena phocaenoides) from the South China Sea. Environ Pollut 144:516–523
Schecter A, Papke O, Tung KC, Staskal D, Birnbaum L (2004) Polybrominated diphenyl ethers contamination of United States food. Environ Sci Technol 38:5306–5311
Schramm KW, Keuttnear T, Weber S, Lützke K (1992) Dioxin hair analysis as monitoring pool. Chemosphere 24:351–358
She J, Holden A, Sharp M, Tanner M, Williams-Derry C, Hooper K (2007) Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from the Pacific Northwest. Chemosphere 67:307–317
Sjödin A, Carlsson H, Thuresson K, Sjödin S, Bergman Å, Östman C (2001) Flame retardants in indoor air at an electronics recycling plant and at other work environments. Environ Sci Technol 35:448–454
Sjödin A, Donald G, Patterson DG, Bergman A (2003) A review on human exposure to brominated flame retardants-particularly polybrominated diphenyl ethers. Environ Int 29:829–839
Sjödin A, Hagmar L, Klasson-Wehler E, Kronholm-Diab K, Jakobsson E, Bergman Å (1999) Flame retardant exposure: polybrominated diphenyl ethers in blood from Swedish workers. Environ Health Perspect 107:643–648
Smolders R, Schramm K-W, Nickmilder M, Schoeters G (2009) Applicability of non-invasively collected matrices for human biomonitoring. Environ Health 8:8–17
Strandam T, Koistinen J, Vartiainen T (2000) Polybrominated diphenyl ethers (PBDEs) in placenta and human milk. Organohalogen Compds 47:61–65
Sudaryanto A, Kajiwara N, Takahashi S, Muawanah, Tanabe S (2008) Geographical distribution and accumulation features of PBDEs in human breast milk from Indonesia. Environ Pollut 151:130–138
Taizhou Economic Committee, China (2007) http://www.tzsjw.gov.cn/index.php (in Chinese)
The World Bank (2005). Environmental Impact assessment of Zhejiang Province for PCB management and disposal demonstration project. http://www-wds.worldbank.org
Tanabe S (2008) Temporal trends of brominated flame retardants in coastal waters of Japan and South China: retrospective monitoring study using archived samples from es-Bank, Ehime University, Japan. Mar Pollut Bull 57:267–274
Thomsen C, Lundanes E, Becher G (2001) Brominated flame retardants in plasma samples from three different occupational groups in Norway. J Environ Monit 3:366–370
Toms LML, Harden FA, Symons RK, Burniston D, Fürst P, Müller JF (2007) Polybrominated diphenyl ethers (PBDEs) in human milk from Australia. Chemosphere 68:797–803
Tsydenova OV, Sudaryanto A, Kajiwara N, Kunisue T, Batoev VB, Tanabe S (2007) Organohalogen compounds in human breast milk from Republic of Buryatia, Russia. Environ Pollut 146:225–232
UNEP (2005) E-waste, the hidden side of IT equipment’s manufacturing and use. http://www.grid.unep.ch/product/publication/download/ew_ewaste.en.pdf
UNEP (2009) Stockholm Convention on Persistent Organic Pollutants (POPs) Press Release: Governments unite to step-up reduction on global DDT reliance and add nine new chemicals under international treaty.
USEPA (2009) DecaBDE phase-out initiative. http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/deccadbe.html (17 December 2009) http://www.unep.org/Documents.Multilingual/Default.asp?DocumentID=585&ArticleID=6158&l=en&t=long
US EPA (1996a) Method 3540C: Soxhlet Extraction. United States Environmental Protection Agency, Washington, DC
US EPA (1996b) Method 3630C: Silica gel cleanup—Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846. US EPA, Washington, DC
US EPA (1996c) Method 3620B: Florisil Cleanup—Solid Waste Analysis SW-846. United States Environmental Protection Agency, Washington, DC
US EPA (2003) US EPA Draft Method 1614: Brominated diphenyl ethers in water, soil, sediment, and tissue by HRGC/HRMS. USEPA, Washington, DC
Wong MH, Wu SC, Deng WJ, Yu XZ, Luo Q, Leung AOW, Wong CSC, Luksemburg WJ, Wong AS (2007) Export of toxic chemicals – A review of the case of uncontrolled electronic-waste recycling. Environ Pollut 149:131–140
Xian Q, Ramu K, Isobe T, Sudaryanto A, Liu X, Gao Z, Takahashi S, Yu H, Tanabe S (2008) Levels and body distribution of polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecanes (HBCDs) in freshwater fishes from the Yangtze River, China. Chemosphere 71:268–276
Xinhua Online. (2007) Seventy percent of worldwide electronic-waste goes to China. Dated January 1, http://news3.xinhuanet.com/tech/2007-01/09/content_5581834.htm (in Chinese)
Yu XZ, Gao Y, Wu SC, Zhang HB, Cheung KC, Wong MH (2006) Distribution of polycyclic aromatic hydrocarbons in soils at Guiyu area of China, affected by recycling of electronic waste using primitive technologies. Chemosphere 65:1500–1509
Zhao GF, Wang ZJ, Dong MH, Rao KF, Luo JP, Wang DH, Zha JM, Huang SB, Xu YP, Ma M (2008) PBBs, PBDEs, and PCBs levels in hair of residents around e-waste disassembly sites in Zhejiang Province, China, and their potential sources. Sci Tot Environ 397:46–57
Zou MY, Ran Y, Gong J, Mai BX, Zeng EY (2007) Polybrominated diphenyl ethers in watershed soils of the Pearl River Delta, China: occurrence, inventory, and fate. Environ Sci Technol 41:8262–8267
Acknowledgements
We thank the mothers who contributed to the study and donated milk, placenta, and hair samples. This research was supported by The Research Grants Council of the University Grants Committee of Hong Kong (Central Allocation Group Research Project HKBU 1/03C), Match Fund from Hong Kong Baptist University, and a private donation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Anna OW Leung and Janet KY Chan contributed equally to this work
Rights and permissions
About this article
Cite this article
Leung, A.O.W., Chan, J.K.Y., Xing, G.H. et al. Body burdens of polybrominated diphenyl ethers in childbearing-aged women at an intensive electronic-waste recycling site in China. Environ Sci Pollut Res 17, 1300–1313 (2010). https://doi.org/10.1007/s11356-010-0310-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0310-6