Abstract
Background, aim, and scope
Dissolved organic matter, measured as dissolved organic carbon (DOC), is an important component of aquatic ecosystems and of the global carbon cycle. It is known that changes in DOC quality and quantity are likely to have ecological repercussions. This review has four goals: (1) to discuss potential mechanisms responsible for recent changes in aquatic DOC concentrations; (2) to provide a comprehensive overview of the interactions between DOC, nutrients, and trace metals in mainly boreal environments; (3) to explore the impact of climate change on DOC and the subsequent effects on nutrients and trace metals; and (4) to explore the potential impact of DOC cycling on climate change.
Main features
We review recent research on the mechanisms responsible for recent changes in aquatic DOC concentrations, DOC interactions with trace metals, N, and P, and on the possible impacts of climate change on DOC in mainly boreal lakes. We then speculate on how climate change may affect DOC export and in-lake processing and how these changes might alter nutrient and metal export and processing. Furthermore, the potential impacts of changing DOC cycling patterns on climate change are examined.
Results
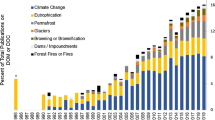
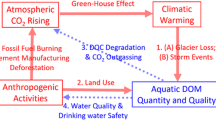
It has been noted that DOC concentrations in lake and stream waters have increased during the last 30 years across much of Europe and North America. The potential reasons for this increase include increasing atmospheric CO2 concentration, climate warming, continued N deposition, decreased sulfate deposition, and hydrological changes due to increased precipitation, droughts, and land use changes. Any change in DOC concentrations and properties in lakes and streams will also impact the acid–base chemistry of these waters and, presumably, the biological, chemical, and photochemical reactions taking place. For example, the interaction of trace metals with DOC may be significantly altered by climate change as organically complexed metals such as Cu, Fe, and Al are released during photo-oxidation of DOC. The production and loss of DOC as CO2 from boreal lakes may also be affected by changing climate. Climate change is unlikely to be uniform spatially with some regions becoming wetter while others become drier. As a result, rates of change in DOC export and concentrations will vary regionally and the changes may be non-linear.
Discussion
Climate change models predict that higher temperatures are likely to occur over most of the boreal forests in North America, Europe, and Asia over the next century. Climate change is also expected to affect the severity and frequency of storm and drought events. Two general climate scenarios emerge with which to examine possible DOC trends: warmer and wetter or warmer and drier. Increasing temperature and hydrological changes (specifically, runoff) are likely to lead to changes in the quality and quantity of DOC export from terrestrial sources to rivers and lakes as well as changes in DOC processing rates in lakes. This will alter the quality and concentrations of DOC and its constituents as well as its interactions with trace metals and the availability of nutrients. In addition, export rates of nutrients and metals will also change in response to changing runoff. Processing of DOC within lakes may impact climate depending on the extent to which DOC is mineralized to dissolved inorganic carbon (DIC) and evaded to the atmosphere or settles as particulate organic carbon (POC) to bottom sediments and thereby remaining in the lake. The partitioning of DOC between sediments and the atmosphere is a function of pH. Decreased DOC concentrations may also limit the burial of sulfate, as FeS, in lake sediments, thereby contributing acidity to the water by increasing the formation of H2S. Under a warmer and drier scenario, if lake water levels fall, previously stored organic sediments may be exposed to greater aeration which would lead to greater CO2 evasion to the atmosphere. The interaction of trace metals with DOC may be significantly altered by climate change. Iron enhances the formation of POC during irradiation of lake water with UV light and therefore may be an important pathway for transfer of allochthonous DOC to the sediments. Therefore, changing Fe/DOC ratios could affect POC formation rates. If climate change results in altered DOC chemistry (e.g., fewer and/or weaker binding sites) more trace metals could be present in their toxic and bioavailable forms. The availability of nutrients may be significantly altered by climate change. Decreased DOC concentrations in lakes may result in increased Fe colloid formation and co-incident loss of adsorbable P from the water column.
Conclusions
Climate change expressed as changes in runoff and temperature will likely result in changes in aquatic DOC quality and concentration with concomitant effects on trace metals and nutrients. Changes in the quality and concentration of DOC have implications for acid–base chemistry and for the speciation and bioavailability of certain trace metals and nutrients. Moreover, changes in DOC, metals, and nutrients are likely to drive changes in rates of C evasion and storage in lake sediments.
Recommendations and perspectives
The key controls on allochthonous DOC quality, quantity, and catchment export in response to climate change are still not fully understood. More detailed knowledge of these processes is required so that changes in DOC and its interactions with nutrients and trace metals can be better predicted based on changes caused by changing climate. More studies are needed concerning the effects of trace metals on DOC, the effects of changing DOC quality and quantity on trace metals and nutrients, and how runoff and temperature-related changes in DOC export affect metal and nutrient export to rivers and lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
Dissolved organic matter, measured as dissolved organic carbon (DOC), is an important component of aquatic ecosystems (see Steinberg et al. 2008) and of the global carbon cycle. As a carbon and energy source for micro-organisms in water and soil, it undergoes and is part of many important chemical and photochemical reactions and transformations. Through its constituent acids, DOC has an effect on the pH of aquatic systems, it imparts color, it attenuates both visible and UV light, thus acting as a sunscreen for aquatic micro-organisms. DOC also binds metals, affecting their toxicity and bioaccumulation, and nutrients such as N, P, Fe, Cu, and Se, thus controlling their bioavailability and mobility. Clearly, changes in DOC quality and quantity are likely to have ecological repercussions.
DOC inputs to boreal lakes are typically dominated by allochthonous sources, especially peatlands (Dillon and Molot 1997a). Water-saturated organic soils such as peat sequester relatively large amounts of organic carbon because saturation inhibits aerobic respiration. Some of the sequestered carbon is released as DIC (CO, CO2, and CH4) to the atmosphere and some is exported as DIC and DOC to adjacent surface waters. Annual DOC export from a catchment is highly correlated with annual runoff (Dillon and Molot 2005), hence DOC loading (sum of all inputs) to a lake is regulated in large part by local hydrology and landscape features.
The leaching of carbon from soils depends on a number of factors. DOC interactions and movement in a landscape comprise many factors (Neff and Asner 2001; Roulet and Moore 2006) so that receiving waters may exhibit a large variability in their response (e.g., CO2 evasion rates) to climate change (Huttunen et al. 2003). Lake characteristics (e.g., size, morphometry) and hydrological and geological characteristics of the catchment also play a role (Xenopoulos et al. 2003).
Having entered a lake, DOC is gradually degraded by biological and photochemical processes (Stumm and Morgan 1996) to dissolved inorganic carbon (DIC) and particulate organic carbon (POC) (Wetzel 2001). The partitioning of DOC losses between DIC (transferred to the atmosphere) and POC (transferred to the sediments) is controlled in part by acidity which promotes photo-mineralization to DIC (Dillon and Molot 1997b; Gennings et al. 2001; Molot et al. 2005) and coagulation by releasing organically bound metals such as Al and Fe (Kopáček et al. 2006). The degradation of DOC also releases bound constituents such as P, N (Wang et al. 2000; Tarr et al. 2001; Vähätalo et al. 2003), and metals (e.g., Kopáček et al. 2005; Shiller et al. 2006; Brooks et al. 2007; Kelton et al. 2007), thereby increasing their bioavailability.
The potential mechanisms responsible for recent changes in aquatic DOC concentrations, such as increasing atmospheric CO2 concentration, climate warming, continued N deposition, decreased sulfate deposition, and hydrological changes, are discussed in this review. Any change in DOC concentrations and properties in lakes and streams will also impact the interactions between DOC, nutrients, and trace metals.
We review how climate change may affect DOC export from terrestrial catchments to lakes and in-lake processing of DOC, and as a result, how these changes might alter nutrient and metal export and processing in lakes. We also explore the potential impact of altered in-lake DOC cycling on climate change.
