Abstract
Background, aim, and scope
Along transects under a mixed woodland of English Oak (Quercus robur) and Common Ash (Fraxinus excelsior) growing on a trichloroethylene (TCE)-contaminated groundwater plume, sharp decreases in TCE concentrations were observed, while transects outside the planted area did not show this remarkable decrease. This suggested a possibly active role of the trees and their associated bacteria in the remediation process. Therefore, the cultivable bacterial communities associated with both tree species growing on this TCE-contaminated groundwater plume were investigated in order to assess the possibilities and practical aspects of using these common native tree species and their associated bacteria for phytoremediation. In this study, only the cultivable bacteria were characterized because the final aim was to isolate TCE-degrading, heavy metal resistant bacteria that might be used as traceable inocula to enhance bioremediation.
Materials and methods
Cultivable bacteria isolated from bulk soil, rhizosphere, root, stem, and leaf were genotypically characterized by amplified rDNA restriction analysis (ARDRA) of their 16S rRNA gene and identified by 16S rRNA gene sequencing. Bacteria that displayed distinct ARDRA patterns were screened for heavy metal resistance, as well as TCE tolerance and degradation, as preparation for possible future in situ inoculation experiments. Furthermore, in situ evapotranspiration measurements were performed to investigate if the degradation capacity of the associated bacteria is enough to prevent TCE evapotranspiration to the air.
Results and discussion
Between both tree species, the associated populations of cultivable bacteria clearly differed in composition. In English Oak, more species-specific, most likely obligate endophytes were found. The majority of the isolated bacteria showed increased tolerance to TCE, and TCE degradation capacity was observed in some of the strains. However, in situ evapotranspiration measurements revealed that a significant amount of TCE and its metabolites was evaporating through the leaves to the atmosphere.
Conclusions and perspectives
The characterization of the isolates obtained in this study shows that the bacterial community associated with Oak and Ash on a TCE-contaminated site, was strongly enriched with TCE-tolerant strains. However, this was not sufficient to degrade all TCE before it reaches the leaves. A possible strategy to overcome this evapotranspiration to the atmosphere is to enrich the plant-associated TCE-degrading bacteria by in situ inoculation with endophytic strains capable of degrading TCE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
Beneficial associations between plants and microorganisms are important in the development of the host plant (Mastretta et al. 2006) and its adaptation to environmental conditions. In addition to the root zone (rhizosphere), where the microbial biomass can be one order of magnitude or more higher than that in bulk soil, bacteria can colonize the interior of their host plant without causing symptoms of disease. These endophytic bacteria, which have been found in numerous plant species, often belong to genera commonly found in soil, including Pseudomonas, Burkholderia, Bacillus, and Azospirillum (Lodewyckx et al. 2002; Mastretta et al. 2006).
Like rhizosphere bacteria, endophytes can affect plant growth and development by fixing atmospheric nitrogen (diazotrophy; Döbereiner et al. 1995; Triplett 1996) and/or synthesizing phytohormones and enzymes involved in plant growth hormone metabolism, such as ethylene (e.g., 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase), auxins, acetoin, 2,3-butanediol and cytokinins (Arshad and Frankenberger 1991; Glick et al. 1994; Glick et al. 1998; Ryu et al. 2003; Glick 2004; Kuklinsky-Sobral et al. 2004). They can also exhibit strong antifungal activities (Hebbar et al. 1992a, b) and antagonisze bacterial pathogens (Coombs and Franco 2003).
In addition to their beneficial effects on plant growth, endophytic bacteria can be exploited for improving phytoremediation of organic contaminants. The fate of organic contaminants in the rhizosphere–root system largely depends on their physical–chemical properties. Plants readily take up organics with a log K ow between 0.5 and 3.5. These compounds seem to enter the xylem faster than the soil and rhizosphere microflora can degrade them, even if the latter is enriched with degradative bacteria (Trapp et al. 2000). Once these contaminants are taken up, plants may metabolize them, although some of them or their metabolites can be toxic (Doucette et al. 1998). For example, TCE can be transformed into TCA. Alternatively, some plants preferentially release volatile pollutants (such as TCE and BTEX) and/or their metabolites into the environment by evapotranspiration via the leaves. This raises questions about the merits of phytoremediation (Burken and Schnoor 1999; van der Lelie et al. 2001; Schwitzguébel et al. 2002; Ma and Burken 2003). The use of engineered endophytic bacteria, which complement the metabolic properties of their host, has the potential to overcome these limitations: while contaminants move through the plant’s vascular system, endophytic bacteria, colonizing the xylem (Germaine et al. 2004), can promote their degradation. This may result in both decreased phytotoxicity and evapotranspiration, provided the bacteria have the genetic information required for efficient degradation of the contaminants. These bacteria can be isolated, subsequently equipped with desirable characteristics and re-inoculated in the host plant to enhance their beneficial effects. Proof of concept was provided by inoculating Lupine plants (Barac et al. 2004) and poplar cuttings (Taghavi et al. 2005) with endophytic bacteria able to degrade toluene, which resulted in decreased toluene phytotoxicity and a significant decrease in toluene evapotranspiration.