2 Climate change and DOC
2.1 Recent changes in DOC concentrations
Increases in DOC concentrations in lake and stream waters during the last 30 years were reported in several studies across much of Europe and North America (Freeman et al. 2001a, 2004; Worrall et al. 2004a; Hongve et al. 2004; Evans et al. 2005; Skjelkvåle et al. 2005; Burns et al. 2006; Vuorenmaa et al. 2006). Several hypotheses have been suggested to explain these increases (discussed in more detail below) including increasing atmospheric CO2 concentration (Freeman et al. 2004), climate warming (Freeman et al. 2001a), continued N deposition (Pregitzer et al. 2004; Findlay 2005), decreased sulfate (SO 2−4 ) deposition (Evans et al. 2006, Monteith et al. 2007), hydrological change including increased precipitation (Freeman et al. 2001a; Hejzlar et al. 2003), droughts, and altered hydrologic pathways (e.g., Hongve et al. 2004; Worrall and Burt 2008). Land use changes are also important factors influencing the transport of DOC from catchments to adjacent surface waters (Worrall et al. 2004b; Raymond and Oh 2007).
In contrast, lakes in central Ontario exhibited small and variable changes in DOC concentrations. Between 1978 and 1998, DOC loading to these lakes (loading is defined as the sum of all tributary export to a lake) did not exhibit a clear increase or decrease. Instead, loading declined during dry conditions in the middle of the study period then increased to earlier levels with loading being strongly associated with runoff patterns (Dillon and Molot 2005). These changes in loading were mirrored by smaller and variable changes in lake DOC concentrations.
2.1.1 Increasing atmospheric CO2
Understanding the effects of elevated atmospheric CO2 concentrations on soil organic matter accumulation and decomposition in wetland ecosystems is important for predicting future carbon dynamics and sequestration. Although wetlands occupy a relatively small percentage of the world’s land area, they store a disproportionate amount of soil C (Lavoie et al. 2005). Elevated CO2 enhances DOC supply in peat soils and possibly into adjacent aquatic ecosystems (Kang et al. 2001). Freeman et al. (2004) observed an increase in DOC exported from peat soils under elevated CO2 conditions which they attributed to elevated net primary productivity and increased root exudation of DOC. They suggested that the labile carbon released by roots stimulate microbial activity, leading to enhanced degradation of soil organic matter; this process is known as the ‘priming mechanism’ (Kuzyakov 2002).
Experiments with elevated CO2 levels significantly increased soil organic matter mineralization by 83–218% in a simulated wetland (Wolf et al. 2007). Based on natural-abundance stable carbon isotope tracing, this study showed that the increase in CO2 production was derived from the mineralization of established soil organic matter, not recently fixed carbon compounds. Fenner et al. (2007b) proposed, however, that the proportion of DOC leachate derived from recently assimilated 13CO2 was significantly increased in the enhanced CO2 peat. They attributed the observed increase in DOC concentrations in the leachate to the switch from predominantly Sphagnum spp. to vascular species (namely Juncus effusus), leading to enhanced exudation and decomposition of the litter and peat, measured as CO2 production. A study by Lavoie et al. (2005) gave inconclusive results for the growth of Sphagnum under enhanced CO2 conditions. A consensus on these findings remains to be established.
Increased atmospheric CO2 concentrations result in increased primary production and, consequently, increased decomposition of wetland soil organic matter, thereby resulting in increased export of DOC to streams and lakes.
2.1.2 Continuing N deposition
The documented increase of NH3 and NOx (NO + NO2) emissions over the last 150 years has accelerated N deposition, compromising air and water quality and altering the functioning of terrestrial and aquatic ecosystems worldwide (Holland et al. 2005). Riverine inputs of N into the North Atlantic basin from Europe have increased by a factor of 3.5–10.6 relative to preindustrial inputs (mid-1800s). Similarly, N fluxes (from direct atmospheric deposition and runoff) into North American rivers have increased by a factor of 1.7–5.3 (Howarth et al. 1996). A possible explanation for the observed differences between Europe and North America may be due to the greater atmospheric transport of N offshore of North America, which is then deposited downwind onto the North Atlantic Ocean or Europe (Galloway et al. 1996; Prospero et al. 1996).
Concentrations of DOC in New York’s Hudson River have doubled over the past 16 years. Temperature, groundwater levels, and land cover have not changed in ways that would make these viable causes for the observed increases in DOC. One plausible mechanism driving these changes is a soil microbial response to N deposition, resulting in greater export of humic material (Findlay 2005). Eight years of experimental nitrate (NO −3 ) additions to four different northern hardwood forests (Great Lakes Region of the USA) dramatically increased leaching losses of DOC (Pregitzer et al. 2004). Other recent studies also acknowledged that N deposition increases DOC and DON production (McDowell et al. 1998; Yano et al. 2000). However, other studies showed no increase in DOC production and export. Currie et al. (1996) found no significant difference in DOC concentrations in soil water from the Oa horizon after 7 years of N addition to pine and hardwood stands in the Harvard Forest, but DON concentrations and ratios of DON:DOC did increase significantly. These disparate findings indicate that other mechanisms are at least partly responsible in determining changes in soil DOC production and export.
The role of continuing N deposition on DOC export from soils is still not completely understood. Results from different field experiments do not agree on the impact of N additions to soils indicating that N deposition is not the only mechanism responsible for observed changes in soil DOC production and export.
2.1.3 Decreasing S deposition
The deposition of strong mineral acids reduces DOC concentrations in low pH (<6) irradiated water through enhanced photochemical oxidation (Gennings et al. 2001; Anesio and Granéli 2004; Molot et al. 2005). Increases in both acidity and ionic strength (associated with a high SO 2−4 loading) have also been shown to reduce soil solution DOC concentrations in a range of laboratory experiments with organic soils horizons (Kalbitz et al. 2000 and references therein). From this last finding, it is inferred that acid deposition may lead to lower DOC loading to lakes and lower DOC concentrations in lake waters.
On the other hand, when acid deposition is reduced DOC loading to lakes should eventually increase. Photochemical oxidation rates will also decline in step with increasing pH leading to an increase in DOC concentration in lakes. As deposition declines, mineral acidity will be partially replaced by organic acidity (Krug and Frink 1983). If declining acid deposition is having a major influence, the rise in DOC concentration may be seen as part of the recovery process in acid-sensitive waters, with “weak” organic acidity increasingly replacing “strong” mineral acidity (Skjelkvåle et al. 2005). Although an inverse relationship has been proposed between mineral acidity and the generation of DOC (Krug and Frink 1983), Freeman et al. (2001b) observed similar proportional increases in DOC concentrations in lakes and streams (UK Acid Waters Monitoring Network) at remote, unacidified sites, as well as at those recovering from anthropogenic acidification, indicating that other mechanisms are also responsible for the observed increases in DOC concentrations.
Several recent studies suggested that changes in SO 2−4 , resulting from either declining deposition or water-table shifts in wetlands, could influence patterns and trends in DOC concentrations in surface waters (Clark et al. 2005, 2006; Vuorenmaa et al. 2006). Evans et al. (2006) reviewed a variety of potential drivers of increases in DOC at 22 UK streams and lakes between 1988 and 2003, and concluded that declines in SO 2−4 deposition and presumed improvements in soil pH (and potential decreases in soil solution ionic strength) were the most likely causes. Monteith et al. (2007) showed, through the assessment of time series data from 522 remote lakes and streams in North America and northern Europe, that rising trends in DOC concentrations between 1990 and 2004 can be explained by a simple model based solely on changes in deposition chemistry and catchment acid sensitivity. They demonstrated that DOC concentrations increased in proportion to the rates at which atmospherically deposited anthropogenic sulfur and sea salt have declined. However, Eimers et al. (2008b) provided an alternative explanation in which negative correlations between SO 2−4 and DOC concentrations are either directly (spring) or indirectly (summer/fall) caused by underlying relationships with hydrology.
The assessment of time series from over 500 acid-sensitive sites in North America and northern Europe showed that the tendency for DOC increases in most regions between 1990 and 2004 could be explained by changes in the acid anion concentration of atmospheric deposition (ICP Waters Report 87/2007). Increases were particularly dominant in the southernmost regions of the Nordic Countries, in the United Kingdom, and in the north-eastern U.S.; these are all areas where sulfur and/or chloride deposition have declined significantly during the 1990–2004 time period. There were strong tendencies for DOC to increase in the northern Nordic region, as well as in Ontario and Quebec, although many of these increases were not significant, suggesting that hydrology may be playing a stronger role in these regions. Atlantic Canada was the only region with little evidence of increasing DOC. For Newfoundland sites, where DOC concentrations declined, the change in SO 2−4 was mostly small and statistically insignificant, whereas Cl− concentrations had increased significantly.
Although decreasing S deposition has been shown to be a major contributor to the observed increases in surface water DOC concentrations in many regions impacted by acidification, other regions, not impacted by acidification, also exhibit increases in surface water DOC concentrations. Furthermore, some acidified regions show insignificant changes in DOC concentrations in response to decreased S deposition. These disparate findings indicate that hydrology may also be an important mechanism in determining surface water DOC concentrations.
2.1.4 Increasing air temperature
Changes in temperature may directly impact DOC export from wetlands by altering DOC production via increased organic matter decomposition and mineralization which are both sensitive to variations in moisture and temperature (Dalva and Moore 1991; Christ and David 1996; Rey et al. 2005). Temperature may also affect net primary production, at least indirectly by changing the length of the growing season. Hence, changes in DOC export will depend in part on how temperature affects the difference between new production and respiration (loss), this difference being the pool that contributes to the leachable component (Schiff et al. 1997).
Increased air temperature may mean increased depth to the water table in organic soils as a result of higher evaporation rates in some regions. If the water table is lowered below the surface, the carbon sink–source relationship is likely to be disturbed because a greater percentage of the peat is available for oxidation in biochemical reactions, thereby increasing the activity of the phenol oxidase enzyme, which degrades the phenolic compounds that inhibit decomposition (Freeman et al. 2001b). Worrall and Burt (2004) associated observed increases in DOC concentrations in UK rivers with severe droughts which triggered the ‘enzymatic latch’ mechanism. The rate of peat decomposition will increase with lowered water tables, and effectively more CO2 and DOC could be available for release. As a potential counter-balance, reduced water tables would result in a reduction in the amount of CH4 (a relatively stronger greenhouse gas than CO2) released because the increase in aerobic conditions will suppress the activity of the anaerobic methanogenic bacteria and decrease the volume of peat in which CH4 oxidation may occur (Holden et al. 2007).
Pastor et al. (2003) observed reduced DOC export from experimentally warmed peat mesocosms as increased evapotranspiration led to reduced discharge, and hence, reduced DOC transport, out of the peat. These experimental findings are consistent with the observations of reduced export from forested catchments during dry conditions in central Ontario (Dillon and Molot 2005). Meyer and Pulliam (1992) interpreted a decrease in DOC inputs to streams to soil warming that results in accelerated respiration of soil organic matter that would otherwise be transported to streams as DOC. The different possible processes responsible for the decreased DOC export to streams indicate that DOC export responses to changes in temperature and runoff will vary geographically, perhaps because peatlands in relatively wet climates like the UK do not experience the same magnitude of aeration as experienced in some parts of North America.
Any rise in temperature that accompanies elevated atmospheric CO2 and CH4 could also intensify aquatic respiration and emission rates (Fenner et al. 2007a). For example, increased temperature results in increased CH4 emissions from the littoral regions of boreal lakes (Kankaala and Bergström 2004) perhaps as a result of more extensive anoxia in shallow sediments. A longer ice-free season may also lead to increased in-lake photochemical and biological consumption of allochthonous DOC, resulting in decreased DOC concentration.
Increased temperature also means a shorter frost season for soils resulting in increased export of nutrients to the lakes (Kortelainen et al. 2006) although this could be offset by decreased streamflow due to changing precipitation patterns which would exist under the warmer and drier scenario. The implications for DOC–nutrient interactions are discussed in Section 4.
Even though increased temperature results in increased decomposition of soil organic matter and production of DOC, it is the availability of water that, in part, controls DOC export. DOC export will also depend on how temperature affects the difference between new production and mineralization.
2.1.5 Change in precipitation
In the previous sections, hydrology has been invoked as a mechanism to explain disparate findings regarding the effects of continuing N deposition and changing S deposition, and air temperature on surface water DOC concentrations. In this and subsequent sections, we will examine the significance of hydrology.
The significance of hydrology on transport of DOC from terrestrial sources to the streams and then to the oceans was discussed by Tranvik and Jansson (2001) who argued that warming can affect DOC export in different ways, depending on whether it is accompanied by increased or decreased precipitation. Precipitation and discharge showed significant increasing trends in the period 1983–2000 in central Europe (Hejzlar et al. 2003). Increases in DOC concentrations and water color and changes in the concentrations of inorganic constituents (significant reduction of sulfate concentrations, but increase in acidity) in Norwegian forest lakes during the last 20 years are well correlated with increasing amounts of precipitation, while no effect was seen with increasing temperature (Hongve et al. 2004). Raymond and Oh (2007) showed a general relationship between the discharge and precipitation in three large-scale watersheds (Mississippi, Missouri, and Ohio River, USA) and concluded that increased precipitation would result in increased DOC export. Similarly, annual DOC export and annual runoff were highly correlated in 20 forested boreal catchments in central Ontario (Dillon and Molot 2005). The substantial increase in DOC concentration in lakes and streams in Sweden during the 1970s and 1980s, despite a reduction of annual temperature (which is in contrast with Freeman et al. 2001a), was explained by the increased precipitation and runoff in these locations.
In contrast, DOC concentrations in a UK upland peatland catchment decreased with increased discharge during autumn storm events, which may be a dilution effect, but remained comparatively flow-invariant at other times of the year (Clark et al. 2007). DOC export increased during individual storm events, however, as DOC export is ultimately controlled by discharge volume, and therefore rainfall. The magnitude of change in discharge was greater than the magnitude of decline in concentrations indicating flushing of stored DOC followed by dilution (Clark et al. 2007).
The implications for long-term DOC trends therefore seem contradictory, as increased rainfall could increase export but cause an overall decrease in DOC concentrations from peatland streams. Alternatively, increased concentrations can occur due to changing DOC production and retention, but with no change in hydrology (Roulet and Moore 2006). We suspect that part of this apparent contradiction is due to the varying response of DOC export and concentrations over different time scales, e.g., short-term precipitation events (hours to days) versus melt events (days to weeks) versus annual estimates. Furthermore, numerous studies have reported long-term trends in DOC concentration; however, some studies consider changes in average measured DOC whereas others compute discharge weighted concentrations (Eimers et al. 2008c).
Eimers et al. (2008b) showed that significant increases in average DOC concentration between 1980 and 2001 at six wetland-dominated catchments (Dorset Study Area, Ontario, Canada) were driven by relatively high DOC concentrations in the latter years of record, consistent with low spring flow in these years, and were not translated into greater DOC export to downstream lakes. Such observations are not surprising since export measured downstream is a result of many interacting processes (Roulet and Moore 2006). Changes in stream flow have an important impact on trends in DOC concentration, and extrapolation of trend results from one region to another should be made cautiously and consider methodological and reporting differences among sites.
2.1.6 Droughts
Major observed changes in DOC concentration and flux were associated with major droughts [in two UK river catchments] (Worrall and Burt 2004). Droughts are considered as a possible driver, as DOC production is thought to increase under aerated conditions, and meteorological observations have shown a change in the seasonality of precipitation in terms of drier summers and wetter winters over the last century (Worrall et al. 2004b).
Droughts could amplify DOC production by causing a drop in the water table below the long-term average position (the acrotelm–catotelm boundary, Holden and Burt 2003) triggering additional aerobic production. Evidence of the role of water table draw-down on DOC production is contradictory. Some authors have observed increased DOC concentrations in soil leachates following periods of draw-down (Tipping et al. 1999), while others have observed a reduction (Pastor et al. 2003; Watts et al. 2001; Worrall and Burt 2008) or no change (Blodau and Moore 2003) in DOC concentrations during droughts.
Several explanations have been put forward to explain the anomalous behavior in drought years: (1) lower DOC export because of reduced runoff (Pastor et al. 2003; Dillon and Molot 2005); (2) delayed/inhibited DOC release from soils because physicochemical changes in the peat structure prevent rewetting (Watts et al. 2001); (3) increased consumption of DOC as a substrate by soil microbes causing increased CO2 efflux (Scott et al. 1998; Pastor et al. 2003; Freeman et al. 2004); and (4) reduced decomposition and production of DOC due to microbial inhibition under dry/acidic conditions (Scott et al. 1998).