Before endophyte-assisted phytoremediation of volatile organic contaminants can be successfully applied under field conditions, several obstacles need to be overcome (Newman and Reynolds 2005). One major point of concern is the persistence and stability of the engineered organisms and their degradation capabilities in field-grown plants, as phytoremediation projects often last for decades. Porteous Moore et al. (2006) have investigated the diversity of endophytic bacteria associated with hybrid poplar trees growing on a BTEX-contaminated site. They have demonstrated that within the diverse bacterial communities found in poplar, several endophytic strains are capable of degrading BTEX compounds. However, Barac et al. (2009) have demonstrated that there only will be an advantage for those endophytic community members possessing the appropriate degradation characteristics as long as a selection pressure is present. Furthermore, this selection pressure is not a guarantee that inoculated strains equipped with the appropriate degradation pathway will become an integrated part of the endogenous endophytic community. Horizontal gene transfer has been shown to play an important role in rapidly adapting a microbial community to a new environmental stress factor (Dong et al. 1998; van Elsas et al. 1998; Ronchel et al. 2000; Top et al. 2002; Devers et al. 2005). Taghavi et al. (2005) have reported the first in planta horizontal gene transfer among poplar-associated endophytic bacteria and demonstrated that such transfer can be used to change natural endophytic microbial communities in order to improve the remediation of organic contaminants.
For this work, a site was chosen where TCE was present in the groundwater at concentrations up to 100 mg l−1 due to former large-scale use of TCE as a degreaser during the production of metal barrels. TCE concentrations were determined in two transects through a small woodland of English Oak (Quercus robur) and Common Ash (Fraxinus excelsior) planted about 25 years ago (Fig. 1). The presence of this woodland seems to be responsible for a sharp decrease in TCE concentrations (Fig. 2) along these transects, since transects outside the planted area did not show this remarkable decrease; concentrations there were stable around 9.5 mg l−1. The main objectives of this work were to investigate (1) the role bacteria that are associated with both tree species can play in the TCE-degradation and (2) if the natural bacterial community is sufficient to prevent evapotranspiration from the leaves to the atmosphere. Given that TCE is one of the most widespread groundwater contaminants, and that English Oak and Common Ash are both native tree species, it is important to explore the cultivable-associated bacterial diversity, and its TCE degradation capacity as part of a larger study on the potential of in situ inoculation with (cultivable) plant-associated bacteria to enhance phytoremediation. Additionally, in 2006, rows of hybrid poplar trees were planted perpendicularly to the contamination plume (see Fig. 1) in order to augment the already existing bioscreen of English Oak and Common Ash.
TCE concentrations crossing the 2 transects through the woodland (monitoring wells are indicated in Fig. 1) of English Oak and Common Ash
2 Materials and methods
2.1 Sampling
In June 2007, the following samples were taken in order to isolate cultivable bacteria associated with the different compartments of English Oak and Common Ash growing in the high TCE concentration zone, nearby monitoring well 1: bulk soil, rhizosphere, and roots were sampled at a depth of 1.5 m and stored in sterile Falcon tubes (50 ml) filled with 20 ml sterile 10 mM MgSO4; leaf and stem samples were transferred in separate plastic bags. For every compartment, samples were taken from three different trees, and they were pooled for further analysis.
2.2 Isolation of bacteria associated with English Oak and Common Ash
Soil samples were diluted up to 10−7 in 10 mM MgSO4 solution and plated on 1/10 strength 869 solid medium (Mergeay et al. 1985) in order to isolate soil bacteria. Rhizosphere samples were vortexed, roots were removed, and serial dilutions up to 10−7 were prepared in 10 mM MgSO4 solution and plated on 1/10 strength 869 solid medium. After 7 days incubation at 30°C, colony-forming units (CFU) were counted and calculated per gram of bulk or rhizosphere soil.
To isolate the endophytic bacteria, English Oak and Common Ash samples were surface sterilized for 10 (roots and leaves) or 5 (stems) min in a 2% (roots and leaves) or a 1% (stems) active chloride solution supplemented with one droplet Tween 80 (Merck) per 100 ml solution, and were subsequently rinsed three times for 1 min in sterile distilled water. The third rinsing solution was plated on 869 medium to check surface sterility (if no growth was observed after 7 days, surface sterilization was considered to be successful). Surface sterile English Oak and Common Ash samples were macerated during 60 (roots and leaves) or 90 (stems) s in 10 ml 10 mM MgSO4 using a Polytron PR1200 mixer (Kinematica A6). Serial dilutions were plated on 1/10 strength 869 solid media and incubated for 7 days at 30°C before the CFU were counted and calculated per gram fresh plant weight.
All morphologically different bacteria were purified three times and plated on selective 284 medium supplemented with C-mix (per liter medium, 0.52 g glucose, 0.35 g lactate, 0.66 g gluconate, 0.54 g fructose, and 0.81 g succinate). The 284 medium contains per liter distilled water 6.06 g Tris–HCl, 4.68 g NaCl, 1.49 g KCl, 1.07 g NH4Cl, 0.43 g NaSO4, 0.20 g MgCl2 × 6H2O, 0.03 g CaCl2 × 2H2O, 40 mg Na2HPO4 × 2H2O, 0.48 mg Fe(III)NH4 citrate, 1 ml microelements solution, final pH 7. The microelement solution contains per liter distilled water: 1.3 ml 25% HCl, 144 mg ZnSO4 × 7H2O, 100 mg MnCl × 4 2H2O, 62 mg H3BO3, 190 mg CoCl2 × 6H2O, 17 mg CuCl2 × 2H2O, 24 mg NiCl2 × 6H2O and 36 mg NaMoO4 × 2H2O.