Clark et al. (2005, 2006) reported that drought-related increases in SO 2−4 concentration at an upland blanket peat bog in the UK were associated with lower than expected DOC concentrations. They argued that declines in pH and/or increases in ionic strength caused by the episodic release of SO 2−4 suppressed DOC solubility in peat solution. Climate-induced drought events have been shown to have a significant influence on SO 2−4 export from forested catchments in central Ontario, subsequently delaying recovery of surface waters from acidification (Aherne et al. 2006) but the associated decreases in DOC export were also strongly correlated with runoff decreases (Dillon and Molot 2005). See Section 2.1.3 for related discussion.
2.1.7 Altered hydrologic pathways
Changed water pathways, due to increased total precipitation and more periods with very intensive rain, have led to more leaching of colored and acidic organic compounds from the upper forest floor. This process has increased the color:DOC ratio and decreased the pH of lake waters (Hongve et al. 2004). Altered DOC in lake water will result in altered bulk properties of lake water including increased color and acidity (e.g., Hongve et al. 2004). In addition, we speculate that this could lead to altered cycling and bioavailability of trace metals, and perhaps altered nutrient (P, Fe, N) bioavailability.
Severe drying of soils can lead to cracking and, thus, the generation of new hydrological pathways. In soils that show hydrophobic behavior upon drying, it is possible that such flowpaths survive beyond the period of the drought that caused the initial cracking. Changes in flowpaths in peat following severe droughts has been proposed as an explanation for long-term increases in DOC concentration in streams draining peat-covered catchments across the northern hemisphere (Worrall and Burt 2008).
2.1.8 Changing land use
Land use changes influence the dynamics of DOC in soils by (1) changing the input of organic matter, (2) changing the substrate quality, and (3) altering the rates, extent, and pathways of microbial degradation and synthesis of organic matter (Cronan et al. 1992; Kalbitz et al. 2000). The soil C stocks decline after land use changes from pasture to tree plantation (i.e., tree farming), native forest to plantation, native forest to crop, and pasture to crop, while the soil C stocks increase after changes from native forest to pasture, crop to pasture, crop to plantation, and crop to secondary forest, the reverse process usually increasing soil carbon (Guo and Grifford 2002). The water quality responses to land use and management practices may be variable (Neal et al. 2005) or exhibit only a short-term impact (Kortelainen and Saukkonen, 1998) that might appear contradictory (Chantigny 2003).
Bellamy et al. (2005) used data from the National Soil Inventory of England and Wales obtained between 1978 and 2003 to show that carbon was lost at a mean rate of 0.6% year−1 from soils across England and Wales over the survey period. Although climate change may be partly responsible (Bellamy et al. 2005), the UK has undergone substantial land use/management changes within the same time period (Dawson and Smith 2007). Worrall et al. (2004a,b) also supposed that DOC changes driven by climate change might be accentuated by land use changes.
Land use changes could alter the relationship between precipitation and carbon export (Raymond and Oh 2007). For example, wetland loss has decreased the export of DOC to lakes and streams in parts of the USA by as much as 20–30% (Raymond et al. 2004).
3 Production and loss of CO2 from boreal lakes
Thus far, we have reviewed how climate change may affect DOC concentrations and export, but the converse is also possible: DOC cycling may impact climate change since one of the sinks for aquatic DOC is its degradation to CO2 and CH4. If the balance between DOC losses to the atmosphere versus transfer to the sediments of lakes is altered, greenhouse gas production and release to the atmosphere can increase or decrease, affecting the severity of climate change. In addition, if lake water levels fall, previously protected organic sediments may be exposed to greater aeration which would lead to greater CO2 evasion to the atmosphere (Mortsch and Quinn 1996; Kling et al. 2003; Benoy et al. 2007), but perhaps less CH4 evasion if the sediments were initially anoxic. Since CH4 is a more powerful greenhouse gas than CO2, the net effect may be further temperature increases, mitigated temperature increases, or no effect at all.
It has been shown that increased precipitation is also linked to increased loss of CO2 from large boreal lakes (Rantakari and Kortelainen 2005) which may have been due to an increase in its allochthonous DOC loading or an increase in photochemical oxidation and biological respiration rates. The link between increased precipitation and loss of CO2 from boreal lakes is not universal, and may be related to other factors such as lake size. Smaller lakes typically have higher concentrations of and emit more CO2, on a unit areas basis, than larger lakes (Kortelainen et al. 2006). Due to their smaller size, these lakes are able to respond more quickly to external changes and thus are probably more sensitive to climatic changes in the short term. However, Kelly et al. (2001), studying boreal lakes in northwest Ontario, smaller than the ones used by Rantakari and Kortelainen (2005), did not find a relationship between CO2 evasion and precipitation. Instead, they found a correlation with regional weather patterns.
Loss of DOC from catchments via gas emissions from lakes is estimated to be relatively important. Dillon and Molot (1997a) estimated that 5% of net ecosystem production is returned to the atmosphere by way of emissions from lakes in central Ontario. Based on a study of Finnish lakes, Huttunen et al. (2003) concluded that carbon release from boreal lakes in general may be an important part of the overall carbon balance of the catchments because lakes cover 7–10% of the total area of Finland and Canada.
Lakes in general tend to be super-saturated with CO2, releasing CO2 to the atmosphere (Huttunen et al. 2003). In the case of Finnish lakes, Kortelainen et al. (2006) found that the super-saturation of CO2 was, in part, due to respiration of organic matter in the sediment. They further found a strong correlation between CO2 super-saturation and O2 under-saturation, but no correlation between CO2 super-saturation and lake TOC concentration nor the proportion of catchment covered by wetland. These findings suggest that degradation of allochthonous DOC was not a major contributor to CO2 super-saturation. These lakes were also sources of CH4 during the ice-free season. In contrast, while O2 under-saturation was correlated with CO2 super-saturation in 33 Quebec lakes, the degree of O2 under-saturation and net carbon flux to the atmosphere were positively correlated with DOC concentration (Prairie et al. 2002). Thus, at least in these Quebec lakes, lower DOC export would lead to a lower carbon flux to the atmosphere.
Using a global chemistry-transport model, Sanderson et al. (2006) showed that climate change will result in an increased amount of nitric acid produced and deposited to soils, acidifying them, resulting in potentially more acidified and eutrophic lakes and, as a consequence, greater CO2 evasion from these. In Section 2.1.2, we saw how continuing N deposition may be contributing to increased DOC production in soils and possibly in increased export to streams and lakes, resulting in increased evasion from these.
4 Interaction of DOC with metals and nutrients
The above sections explored possible mechanisms influencing the export and fate of DOC in aquatic ecosystems. The potential impact of these mechanisms on DOC interactions with trace metals and nutrients is as complex as the chemistry and properties of DOC itself.
The interaction of trace metals with DOC may be significantly altered by climate change. Copper, which is most toxic in its free Cu2+ form, is less bioavailable when it is bound to DOC. Should climate change result in altered DOC chemistry (e.g., significantly fewer and/or weaker binding sites for Cu), the result could be more copper being present in its toxic and bioavailable Cu2+ form, assuming constant Cu loading. Presumably, this applies to other toxic trace metals exhibiting similar behavior with DOC. Under the scenario of decreased DOC export to lakes (Schindler et al. 1997; Dillon and Molot 2005) as well as increased photo-oxidation (due to increased UV light) of DOC, the toxicity of trace metals may increase. Brooks et al. (2007) have shown that photo-oxidation of river DOC decreases Cu–DOC complexes in most cases used in their study. The work by Shiller et al. (2006) also indicated that organically complexed metals such as Cu and Fe are released during photo-oxidation of DOC, while Kopáček et al. (2003, 2005) found it to be the case that Al and Fe were preferentially released. Unfortunately, there are no data on Cu export; however, similarly to Fe, we would expect changes in Cu export to be linked to changes in runoff and DOC export.