2.3 Genotypic characterization
Total genomic DNA was extracted from the purified bacteria (Bron and Venema, 1972). Polymerase chain reaction (PCR) amplification of 16 S rRNA genes was carried out in mixtures containing 100 ng μl−1 DNA, 1× High Fidelity PCR buffer (Invitrogen, Carlsbad, CA, USA), 0.2 mM of each of the four deoxynucleoside triphosphates, 2 mM MgCl2, 0.2 μM each of the forward and reverse primers, and 1 U of High Fidelity Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) per 50 μl. The universal 1392R (5′-ACGGGCGGTGTGTRC-3′) and the bacteria-specific 26F (5′-AGAGTTTGATCCTGGCTCAG-3′) primers were used for prokaryotic 16 S rRNA gene amplification. Cycling conditions consisted of: one denaturation cycle at 94 °C for 5 min, followed by 30 cycles at 94 °C for 1 min, 45 °C for 45 s, and 72 °C for 1.5 min, and completed with an extension cycle of 10 min at 72 °C. PCR products were purified using the QIAquick PCR purification columns (Qiagen, Valencia, CA, USA) and quantified spectrophotometrically, using the Nanodrop spectrophotometer (ND-1000, Isogen Life Science). The PCR products were directly used for ARDRA and sequencing.
For amplified 16 S rDNA restriction analysis (ARDRA), aliquots of the PCR products were digested overnight at 37°C with 1 U of the four-base-specific restriction endonuclease HpyCH4 IV in 1× NEB buffer 1 (New England Biolabs, Beverly, MA, USA). The digestion products obtained were separated by electrophoresis in a 1.5% agarose gel, and visualized by ethidium bromide staining and UV illumination. Bacterial strains with the same ARDRA patterns were grouped, and one representative strain of each group was selected for sequencing.
Purified PCR products (QIAquick column) of 16 S rRNA genes were sequenced using the Prism Big Dye Terminator sequencing kit (Applied Biosystems, Foster City, CA, USA) with 100 ng of template DNA. The extended sequences were obtained with universal primers 26F and 1392R. DNA sequences were determined on a 16 Capillary DNA Sequencer (Applied Biosystems, Foster City, CA, USA). Sequence Match at the Ribosome Database Project II (http://rdp.cme.msu.edu/index.jsp) was used for nearest neighbor and species identification. In order to verify the identification, a neighbor-joining analysis was performed. Prior to this analysis, the sequences were aligned using Clustal X (Thompson et al. 1997). A neighbor-joining tree was constructed with PAUP*4.0b10 (Swofford 2003), using default settings. In order to assess branch supports, bootstrap values were calculated with 2,000 pseudoreplicates.
2.4 Phenotypic characterization
Bacterial strains that displayed distinct ARDRA patterns were screened for heavy metal resistance (these strains can be used in future experiments to trace inoculated bacteria), as well as TCE and toluene (as a model BTEX compound) tolerance and degradation, as preparation for possible future in situ inoculation experiments (see discussion). To test metal resistance, all different bacteria were plated on selective 284 medium with the addition of 1 mM nickel, 2 mM zinc, or 0.8 mM cadmium. A carbon mix (per liter medium, 0.52 g glucose, 0.35 g lactate, 0.66 g gluconate, 0.54 g fructose, and 0.81 g succinate) was added, and cultures were incubated at 30°C for 7 days. In order to screen the bacteria for toluene and TCE tolerance and degradation, strains were plated on selective medium and incubated for 7 days at 30°C in sealed 10-l vessels with addition of 600 µl toluene or TCE to have a TCE or toluene-saturated atmosphere. To detect autotrophic strains, bacteria were also plated on selective medium without any carbon source.
After this screening, head space gas chromatography was used in order to confirm toluene and TCE degradation. For this experiment, bacteria were grown in 40 ml Schatz medium (Schatz and Bovell 1952) with the addition of 100 mg l−1 toluene, 100 mg l−1 TCE, or 100 mg l−1 toluene and 100 mg l–1 TCE, and in Schatz medium supplemented with C-mix (per liter medium, 0.52 g glucose, 0.35 g lactate, 0.66 g gluconate, 0.54 g fructose, and 0.81 g succinate) and 100 mg l−1 TCE. Samples of 10 ml were taken at the beginning of the experiment and after 3 days, and placed in 20-ml head space vials to which 4 g Na2Cl was added to stop all bacterial activity. Samples were analyzed by head space (Teledyne Tekmar HT3™) gas chromatography (Trace GC Ultra, Interscience). The volatilization of toluene and TCE was taken into account by measuring control samples (without addition of bacteria), and degradation was calculated as a percentage of the nonvolatilized fraction.
2.5 In situ evapotranspiration
In June 2008, the in situ evapotranspiration was measured for English Oak and Common Ash. The system designed for these measurements is shown in Fig. 3. Gas sampling pumps (ADC BioScientific) were connected to Teflon sampling bags (Chemware Laboratory products) via Teflon tubes and Chromosorb 106 traps. A column with CaCl2 was placed between the sampling bags and the Chromosorb traps to prevent water condensation in the traps. In order to have an inflow free of TCE, a column with CaCl2 and Chromosorb 106 traps were also placed before the inflow of the sampling bags. For each tree species, three measurements were performed. Twigs with five to six leaves were placed into the sampling bag which was made gas-tight around the twig, and an airflow of 5 l h–1 was created for 3 h. After sampling, the leaves were collected in plastic bags and stored at 4°C until leaf surface area analysis. The Chromosorb traps were analyzed by gas chromatography-mass spectrometry (GC-MS) with an ATD400 automatic thermal desorption system, an Auto System XLL gas chromatograph, and a Turbo mass spectrometer (Perkin-Elmer). The amount of evapotranspired TCE was calculated per hour and unit of leaf surface.
3 Results
3.1 Isolation of bacteria associated with English Oak and Common Ash
Bacteria were isolated from bulk soil, rhizosphere, root, stem, and leaf from English Oak and Common Ash. For both tree species, the number of cultivable bacteria recovered was an order of magnitude higher for rhizosphere than for soil samples (Table 1). The number of endophytic bacteria recovered was the highest in roots and stems, and was lower in the leaves. For both tree species, the number of different bacterial morphotypes was the highest in the rhizosphere, rather similar in roots and stems, and the lowest in the leaves. For soil, the number of morphologically different species was clearly higher in association with Common Ash than in association with English Oak.
3.2 Genotypic characterization
After purification, all morphologically different bacteria were characterized by ARDRA using HpyCH4 IV. Closely related strains were determined, and out of these strains, 16S rRNA genes of representative members were sequenced for species identification by means of Sequence Match at the Ribosome Database Project II (Fig. 4). The sequence match numbers marked in Fig. 4 were all (except bacterial strain 2) higher than 0.900, which indicated that the identification to the genus level was confident. Moreover, in the neighbor-joining tree, strains belonging to the same genus cluster together in distinct clades (bootstrap values of 100%), which confirmed the results of the 16S rRNA identification procedure. The 16S rRNA-based identification resulted in 41 and 30 genotypically different bacterial strains associated with English Oak and Common Ash, respectively. We have numbered the different bacterial strains (numbers 1–56 in Fig. 4). These numbers are further used in Figs. 5 and 6 and in Table 2.
Neighbor-joining tree of 16 S rDNA of cultivable bacteria associated with English Oak and Common Ash growing on the TCE-contaminated groundwater plume. On the right of the ARDRA fingerprint, the sequence match number, the 16 S rDNA identification (with unique number), the accession number of the closest reference strain, and the associated tree are shown. QR Quercus robur (Oak), FE Fraxinus excelsior (Ash). The TCE-degrading strains are marked with a black box
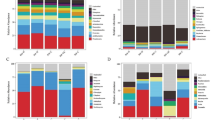
a Diversity of cultivable bacteria in the soil and rhizosphere associated with English Oak; b Diversity of cultivable endophytic strains associated with English Oak. c Diversity of cultivable bacteria in the soil and rhizosphere associated with Common Ash; d Diversity of cultivable endophytic strains associated with Common Ash. Central pie shows percentages by phyla; each outer ring progressively breaks these down by finer taxonomic levels with the bacterial number (see Fig. 4) in the outermost ring. Numbers in parentheses indicate the relative abundance, expressed as a percentage, of the total number of cultivable isolates per gram fresh weight that are present in the soil and rhizosphere (a) and inside (b) of English Oak, and in the soil and rhizosphere (c) and inside (d) of Common Ash. Pie diagrams were generated using sigmaplot
Schematic representation of endophytic strains as appearing within the different compartments of English Oak (a) and Common Ash (b). The numbers in parentheses refer to the bacterial strain numbers used in Fig. 4
To visualize the diversity of cultivable bacteria associated with Oak and Ash (Fig. 5), soil and rhizosphere bacteria were distinguished from endophytic bacteria. The relative abundance of each genotypically different strain was expressed as a percentage of the total number of cultivable isolates per gram fresh weight in soil and rhizosphere (Oak, 100% = 14.7 × 104 + 37.1 × 105 = 38.6 × 105; Ash, 100% = 19.1 × 104 + 14.8 × 105 = 16.7 × 105; see Table 1) or inside the plant (Oak, 100% = 94.7 × 103 + 28.2 × 104 + 13.9 × 103 = 39.1 × 104; Ash, 100% = 18.0 × 104 + 28.2 × 104 + 13.9 × 103 = 47.6 × 104; see Table 1).
In the soil and rhizosphere associated with English Oak (see Fig. 5A), 58.6% of the total number of isolates were Actinobacteria mostly of the genera Streptomyces (48.9%) and Arthrobacter (9.7%). Firmicutes made up 31.4% of the total number of isolates represented by Paenibacillaceae (18.6%) and Bacillaceae (12.8%). The remaining 10.0% of the collection was represented by Proteobacteria with a majority of gamma-Proteobacteria (8.3%) with Pseudomonas as dominant genus, and 1.7% of beta-Proteobacteria. The cultivable endophytic bacterial community associated with English Oak (see Fig. 5B) was also dominated by Actinobacteria (65.1%), with Frigobacterium spp. (45.0%) and Okibacterium spp. (13.0%) forming the majority of the group. Arthrobacter (3.7%) and Streptomyces (2.0%) were much less represented. Proteobacteria represented 23.1% of the endophytic collection associated with English Oak and were dominated by gamma-Proteobacteria (17.9%) including Pseudomonas spp. (9%), Xanthomonas spp. (4.6%), Enterobacter spp. (3.4%), and Erwinia (0.8%). The remaining part of the endophytic community associated with English Oak were Firmicutes (11.8%) with 8.8% Bacillaceae and 3.0% Paenibacillaceae. The compartmentalization of the dominant endophytic taxa in the different plant parts associated with English Oak is shown in Fig. 6A.
The community of cultivable soil and rhizosphere bacteria associated with Common Ash (see Fig. 5C) was dominated by Bacteroidetes, more specifically species of Flavobacterium (36.1%), and by Firmicutes (35.0%), including 29.4% Bacillaceae and 5.6% Paenibacillaceae. Actinobacteria made up 18.9% of the community (9.5% Arthrobacter spp. and 9.4% Streptomyces spp.) and Proteobacteria represented 10.1% (5.1% Pseudomonas spp. and 5.0% Collimonas). Proteobacteria accounted for 67.4% of the Common Ash endophyte isolates (see Fig. 5D). Nearly all of these (67.3%) were gamma-Proteobacteria of the genus Pseudomonas; the remaining 0.1% were identified as alpha-Proteobacterial Sinorhizobium. Further, Actinobacteria made up 22.1% of the endophytic community, including a majority of 19.9% Streptomyces and a minority of 2.2% Arthrobacter. The remaining part of the Common Ash associated endophytic community were Firmicutes (10.5%), comprising mainly Bacillaceae (9.9%), and a small fraction of Paenibacillaceae (0.6%). The compartmentalization of the bacterial endophytic community associated with Common Ash is presented in Fig. 6B. No typical leaf strains could be isolated from Common Ash.