To the authors’ knowledge, there are few studies examining how the presence of trace metals affects the chemistry of aquatic DOC. Such knowledge would allow for a better understanding of other climate change-dependent effects. Recent work by Kelton (2006) has demonstrated that iron enhances the formation of POC during irradiation of lake water with UV light and therefore may be an important pathway for transfer of allochthonous DOC to sediments. She hypothesized a physical process that excludes any conclusions regarding the chemical alteration of DOC other than removal of adsorbable fractions of DOC by settling amorphous Fe; this process has important implications for the fate of DOC in lakes. Kopáček et al. (2006) showed that not only Fe but also Al can significantly contribute to the sedimentation of POC. Increased sedimentation of DOC (in the form of POC) implies less DOC being lost from these lakes as CO2. If there is a reduced Fe input to non-acid lakes, then reduced POC formation may result in relatively more DOC being evaded as CO2 from these lakes or discharged downstream and a decrease in the relative amount of DOC transferred from the water column as POC to the sediment. Decreases in Fe export that were larger than decreases in DOC export under dry conditions in central Ontario (Dillon and Molot 2005) could have led to relatively lower rates of POC formation in lakes. Such activity could increase the importance of these lakes in the global C cycle as a source of CO2, although the effects of decreased runoff on a regional scale of CO2 fluxes must be considered.
Not only does Fe affect DOC but there is also a reverse effect whereby DOC affects the fate of Fe and, in turn, other nutrients. The rate of loss of Fe from lake water is strongly and negatively associated with DOC concentration (Molot and Dillon 2003) suggesting that DOC retards the formation of Fe colloids, which precipitate out of the water column to the sediment. Since Fe colloids have a strong affinity for P, DOC indirectly affects loss of P from the water column: decreased DOC concentrations in lakes would result in increased Fe colloid formation and co-incident loss of adsorbable P from the water column. Kopáček et al. (2003, 2005) found that Al and Fe were preferentially released during photo-oxidation of DOC, which is consistent with the findings of Molot and Dillon (2003). They also suggested that the Al and Fe hydroxides formed may bind PO 3−4 resulting in its decreased availability. The Al and Fe hydroxides would then precipitate to the sediment, lowering the P concentration in lake water. A competing internal mechanism was reported by Komatsu et al. (2007) who predicted that increased lake water temperatures (and a longer ice-free season) will result in increased P in the water column from increased internal loading because of greater anoxia, thus leading to increased trophic conditions. However, the formation of anoxic areas in oligotrophic lakes (the vast majority of boreal lakes are oligotrophic) is restricted to lakes that are small and sheltered.
Dillon and Evans (2001) found that there was a decrease in Fe retention (on a mass basis) over a 14-year period (1978–1992) in soft-water lakes in central Ontario, located on the Canadian Shield. It is believed that processes controlling Fe retention in these lakes probably remained unchanged during the study period, and that the lower mass retention in lakes was due to lower Fe export from their catchments under drier conditions, likely due to more oxidizing conditions in the surface of peatlands (Dillon and Molot 2005). The implications of lower Fe in boreal lakes may be reduced formation of POC, and perhaps greater DIC formation and subsequent evasion. In other words, the fate of DOC in these lakes may be altered.
Reduced DOC and Fe inputs to lake water as described by Dillon and Molot (2005) may also limit the burial of sulfate, as FeS, in lake sediments, thereby contributing acidity to the water by increasing the formation of H2S. Increased acidity enhances the photochemical degradation of DOC (Dillon and Molot 1997b; Gennings et al. 2001; Molot et al. 2005), further decreasing its concentration, but also results in the release of organically bound metals such as Al and Fe (Kopáček et al. 2006), organically bound nutrients such as P, N, (Wang et al. 2000; Tarr et al. 2001; Vähätalo et al. 2003), and S. Regardless of the limitation of the burial of Fe as FeS, sediments of boreal lakes likely have large stores of Fe (Kortelainen et al. 2004) due to co-sedimentation of humic substances with iron oxide particles (Tipping and Woof 1983).
N and P are constituents of DOC which explains in large part why N and P export are correlated with DOC export (Dillon and Molot 1997a, 2005). Dillon and Molot (2005) speculated that permanently drier conditions with less runoff would likely lead to clearer lakes that are less productive because of reduced nutrient and DOC export. Conversely, increased runoff would lead to more colored and productive lakes.
Photochemical processes play a role in allochthonous nutrient cycling: inorganic P, nitrate plus nitrite, ammonia and amino acids are released from DOC by photo-oxidation (Wang et al. 2000; Tarr et al. 2001; Vähätalo et al. 2003) which results in increased phytoplankton production.
Increased trophic conditions (due to increased P and N) also yields increased decomposition of easily degradable organic matter, increased O2 consumption both in the water column and sediments, and increased CO2 evasion (Huttunen et al. 2003). Huttunen et al. (2003) have shown that CO2 concentrations in Finnish lakes correlate positively with lake P and N concentrations. Kortelainen et al. (2006) found that CO2 evasion from boreal lakes is related to the trophic status of the lakes. The consumption of O2 during mineralization of DOC may result in anaerobic conditions in the sediments and increased CH4 emission from these (Huttunen et al. 2003). Huttunen et al. (2003) also found that while all Finnish lakes and reservoirs in their study set were net evaders of CO2, CO2 fluxes were higher from lakes and reservoirs with peatlands or managed forests in their catchment area and from eutrophic lakes.
5 Impact of changing DOC on lake water chemistry
Any change in DOC concentrations and properties in lakes and streams will also impact the acid–base chemistry of these waters and, presumably, the chemical and photochemical reactions taking place and the production of CO2. Hongve et al. (2004) observed an increased color: DOC ratio and a decreased pH of lake waters due to altered hydrologic pathways resulting from climate change. They speculate that the different color of the water is an indication of changing DOC quality. Molot et al. (2005) also noted that pH affects photochemical DOC loss and photobleaching rates differently, hence climate mediated changes in lake pH are likely to affect water clarity even where DOC concentration remains unchanged.
Bertilsson and Tranvik (2000) suggest that although there is a large variability in water chemistry from lake to lake, photoproducts such as DIC and low molecular weight acids are universally produced in lake water exposed to sunlight. They further speculate that the variability in DIC production rates in lakes (after normalizing for absorbed radiation energy) could partly be attributed to general water chemistry parameters such as pH, iron concentration, and conductivity. There is no evidence currently available to indicate whether end products other than the amount of DIC and the type of low molecular weight acids produced will differ as a result of climate change, but we can speculate that the rate at which DOC is degraded will be altered, because DOC degradation rates are temperature-dependent (Cabaniss et al. 2005). A comprehensive review of the interactive effects of ozone depletion and climate change on biogeochemical cycles was presented by Zepp et al. (2003).
6 Future climate trends and their effect on DOC cycling
While climate change models predict that higher temperatures are likely to occur throughout most of the boreal forest regions in North America, Europe and Asia over the next century, model predictions of regional changes in precipitation, evapotranspiration and, hence, runoff are much more uncertain (see review by Lavoie et al. 2005). Models also suggest that climate change is unlikely to be uniform spatially. Some regions may become wetter while others become drier. Climate change is also expected to change the severity and frequency of storm and drought events, change seasonal runoff patterns, and increase the length of the ice-free season. Rates of change in DOC export and concentrations will thus vary regionally and the changes may be non-linear (e.g., ICP Waters Report 87/2007; Eimers et al. 2008a). The effects of changing temperature and precipitation will be compounded by continued elevated N and decreasing S deposition.
Climate change models predict that higher temperatures are likely to occur over most of the boreal forest regions in North America, Europe and Asia over the next century. While it is difficult to predict regional climate trends and, thus, future regional trends in DOC export and concentrations, two general climate scenarios emerge with which to examine possible DOC trends: warmer and wetter and warmer and drier. We also need to consider a scenario with warmer temperatures but no change in runoff. Whatever the future brings, increasing temperature and hydrological changes are likely to lead to changes in DOC export and DOC processing rates in lakes which will alter concentrations of DOC and its constituents.