3.3 Phenotypic characterization
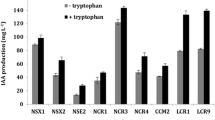
In a first test, all genotypically different bacteria associated with English Oak and Common Ash were phenotypically characterized for their heavy metal resistance and for their tolerance to the target pollutants TCE and toluene (see Table 2). Metal resistance was also tested because, in future experiments, metal-resistant strains may allow an easier tracebility. From all the isolated bacteria, six strains (6, 20, 36, 40, 41, and 49) could grow in the presence of Ni, and one strain (23) was resistant to Cd and Zn. The screening for TCE and toluene tolerance resulted in 82% of the bacteria that could grow in the presence of TCE, and 77% in the presence of toluene. In order to test TCE and toluene degradation capacity, a selection was made of bacteria that probably degrade TCE and/or toluene, based on the fact that these bacteria were growing better in a TCE and/or toluene-saturated atmosphere compared to autotrophic conditions, which suggests that they were able to use these components as a carbon source. These bacteria (strains 4, 19, 27, 34 and 37) were screened for TCE and/or toluene degradation. Strains 4 (Arthrobacter sp.) and 19 (Streptomyces turgidiscabies), both rhizosphere bacteria associated with Common Ash, showed a 100% toluene degradation capacity and a maximum TCE degradation capacity of 22% and 14%, respectively, over a 3-day testing period in Schatz medium with addition of 100 mg l−1 TCE (Fig. 7). The other bacterial strains (27, 34, and 37) did not show any TCE or toluene degradation capacity.
Toluene and TCE degradation tested by head space chromatography. Bacteria were grown in Schatz medium with addition of 100 mg l–1 toluene, 100 mg l–1 TCE or 100 mg l–1 toluene and 100 mg l–1 TCE, and in Schatz medium supplemented with C-mix (per liter medium, 0.52 g glucose, 0.35 g lactate, 0.66 g gluconate, 0.54 g fructose, and 0.81 g succinate) and 100 mg l–1 TCE. Samples were taken at the beginning of the experiment and after 3 days. The volatilization of toluene and TCE was taken into account by measuring control samples (without addition of bacteria), and degradation was calculated as a percentage of the nonvolatilized fraction
3.4 In situ evapotranspiration
In Common Ash 10.84 × 10−3 ± 1.17 × 10−3 ng TCE cm−² h−1 was evapotranspired to the atmosphere. The amount of transpired TCE from the leaves of English Oak was 6.35 × 10−3 ± 0.18 × 10–3 ng cm–² h–1. Since (1) the most common TCE degradation products in plant tissues are trichloroethanol, trichloroacetic acid, dichloroacetic acid, and trichloroethanol glycoside (Burken and Ma 2006) and (2) aerobic degradation of TCE has been reported for bacterial strains possessing the toluene ortho-monooxygenase (TomA) genes with TCE-epoxide, dichloroacetate, glyoxylate, and formate as metabolites, these oxidative metabolites were also analyzed. Although they have been identified in controlled cell culture experiments, whole-plant laboratory experiments, and full-scale field settings, none of these degradation products could be detected in these evapotranspiration measurements.
4 Discussion
Poplar trees are frequently used for phytoremediation of groundwater contaminated with organic solvents (Schnoor et al. 1995; Ferro et al. 1997; Barac et al. 2009), Their fast growth and large transpiration potential make them “trees of choice” for phytoremediation purposes (Schnoor et al. 1995; Shim et al. 2000). In this study, the potential of English Oak and Common Ash and their associated microorganisms for the phytoremediation of a TCE-contaminated site was investigated. Since they are both widely distributed native tree species in Europe possessing a relatively high transpiration capacity, it seemed very interesting to explore the diversity and remediation potential of their associated bacterial community. Since this work is part of a larger study on the potential role of traceable plant-associated bacteria to enhance in situ phytoremediation with an ultimate aim in situ (re-)inoculation of plant-associated bacteria equipped with the required degradation pathways, only the cultivable bacteria were characterized.
Cultivable bacteria were isolated from bulk soil, rhizosphere, root, stem, twig, and leaf from English Oak and Common Ash (see Table 1). For both tree species, the total amount of bacteria in the rhizosphere was one order of magnitude higher than in the bulk soil which can be explained by the “rhizosphere effect.” The number of cultivable endophytes was highest in roots and stems and was lower in leaves, suggesting that colonization was mainly taking place through the roots.
The 16S rDNA identification of all isolated bacteria that were found to be different after ARDRA analysis resulted in 56 different bacterial strains comprising 26 strains exclusively associated with English Oak, 15 strains exclusively associated with Common Ash and 15 strains associated with both tree species (see Fig. 4). This indicates that both tree species were associated with both a specific bacterial population and a nonspecific bacterial population.
In case of English Oak, the bacterial diversity (number of different genera) in soil and rhizosphere seemed similar to the endophytic bacterial diversity, except that the fraction of Proteobacteria was higher, and the fraction of Firmicutes was lower in the endophytic community (see Fig. 5A,B). However, a closer look at the results shows that some taxa indeed were occurring in the bulk soil and the rhizosphere as well as inside the plant (e.g., Arthrobacter, Streptomyces, Bacillus, Paenibacillus, Pseudomonas). Nevertheless, other taxa were exclusively found inside the plant (Frigobacterium, Okibacterium, Curtobacterium, Aeromicrobium, Enterobacter, and Erwinia). This suggests that the endophytic community of English Oak might be composed of both facultative endophytes colonizing the tree via the roots and obligate endophytes transferred from one generation to the other through the seeds. Furthermore, the endophytes exhibited a marked spatial compartmentalization, suggesting that in English Oak, some taxa of bacteria only occur in a specific plant part (e.g., Paenibacillus), while others did not show any compartment specificity (e.g., Pseudomonas; see Fig. 6A).