6.1 Warmer and constant runoff scenario
A scenario with a warmer climate with runoff remaining constant may lead to further increases in DOC concentrations, with complex consequences for surface waters including increased organic acidity, increased buffering of changes in pH, increased water coloration, and decreased visible light and UV-B penetration within the water column (Skjelkvåle et al. 2005). Alternatively, this scenario may not lead to further increases in DOC concentrations, depending on the net difference between soil production and respiration rates, especially in wetlands (see section 2.1.4). Increasing DOC concentrations in boreal soil water would have a significant negative impact on soil recovery from acidification, with elevated organic acidity lowering soil water pH by severely depleting soil base saturation (Evans 2005).
6.2 Warmer and wetter scenario
Several studies have shown that increased precipitation and runoff lead to increased DOC export and concentrations regardless of temperature changes (Worrall and Burt 2004; Dillon and Molot 2005). A warmer and wetter climate and increased concentrations of atmospheric CO2 can increase primary productivity (Freeman et al. 2004), leading to more plant material in the soil being available for microbial production of DOC. Increased leaching may lead to higher surface water DOC concentrations (Hongve et al. 2004).
6.3 Warmer and drier scenario
On the other hand, if temperatures increase in conjunction with decreased runoff then increased DOC export and concentrations may not materialize and may, in fact, decrease (Schindler et al. 1997; Dillon and Molot 2005). Warmer and drier conditions generally resulted in decreased inputs of silica, DOC, base cations, and phosphorus from streams to the lakes in the Experimental Lake Area (Schindler et al. 1996).
7 Conclusions
There are several potential mechanisms responsible for recent changes in aquatic DOC concentrations, including increasing atmospheric CO2 concentration, climate warming, continued N deposition, decreased sulfate deposition, and hydrological changes.
Climate change appears to be a significant driver of observed changes in aquatic DOC concentrations. Climate change models predict that higher temperatures are likely to occur over most of the boreal forests in North America, Europe and Asia over the next century. Two general climate scenarios emerge, namely warmer and wetter or warmer and drier. Climate change expressed via changes in runoff and temperature will likely result in changes in aquatic DOC concentrations with concomitant effects on trace metals and nutrients.
Changes in the quality and concentration of aquatic DOC have implications for lake acid–base chemistry and for the speciation and bioavailability of certain trace metals and nutrients. The interaction of trace metals with DOC may be significantly altered by climate change as organically complexed metals such as Cu, Fe, and Al are released during photo-oxidation of DOC. If climate change results in altered DOC chemistry (e.g., fewer and/or weaker binding sites), more trace metals could be present in their toxic and bioavailable forms.
Moreover, changes in DOC, metals and nutrients are likely to drive changes in rates of C evasion and storage in lake sediments, as the partitioning of DOC to POC (goes to sediment) and DIC (evaded as CO2) is altered. However, the key controls (runoff, temperature, soil chemistry) on allochthonous DOC quality and catchment export are still not fully understood. Furthermore, the partitioning of DOC between lake sediments and the atmosphere is a function of pH.
Predicted future climate scenarios include a warmer and drier scenario where, if lake water levels fall, previously stored organic sediments may be exposed to greater aeration, which would lead to greater CO2 evasion to the atmosphere. Under a warmer and wetter scenario, long-term DOC trends seem contradictory, as increased rainfall could increase export but cause an overall decrease in DOC concentrations from peatland streams to lakes. Alternatively, increased concentrations can occur due to changing DOC production and retention, but with no change in hydrology. Part of this apparent contradiction is due to the varying response of DOC export and concentrations over different time scales as well as how DOC changes are measured, whether changes in average measured DOC or discharge weighted DOC concentrations.
Contradictory findings reported herein suggest that descriptive (e.g., correlative) studies have their limitations and that detailed modeling studies that integrate key controls are needed to allow testing of various scenarios. Similarly, more studies are also needed to explore how runoff and temperature-related changes in DOC export affect metal and nutrient export to rivers and lakes. Models are typically developed using results from small areas, hence, attention needs to be paid to scaling up the models to much larger regional scales.
References
Aherne J, Larssen T, Cosby BJ, Dillon PJ (2006) Climate variability and forecasting surface water recovery from acidification: modelling drought-induced sulphate release from wetlands. Sci Total Environ 365:186–199
Anesio AM, Granéli W (2004) Photochemical mineralization of dissolved organic carbon in lakes of differing pH and humic content. Arch Hydrobiol 160:105–116
Bellamy PH, Loveland PJ, Bradley RI, Lark RM, Kirk GJD (2005) Carbon losses from all soils across England and Wales 1978–2003. Nature 437:245–248
Benoy G, Cash K, McCauley E, Wrona F (2007) Carbon dynamics in lakes of the boreal forest under a changing climate. Environ Rev 15:175–189
Bertilsson S, Tranvik LJ (2000) Photochemical transformation of dissolved organic matter in lakes. Limnol Oceanogr 45(4):753–762
Blodau C, Moore TR (2003) Experimental response of peatland carbon dynamics to a water table fluctuation. Aquat Sci 65:47–62
Brooks ML, McKnight DM, Clements WH (2007) Photochemical control of copper complexation by dissolved organic matter in Rocky Mountain streams, Colorado. Limnol Oceanogr 52:766–779
Burns DA, McHale MR, Driscoll CT, Roy KM (2006) Response of surface water chemistry to reduced levels of acid precipitation: comparison of trends in two regions of New York, USA. Hydrol Processes 20:1611–1627
Cabaniss SE, Madey G, Leff L, Maurice PA, Wetzel R (2005) A stochastic model for the synthesis and degradation of natural organic matter. Part I. Data structures and reaction kinetics. Biogeochemistry 76:319–347
Chantigny MH (2003) Dissolved and water-extractable organic matter in soils: a review on the influence of land use and management practices. Geoderma 113:357–380
Christ MJ, David MB (1996) Temperature and moisture effects on the production of dissolved organic carbon in a spodosol. Soil Biol Biochem 28:1171–1179
Clark JM, Chapman PJ, Adamson JK, Lane SN (2005) Influence of drought-induced acidification on the mobility of dissolved organic carbon in peat soils. Global Change Biol 11:791–809
Clark JM, Chapman PJ, Heathwaite AL, Adamson JK (2006) Suppression of dissolved organic carbon by sulfate induced acidification during simulated droughts. Environ Sci Technol 40:1776–1783
Clark JM, Lane SN, Chapman PJ, Adamson JK (2007) Export of dissolved organic carbon from an upland peatland during storm events: implications for flux estimates. J Hydrol 347:438–447
Cronan CS, Lakshman S, Patterson HH (1992) Effects of disturbance and soil amendments on dissolved organic carbon and organic acidity in red pine forest floors. J Environ Qual 21:457–463
Currie WS, Aber JD, McDowell WH, Boone RD, Magill AH (1996) Vertical transport of dissolved organic C and N under long-term N amendments in pine and hardwood forests. Biogeochemistry 35:471–506
Dalva M, Moore TR (1991) Sources and sinks of dissolved organic carbon in a forested swamp catchment. Biogeochemistry 15:1–19
Dawson JJC, Smith P (2007) Carbon losses from soil and its consequences for land-use management. Sci Total Environ 382:165–190
Dillon PJ, Molot LA (1997a) The effect of landscape form on the export of dissolved substances from forested stream catchments. Water Resour Res 33:2591–2600
Dillon PJ, Molot LA (1997b) Dissolved organic and inorganic carbon mass balances in central Ontario lakes. Biogeochemistry 36:29–42
Dillon PJ, Evans HE (2001) Comparison of iron accumulation in lakes using sediment core and mass balance calculations. Sci Total Environ 266:211–219
Dillon PJ, Molot LA (2005) Long-term trends in catchment export and lake retention of dissolved organic carbon, dissolved organic nitrogen, total iron and total phosphorus: The Dorset, Ontario study, 1978–1998. J Geophys Res—Biogeosciences 110, No.G01002, doi:1029/2004JG000003
Eimers MC, Buttle JM, Watmough SA (2008a) Influence of seasonal changes in runoff and extreme events on DOC trends in wetland and upland-draining streams. Can J Fish Aquat Sci 65:796–808
Eimers MC, Watmouth SA, Buttle JM, Dillon PJ (2008b) Examination of the potential relationship between droughts, sulphate and dissolved organic carbon at a wetland-draining stream. Global Change Biol 14:938–948
Eimers MC, Watmough SA, Buttle JM (2008c) Long-term trends in dissolved organic carbon concentration: a cautionary note. Biogeochemistry 87:71–81
Evans CD (2005) Modelling the effects of climate change on an acidic upland stream. Biogeochemistry 74:21–46
Evans CD, Monteith DT, Cooper DM (2005) Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environ Pollut 137:55–71
Evans CD, Chapman PJ, Clark JM, Monteith DT, Cresser MS (2006) Alternative explanations for rising dissolved organic carbon export from organic soils. Global Change Biol 12:2044–2053
Fenner N, Freeman C, Lock MA, Harmens H, Reynolds B, Sparks T (2007a) Interactions between elevated CO2 and warming could amplify DOC Exports from Peatland Catchments. Environ Sci Tech 41:3146–3152
Fenner N, Ostle NJ, McNamara N, Sparks T, Harmens H, Reynolds B, Freeman C (2007b) Elevated CO2 effects on peatland plant community carbon dynamics and DOC production. Ecosystems 10:635–647
Findlay SEG (2005) Increased carbon transport in the Hudson River: unexpected consequence of nitrogen deposition? Front Ecol Environ 3:133–137
Freeman C, Ostle N, Kang H (2001a) An enzymatic ‘latch’ on a global carbon store. Nature 409:149
Freeman C, Evans CD, Monteith DT, Reynolds B, Fenner N (2001b) Export of organic carbon from peat soils. Nature 412:785
Freeman C, Fenner N, Ostle NJ, Kang H, Dowrick DJ, Reynolds B, Lock MA, Sleep D, Hughes S, Hudson J (2004) Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 430:195–198
Galloway JN, Howarth RW, Michaels AF, Nixon SW, Prospero JM, Dentener FJ (1996) Nitrogen and phosphorus budgets of the North Atlantic Ocean and its watershed. Biogeochemistry 35:3–25
Gennings C, Molot LA, Dillon PJ (2001) Enhanced photochemical loss of DOC in acidic waters. Biogeochemistry 52:339–354
Guo LB, Grifford RM (2002) Soil carbon stocks and land use change: a meta analysis. Global Change Biol 8:345–360
Hejzlar J, Dubrovský M, Buchtele J, Růžička M (2003) The apparent and potential effects of climate change on the inferred concentration of dissolved organic matter in a temperate stream (the Malse River, south Bohemia). Sci Total Environ 310:143–152
Holden J, Burt TP (2003) Hydrological studies on blanket peat: the significance of the acrotelm–catotelm model. J Ecol 91(1):86–102
Holden J, Shotbolt L, Bonn A, Burt TP, Chapman PJ, Dougill AJ, Fraser EDG, Hubacek K, Irvine B, Kirkby MJ, Reed MS, Prell C, Stagl S, Stringer LC, Turner A, Worrall F (2007) Environmental change in moorland landscapes. Earth-Sci Rev 82:75–100
Holland EA, Braswell BH, Sulzman J, Lamarque J-F (2005) Nitrogen deposition onto the United States and Western Europe: synthesis of observations and models. Ecol Appl 15:38–57
Hongve D, Riise G, Kristiansen JF (2004) Increased colour and organic acid concentrations in Norwegian forest lakes and drinking water—a result of increased precipitation? Aquat Sci 66:231–238
Howarth RW, Billen G, Swaney D, Townsend A, Jaworski N, Lajtha K, Downing JA, Elmgren R, Caraco N, Jordan T, Berendse F, Freney J, Kudeyarov V, Murdoch P, Zhao-Liang Z (1996) Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry 35:75–139
Huttunen JT, Alm J, Liikanen A, Juutinen S, Larmola T, Hammar T, Silvola J, Martikainen PJ (2003) Fluxes of methane, carbon dioxide and nitrous oxide in boreal lakes and potential anthropogenic effects on the aquatic greenhouse gas emissions. Chemosphere 52:609–621
ICP Waters Report 87/2007. International Cooperative Programme on Assessment and Monitoring of Acidification of Rivers and Lakes: Trends in Surface Water, Chemistry, and Biota. Norwegian Institute for Water Research
IPCC (2001) Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (eds. Houghton JT, Ding Y, Griggs DJ, et al.):, http://www.grida.no/climate/ipcc_tar/wg1/index.htm
Kalbitz K, Solinger S, Park J-H, Michalzik B, Matzner E (2000) Controls on the dynamics of organic matter in soils: a review. Soil Sci 165:277–304
Kang H, Freeman C, Ashendon TW (2001) Effects of elevated CO2 on fen peat biogeochemistry. Sci Total Environ 279:45–50
Kankaala P, Bergström I (2004) Emission and oxidation of ethane in Equisetum fluviatile stands growing on organic sediment and sand bottoms. Biogeochemistry 67:21–37
Kelly CA, Fee E, Ramlal PS, Rudd JWM, Hesslein RH, Anema C, Schindler EU (2001) Natural variability of carbon dioxide and net epilimnetic production in the surface waters of boreal lakes of different sizes. Limnol Oceanogr 46:1054–1064
Kelton N (2006) Effects of UV–visible radiation on DOC–Fe interaction in streams. PhD dissertation, York University, Toronto, Canada
Kelton N, Molot LA, Dillon PJ (2007) Effect of ultraviolet and visible radiation on iron lability in boreal and artificial waters. Aquat Sci 69:86–95
Kling GW, Hayhoe K, Johnson LB, Magnuson JJ, Polasky P, Robinson SK, Shuter BJ, Wander MM, Wuebbles DJ, Zak DR, Lindroth RL, Moser SC, Wilson ML (2003) Confronting Climate Change in the Great Lakes Region: Impacts on our Communities and Ecosystems. Union of Concerned Scientists, Cambridge MA, and Ecological Society of America, Washington, DC
Komatsu E, Fukushima T, Harasawa H (2007) A modeling approach to forecast the effect of long-term climate change on lake water quality. Ecol Model 209:351–366
Kopáček J, Hejzlar J, Kaňa J, Porcal P, Klementová Š (2003) Photochemical, chemical, and biological transformations of dissolved organic carbon and its effect on alkalinity production in acidified lakes. Limnol Oceanogr 48(1):106–117
Kopáček J, Klementová Š, Norton SA (2005) Photochemical production of ionic and particulate aluminum and iron in lakes. Environ Sci Technol 39:3656–3662
Kopáček J, Marešová M, Norton SA, Porcal P, Veselý J (2006) Photochemical source of metals for sediments. Environ Sci Technol 40:4455–4459
Kortelainen P, Saukkonen S (1998) Leaching of nutrients, organic carbon and iron from Finnish forestry land. Water Air Soil Poll 105:239–250
Kortelainen P, Pajunen H, Rantakari M, Saarnisto M (2004) A large carbon pool and small sink in boreal Holocene lake sediments. Glob Change Biol 10:1648–1653
Kortelainen P, Rantakari M, Huttunen JT, Mattsson T, Alm J, Juutinen S, Larmola T, Silvola J, Martikainen PJ (2006) Sediment respiration and lake trophic state are important predictors of large CO2 evasion from small boreal lakes. Global Change Biol 12:1554–1567
Krug EC, Frink CR (1983) Acid rain and acid soil: a new perspective. Science 221:520–525
Kuzyakov Y (2002) Review: Factors affecting rhizosphere priming effects. J Plant Nut Soil Sci 165:382–396
Lavoie M, Paré D, Bergeron Y (2005) Impact of global change and forest management on carbon sequestration in northern forested peatlands. Environ Rev 13:199–240