As to Common Ash, a clear difference could be noticed in the composition of the bacterial community in the soil and rhizosphere and the endophytic bacterial community, since Bacteroidetes represented 36.1% of the soil and rhizosphere bacteria, while they were completely lacking in the endophytic bacterial community (see Fig. 5C,D). Moreover, species of Proteobacteria dominate the endophytic bacterial community, while they only represented 10.1% of the soil and rhizosphere bacteria. In contrast with the bacterial endophytes associated with English Oak, the endophytic bacterial community associated with Common Ash contained almost no species exclusively present inside the plant, which might indicate that the endophytic population consists mainly of facultative endophytes. This hypothesis is supported by the compartmentalization of the Common Ash associated endophytes (see Fig. 6B), since most of the endophytes were localized in the roots and the stems, which is typical for facultative endophytes (Mastretta et al. 2006). Given that obligate endophytes have to be transferred through the seeds, this difference in endophyte-type between English Oak and Common Ash can possibly be explained by both the size and the structure of the seeds. Firstly, the seed is distinctly bigger and more robust in case of English Oak. In addition, in seeds of English Oak, the embryo possesses large cotyledons filling up the entire space within the seed coat, while in case of Common Ash, the cotyledons are tiny, and the embryo’s food reserve is almost entirely located in the extra-embryonic endosperm. Due to these clear differences, survival sites for endophytes might be different. Therefore, in a future experiment, the seed endophytes of English Oak and Common Ash growing on the TCE-contaminated field site will be isolated and characterized. The importance of seed endophytes as a vector for beneficial bacteria has already been demonstrated by Cankar et al. (2005) and Mastretta et al. (2006). Beside plant species-specific bacteria (see Fig. 4), several taxa (e.g., Pseudomonas, Bacillus, Paenibacillus, and Arthrobacter) that were found in association with both English Oak and Common Ash were found in association with hybrid poplar (Populus trichocarpa x deltoides; Porteous Moore et al. 2006). Moreover, we will characterize the bacterial community associated with the hybrid poplar trees newly planted on the TCE-contaminated site and compare this with the populations associated with English Oak and Common Ash growing on the same site and with the bacterial community associated with hybrid poplar growing on a BTEX-contaminated site (Porteous Moore et al. 2006).
In order to determine whether there was selection for specific bacterial phenotypes in the presence of TCE, representatives of all ARDRA types were tested for TCE and toluene tolerance. It is obvious that TCE concentrations in the groundwater up to 100 mg l−1 resulted for both tree species in a bacterial population that was dominated by TCE tolerant strains (see Table 2; Barac et al. 2009). This enrichment of TCE-tolerant strains in response to the TCE contamination is consistent with the observations of Siciliano et al. (2001). Beside TCE tolerant bacteria, there was also an enrichment of toluene-tolerant bacteria. The combined occurrence of TCE and toluene tolerance might be due to common mechanisms, which have been demonstrated for aerobic degradation of these compounds. Aerobic degradation of both toluene and TCE has been reported for bacterial strains possessing the toluene ortho-monooxygenase (TomA) genes of Burkholderia cepacia G4 (Mars et al. 1996; Yee et al. 1998), and for toluene-o-xylene monoxygenase (TomO) of Pseudomonas stutzeri OX1 (Ryoo et al. 2000, 2001; Shim et al. 2000, 2001). The fact that some strains (e.g., strains 4, 19, 34, 37) showed better growth on the 284 medium in the presence of TCE (and toluene; see Table 2) suggests that TCE (and toluene) can be used as a substrate. The results of the degradation experiments indeed showed TCE (and toluene) degradation capacity (see Fig. 7), which was already suggested by the remarkable decrease in TCE concentration through the transects at the field site (see Fig. 2). Although these results support the hypothesis of a causal relationship between the strong decrease in TCE concentration through the transects (see Fig. 1) and the TCE (and/or toluene) degradation capacity of the bacterial population associated with the English Oak and Common Ash, still a significant amount of TCE (English Oak, 6.35 × 10–3 ± 0.18 × 10–3 ng cm−² h−1; Common Ash, 10.84 × 10–3 ± 1.17 × 10−3 ng TCE cm−² h−1) was evapotranspired from the leaves to the atmosphere. This implies that the natural bacterial community has insufficient capacity to degrade all the TCE taken up by the roots before it reaches the leaves. Therefore, it might be worth attempting to inoculate the TCE-degrading bacteria that were isolated from Oak and Ash growing on this field, in order to enrich the quantity of degrading strains resulting in an improved remediation capacity. Furthermore, the newly planted poplar trees could be inoculated with TCE-degrading poplar endophytes, such as Pseudomonas putida W619 (Taghavi et al. 2005), to improve the degradation capacity of the endogenous endophytic populations through horizontal gene transfer.
For these inoculation experiments, it is favorable to work with bacteria that can easily be re-isolated, such as bacteria possessing heavy metal resistance. Such strains may allow an easier traceability for bioaugmentation of polluted sites. For this reason, all bacteria that were found to be different after ARDRA analysis were also tested for heavy metal resistance. Only a very limited number of strains were found to be resistant to the heavy metals Ni, Cd, and/or Zn, which can be explained by the absence of selection pressure. Metal concentrations measured were indeed within the range of background values.
5 Conclusions and perspectives
The results obtained in this study show that the bacterial community associated with English Oak and Common Ash growing on a TCE contaminated groundwater plume, was strongly enriched with toluene and/or TCE tolerant strains, but that this was not sufficient to degrade all TCE before it reaches the leaves. Although both tree species were exposed to the same type and level of contamination and were growing side by side in the same woodland, their associated bacterial populations clearly differed in composition. The endophytic bacterial community associated with English Oak contained significantly more species-specific, most likely obligate endophytes. This might be related to the seed type. Furthermore, the remediation capacity of English Oak and Common Ash possibly might be improved by in situ inoculation of the TCE degrading, plant-associated bacteria that were isolated. Since English Oak and Common Ash are both common native and widely spread species in Europe, this in situ inoculation strategy could have a large application potential. In addition, the newly planted poplar trees can be inoculated with Pseudomonas putida W619 (Taghavi et al. 2005), to improve the degradation capacity of the endogenous endophytic populations through horizontal gene transfer.
References
Arshad M, Frankenberger WT (1991) Microbial production of plant hormones. Kluwer Academic Publishers, Dordrecht, the Netherlands
Barac T, Taghavi S, Borremans B, Provoost A, Oeyen L, Colpaert JV, Vangronsveld J, van der Lelie D (2004) Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat Biotechnol 22:583–588
Barac T, Weyens N, Oeyen L, Taghavi S, van der Lelie D, Dubin D, Spliet M, Vangronsveld J (2009) Field note: hydraulic containment of a BTEX plume using poplar trees. Int J Phytorem 11:416–424
Bron S, Venema G (1972) Ultraviolet inactivation and excision-repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mut Res 15:1–10
Burken JG, Schnoor JL (1999) Distribution and volatilization of organic compounds following uptake by hybrid Poplar trees. Int J Phytorem 1:139–151
Burken JG, Ma X (2006) Phytoremediation of volatile organic compounds. In: Mackova M, Dowling DN, Macek T (eds), Phytoremediation and Rhizoremediation Theoretical Background. pp 199–216
Cankar K, Kraigher H, Ravnikar M, Rupnik M (2005) Bacterial endophytes from seeds of Norway spruce (Picea abies L. Karts). FEMS Microbiol Lett 244:341–345
Coombs JT, Franco CMM (2003) Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl Environ Microbiol 69:5603–5608
Devers M, Henry S, Hartmann A, Martin-Laurent F (2005) Horizontal gene transfer of atrazine-degrading genes (atz) from Agrobacterium tumefaciens St96-4pADPA:Tn5 to bacteria of maize-cultivated soil. Pest Manag Sci 61:870–880
Döbereiner J, Urquiaga S, Boddey RM (1995) Alternatives for nitrogen nutrition of crops in tropical agriculture. Fert Res 42:339–346
Dong Q, Springael D, Schoeters J, Nuyts G, Mergeay M, Diels L (1998) Horizontal transfer of bacterial heavy metal resistance genes and its applications in activated sludge systems. Water Sci Technol 37:465–468
Doucette WJ, Bugbee B, Hayhurst S, Plaehn WA, Downey DC, Taffinder SA, Edwards R (1998) Phytoremediation of dissolved phase trichloroethylene using mature vegetation. In: Wickramanayake GB, Hinchee HE (eds) Bioremediation and Phytoremediation: Chlorinated and Recalcitrant Compounds. Batelle Press, Columbus, USA, pp 251–256
Ferro AM, Rieder JP, Kennedy J, Kjelgren R (1997) Phytoremediation of groundwater using Poplar trees. In: Thibault CA (ed) Phytoremediation. Inc, International Business Communications, pp 202–212
Germaine K, Keogh E, Borremans B, van der Lelie D, Barac T, Oeyen L, Vangronsveld J, Porteus Moore F, Moore ERB, Campbel CD, Ryan D, Dowling D (2004) Colonisation of Poplar trees by gfp expressing endophytes. FEMS Microbiol Ecol 48:109–118
Glick BR (2004) Bacterial ACC deaminase and the alleviation of plant stress. Adv Appl Microbiol 56:291–312
Glick BR, Jacobson CB, Schwarze MK, Pasternak JJ (1994) 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR 12–2 do not stimulate canola root elongation. Can J Microbiol 40:911–915
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J Theor Biol 190:63–68
Hebbar KP, Davey AG, Merrin J, Dart PJ (1992a) Rhizobacteria of maize antagonistic to Fusarium moniliforme, a soil-borne fungal pathogen: colonization of rhizosphere and roots. Soil Biol Biochem 24:989–997
Hebbar KP, Davey AG, Merrin J, McLoughlin TJ, Dart PJ (1992b) Pseudomonas cepacia, a potential suppressor of maize soil-borne diseases-seed inoculation and maize root colonization. Soil Biol Biochem 24:999–1007
Kuklinsky-Sobral J, Araujo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL (2004) Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol 6:1244–51
Lodewyckx C, Vangronsveld J, Porteous F, Moore ERB, Taghavi S, Mergeay M, van der Lelie D (2002) Endophytic bacteria and their potential applications. Critical Rev Plant Sci 21:583–606
Ma X, Burken JG (2003) TCE diffusion to the atmosphere in phytoremediation applications. Environ Sci Technol 37:2534–2539
Mars AE, Houwing J, Dolfing J, Janssen DB (1996) Degradation of toluene and trichloroethylene by Burkholderia cepacia G4 in growth-limited fed-batch culture. Appl Environ Microbiol 62:886–891