McDowell WH, Currie WS, Aber JD, Yano Y (1998) Effects of chronic nitrogen amendments on production of dissolved organic carbon and nitrogen in forest soils. Water Air Soil Poll 105:17–182
Meyer JL, Pulliam WM (1992) Modification of terrestrial–aquatic interactions by a changing climate. In: Firth P, Fisher SG (eds) Global climate change and freshwater ecosystems. Springer, New York, pp 177–191
Molot LA, Dillon PJ (2003) Variation in iron, aluminum and dissolved organic carbon mass transfer coefficients in lakes. Water Res 37:1759–1768
Molot LA, Hudson JJ, Dillon PJ, Miller SA (2005) Effect of pH on photo-oxidation of dissolved organic carbon by hydroxyl radicals in a coloured, softwater stream. Aquat Sci 67:189–195
Monteith DT, Stoddard JL, Evans CD, de Wit H, Forsius M, Høåsen T, Wilander A, Skjelkvåle BL, Jeffries DS, Vuorenmaa J, Keller B, Kopáček J, Veselý J (2007) Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450:537–541
Mortsch LD, Quinn FH (1996) Climate change scenarios for Great Lakes Basin ecosystem studies. Limnol Oceanogr 41:903–911
Neff JC, Asner GP (2001) Dissolved organic carbon in terrestrial ecosystems: synthesis and a model. Ecosystems 4:29–48
Neal C, Robson AJ, Neal M, Reynolds B (2005) Dissolved organic carbon for upland acidic and acid sensitive catchments in mid-Wales. J Hydrol 304:203–230
Pastor J, Solin J, Bridgham SD, Updegraff K, Harth C, Weishampel P, Dewey B (2003) Global warming and the export of dissolved organic carbon from boreal peatlands. Oikos 100:380–386
Prairie YT, Bird DF, Cole JJ (2002) The summer metabolic balance in the epilimnion of southeastern Quebec lakes. Limnol Oceanogr 47:316–321
Pregitzer K, Zak DR, Burton AJ, Ashby JA, MacDonald NW (2004) Chronic nitrate additions dramatically increase the export of carbon and nitrogen from northern hardwood ecosystems. Biogeochemistry 68:179–197
Prospero JM, Barrett K, Church T, Dentener F, Duce RA, Galloway JN, Levy H, Moody J, Quinn P (1996) Atmospheric deposition of nutrients to the North Atlantic Basin. Biogeochemistry 35:27–73
Rantakari M, Kortelainen P (2005) Interannual variation and climatic regulation of the CO2 emission from large boreal lakes. Global Change Biol 11:1368–1380
Raymond PA, Oh NH (2007) An empirical study of climatic controls on riverine C export from three major U.S. watersheds. Global Biogeochem Cy 21:GB2022
Raymond PA, Bauer JE, Caraco NF, Cole JJ, Longworth B, Petsch ST (2004) Controls on the variability of organic matter and dissolved inorganic carbon ages in northeast US rivers. Mar Chem 92:353–366
Rey A, Petsikos C, Jarvis PG, Grace J (2005) Effect of temperature and moisture on rates of carbon mineralization in a Mediterranean oak forest soil under controlled and field conditions. Eur J Soil Sci 56:589–599
Roulet N, Moore TR (2006) Browning the waters. Nature 444:283–284
Sanderson MG, Collins WJ, Johnson CE, Derwent RG (2006) Present and future acid deposition to ecosystems: the effect of climate change. Atmos Environ 40:1275–1283
Schiff SL, Aravena R, Trumbore SE, Hinton MJ, Elgood R, Dillon PJ (1997) Export of DOC from forested catchments on the Precambrian Shield of Central Ontario: clues from C-13 and C-14. Biogeochemistry 36:43–65
Scott MJ, Jones MN, Woof C, Tipping E (1998) Concentrations and fluxes of dissolved organic carbon in drainage water from an upland peat system. Environ Int 24:537–546
Schindler DW, Bayley SE, Parker BA, Beaty KG, Cruikshank DR, Fee EJ, Shindler EU, Stainton MP (1996) The effects of climatic warming on the properties of boreal lakes and streams at the Experimental Lakes Area, northwestern Ontario. Limnol Oceanogr 4l(5):1004–1017
Schindler DW, Curtis PJ, Bayley SE, Parker BR, Beaty KG, Stainton MP (1997) Climate-induced changes in the dissolved organic carbon budgets of boreal lakes. Biogeochemistry 36:9–28
Shiller AM, Duan S, van Erp P, Bianchi TS (2006) Photo-oxidation of dissolved organic matter in river water and its effect on trace element speciation. Limnol Oceanogr 51:1716–1728
Skjelkvåle BL, Stoddard JL, Jeffries DS, Tørseth K, Hogasen T, Bowman J, Mannio J, Monteith DT, Mosello R, Rogora M, Rzychon D, Veselý J, Wieting J, Wilander A, Worsztynowicz A (2005) Regional scale evidence for improvements in surface water chemistry 1990–2001. Environ Pollut 137:165–176
Steinberg CEW, Meinelt T, Timofeyev MA, Bittner M, Menzel R (2008) Humic Substances (review series): Part 2: interactions with organisms. Environ Sci Pollut Res 15(2):128–135
Stumm W, Morgan JJ (1996) Aquatic chemistry, chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York 1022 p
Tarr MA, Wang W, Bianchi T, Engelhaupt E (2001) Mechanisms of ammonia and amino acid photoproduction from aquatic humic acid and colloidal matter. Water Res 35:3688–3696
Tipping E, Woof C (1983) Elevated concentrations of humic substances in a seasonally anoxic hypolimnion: evidence for co-accumulation with iron. Arch Hydrobiol 98:137–145
Tipping E, Woof C, Rigg E, Harrison AF, Ineson P, Taylor K, Benham D, Poskitt J, Rowland AP, Bol R, Harkness DD (1999) Climatic influences on the leaching of dissolved organic matter from upland UK Moorland soils, investigated by a field manipulation experiment. Environ Int 25:83–95
Tranvik LJ, Jansson M (2001) Terrestrial export of organic carbon. Nature 415:861–862
Vähätalo AV, Salonen K, Münster U, Järvinen M, Wetzel RG (2003) Photochemical transformation of allochthonous organic matter provides bioavailable nutrients in a humic lake. Arch Hydrobiol 156:287–314
Vuorenmaa J, Forsius M, Mannio J (2006) Increasing trends of total organic carbon concentrations in small forest lakes in Finland from 1987 to 2003. Sci Total Environ 365:47–65
Wang W, Tarr MA, Bianchi TS, Engelhaupt E (2000) Ammonium photoproduction from aquatic humic and colloidal matter. Aquat Geochem 6:275–292
Watts CD, Naden PS, Machell J, Banks J (2001) Long-term variation in water colour from Yorkshire catchments. Sci Total Environ 278:57–72
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic, New York
Wolf AA, Drake BG, Erickson JE, Megonigal JP (2007) An oxygen-mediated positive feedback between elevated carbon dioxide and soil organic matter decomposition in a simulated anaerobic wetland. Global Change Biol 13:2036–2044
Worrall F, Burt T (2004) Time series analysis of long-term river dissolved organic carbon records. Hydrol Processes 18:893–911
Worrall F, Burt T (2008) The effect of severe drought on the dissolved organic carbon (DOC) concentration and flux from British rivers. J Hydrol 361:262–274
Worrall F, Harriman R, Evans CD, Watts CD, Adamson J, Neal C, Tipping E, Burt T, Grieve I, Monteith D, Naden PS, Nisbet T, Reynolds B, Stevens P (2004a) Review of riverine DOC trends in the UK. Biogeochemistry 70:369–402
Worrall F, Burt T, Adamson J (2004b) Can climate change explain increases in DOC flux from upland peat catchments? Sci Total Environ 326:95–112
Xenopoulos MA, Lodge DM, Frentress J, Kreps TA, Bridham SD, Grossman E, Jackson CJ (2003) Regional comparisons of watershed determinants of dissolved organic carbon in temperate lakes from the Upper Great Lakes region and selected regions globally. Limnol Oceanogr 48:2321–2334
Yano Y, McDowell WH, Aber JD (2000) Biodegradable dissolved organic carbon in forest soil solution and effects of chronic nitrogen deposition. Soil Biol Biochem 32:1743–1751
Zepp RG, Callaghan TV, Erickson DJ III (2003) Interactive effects of ozone depletion and climate change on biogeochemical cycles. Photochem Photobiol Sci 2:51–61
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Christian Steinberg
Rights and permissions
About this article
Cite this article
Porcal, P., Koprivnjak, JF., Molot, L.A. et al. Humic substances—part 7: the biogeochemistry of dissolved organic carbon and its interactions with climate change. Environ Sci Pollut Res 16, 714–726 (2009). https://doi.org/10.1007/s11356-009-0176-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-009-0176-7