Mastretta C, Barac T, Vangronsveld J, Newman L, Taghavi S, van der Lelie D (2006) Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. Biotech Gen Eng Rev 23:175–207
Mergeay M, Nies D, Schlegel HG, Gerits J, Charles P, Van Gijsegem F (1985) Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol 162:328–334
Newman LA, Reynolds CM (2005) Bacteria and phytoremediation: new uses for endophytic bacteria in plants. Trends Biotechnol 23:6–8
Porteous Moore F, Barac T, Borremans B, Oeyen L, Vangronsveld J, van der Lelie D, Campbell CD, Moore ERB (2006) Endophytic bacterial diversity in Poplar trees growing on a BTEX-contaminated site: the characterisation of isolates with potential to enhance phytoremediation. Sys App Micro 29:539–556
Ronchel MC, Ramos-Diaz MA, Ramos JL (2000) Retrotransfer of DNA in the rhizosphere. Environ Microbiol 2:319–323
Ryoo D, Shim H, Canada K, Barbieri P, Wood TK (2000) Aerobic degradation of tetrachloroethylene by toluene-o-xylene monooxygenase of Pseudomonas stutzeri OX1. Nat Biotechnol 18:775–778
Ryoo D, Shim H, Arenghi FLG, Barbieri P, Wood TK (2001) Tetrachloroethylene, trichloroethylene, and chlorinated phenols induce toluene-o-monooxygenase activity in Pseudomonas stutzeri OX1. Appl Microbiol Biotechnol 56:545–549
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932
Schatz A, Bovell C (1952) Growth and hydrogenase activity of a new bacterium Hydrogenomonas facilis. J Bacteriol 63:87–98
Schnoor LJ, Licht AL, McCutchon CS, Wolfe NL, Carreira HL (1995) Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29:318A–323A
Schwitzguébel JP, van der Lelie D, Glass DJ, Vangonsveld J, Baker AJM (2002) Phytoremediation: European and American trends, successes, obstacles and needs. J Soils Sediments 2:91–99
Shim H, Chauhan S, Ryoo D, Bowers K, Thomas SM, Canada KA, Burken J, Wood TK (2000) Rhizosphere competitiveness of trichloroethylene-degrading, poplar colonizing recombinant bacteria. Appl Environ Microbiol 66:4673–4678
Shim H, Ryoo D, Barbieri P, Wood TK (2001) Aerobic degradation of mixtures of tetrachloroethylene, trichloroethylene, dichloroethylenes, and vinyl chloride by toluene-o-xylene monooxygenase of Pseudomonas stutzeri OX1. Appl Microbiol Biotechnol 56:265–269
Siciliano SD, Fortin N, Mihoc A, Wisse G, Labelle S, Beaumier D, Ouellette D, Roy R, Whyte LG, Banks MK, Schwab P, Lee K, Greer CW (2001) Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl Environ Microbiol 67:2469–2475
Swofford DL (2003) PAUP*—Phylogenetic Analysis Using Parsimony (*and Other Methods), Ver. 4. [Computer Software and Manual.]. Sinauer Associates, Sunderland, MA
Taghavi S, Barac T, Greenberg B, Borremans B, Vangronsveld J, van der Lelie D (2005) Horizontal gene transfer to endogenous endophytic bacteria from Poplar improves phytoremediation of toluene. Appl Environ Microbiol 71:8500–8505
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequences aided by quality analysis tools. Nucleic Acid Res 25:4876–4882
Top EM, Springael D, Boon N (2002) Catabolic mobile genetic elements and their potential use in bioaugmentation of polluted soils and waters. FEMS Microbiol Ecol 42:199–208
Trapp S, Zambrano KC, Kusk KC, Karlson U (2000) A phytotoxicity test using transpiration of willows. Arch Env Contam Toxicol 39:154–160
Triplett EW (1996) Diazotrophic endophytes: progress and prospects for nitrogen fixation in monocots. Plant Soil 186:29–38
van der Lelie D, Schwitzguébel JP, Vangronsveld J, Baker AJM (2001) Assessing phytoremediation’s progress in the United States and Europe. Environ Sci Technol 35:446A–452A
Van Elsas JD, Gardener BB, Wolters AC, Smit E (1998) Isolation, characterization, and transfer of cryptic gene-mobilizing plasmids in the wheat rhizosphere. Appl Environ Microbiol 64:880–889
Yee DC, Maynard JA, Wood TK (1998) Rhizoremediation of trichloroethylene by a recombinant, root-colonizing Pseudomonas fluorescens strain expressing toluene ortho-monooxygenase constitutively. Appl Environ Microbiol 64:112–118
Acknowledgements
This research was funded by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) for N.W. and by the Fund for Scientific Research Flanders (FWO-Vlaanderen), Ph.D. grant for J.B. and postdoc grant for T.B. This work was also supported by the UHasselt Methusalem project 08M03VGRJ. D.v.d.L. and S.T. are supported by the US Department of Energy, Office of Science, BER, project number KP1102010 under contract DE-AC02-98CH10886, and by Laboratory Directed Research and Development funds (LDRD05-063) at the Brookhaven National Laboratory under contract with the U.S. Department of Energy. We thank A. Wijgaerts and C. Put for their help with the isolation and J. Put, J. Czech, and R. Carleer for GC analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editors: Peter Schröder, Jean-Paul Schwitzguébel
Rights and permissions
About this article
Cite this article
Weyens, N., Taghavi, S., Barac, T. et al. Bacteria associated with oak and ash on a TCE-contaminated site: characterization of isolates with potential to avoid evapotranspiration of TCE. Environ Sci Pollut Res 16, 830–843 (2009). https://doi.org/10.1007/s11356-009-0154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-009-0154-0