Abstract
Japanese gardens play an important role in the conservation of bryophyte diversity. Previous studies have indicated that diverse garden landscapes and their maintenance as well as garden microclimates can be related to high bryophyte diversity, although it has not been examined how these microclimates differ from those in other land use types and how they affect bryophyte diversity. Therefore, this study analyzed the relationships among microclimate, garden landscape, and bryophyte diversity. The study sites comprised the renowned Saihoji Temple in Kyoto, Japan, and surrounding residential areas and forests. Seventeen 1-m-radius circular plots were established within the study sites, and bryophyte species richness and cover, landscape elements, and microclimates (temperature and relative humidity) were recorded in these plots. Sixty-seven species were identified, including four endangered species, and garden plots showed higher species richness and cover than surrounding areas. Garden microclimates were characterized by significantly lower temperature and higher relative humidity, which can be attributed to a combined influence of garden elements such as large vegetation cover and water surface. Notably, these microclimates can significantly and positively affect bryophyte diversity by mitigating drought stress. Thus, Japanese gardens featuring large vegetation cover and water surface can function as conservation sites for drought-sensitive species, increasing urban bryophyte diversity. Conservation of bryophytes might be beneficial to urban biodiversity and resilience reinforcement by promoting biological interactions among several species and improving ecological functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ongoing global advance of urbanization and associated fragmentation of natural environments highlight the increasing importance of urban greenery as a refuge for native biodiversity (Goddard et al. 2010). The contribution of private gardens to biodiversity conservation has recently attracted considerable attention (Goddard et al. 2010) because gardens often constitute major components of urban greenery (Loram et al. 2007), and their potential value for biodiversity is relatively high (Davies et al. 2009; Goddard et al. 2010). Owing to these advantages, an increasing number of cities aim to use gardens for the conservation of plants and animals (Goddard et al. 2010).
The importance of garden landscapes and their management for biodiversity has been reported worldwide. For example, avian diversity in Tasmanian suburban areas has been closely related to garden floristics, and therefore gardens can be designed and managed to conserve particular avian species and species assemblages (Daniels and Kirkpatrick 2006). Other studies have shown that the spatial arrangement of flowers in urban gardens is a strong predictor of bee abundance and richness (Plascencia and Philpott 2017). In a review of gardens and biodiversity, the need for coordination of garden management within the context of the surrounding landscape was proposed as a positive action for conservation (Goddard et al. 2013).
There are many types of gardens in urban areas. Among these, the role of Japanese gardens in the conservation of bryophytes is notable, because they contain a considerably high diversity of these plants compared with other urban green spaces (Oishi 2012). Some Japanese gardens have been reported to contain more than 100 bryophyte species, including endangered species (e.g., Hasegawa 2002). This high diversity is explained by both landscape elements and meticulous daily maintenance (Oishi 2012). The design of Japanese gardens consists of miniatures of natural landscapes (e.g., mountain, ocean, and island) that symbolize world habitats, thereby creating a variety of environments and promoting bryophyte diversity. Moreover, daily maintenance of garden landscapes prevents bryophytes from being covered by fallen leaves and weeds.
Several studies have empirically demonstrated that garden landscapes provide suitable microclimates for bryophytes. Ornamental deciduous trees planted in Japanese gardens (e.g., maples) maintain humidity beneath them and provide suitable light conditions for photosynthesis (Mizutani 1975). In addition, surrounding hedges comprising evergreen trees prevent drier air from flowing into the garden (Mizutani 1975). These hedges can also provide calm and cool air conditions within gardens that promote the occurrence of morning dews, which are an important source of water for bryophytes (Iida et al. 2010).
The notion that garden design may alleviate drought stress for bryophytes is also worthy of detailed discussion, as it is a major factor influencing urban biodiversity. Urbanization increases temperature and decreases humidity, owing to anthropogenic heat release and to the replacement of green areas by impervious surfaces (Ryu and Baik 2012). These changes in climate (urban heat islands, UHIs) favor plants that are able to cope with drought or that avoid drought (Knapp et al. 2008). Forest fragmentation by urbanization also causes drought stress. At the edges of fragmented forests, pronounced drought stress is observed (“edge effects”; Murcia 1995), and this environmental change results in a decrease in drought-sensitive species (Zartman 2003; Laurance et al. 2007; Oishi 2009; Aragón et al. 2015; Oishi and Morimoto 2016). Besides, an increase in alien species at these edges was also reported for tracheophytes (Pauchard and Alaback 2006).
Thus, whereas drought stress has a negative influence on urban biodiversity, garden design can be beneficial to mitigate this stress for improving diversity, an effect that can be reflected well in the relationships between Japanese garden and bryophyte diversity. Therefore, this study first examined the characteristics of microclimates in a Japanese gardens and their relation to the landscape elements, and then assessed how these garden microclimates affected bryophyte diversity. These results will be applied to the design of urban greenery, contributing to biodiversity conservation by mitigating drought stress.
Materials and methods
Study site and data collection
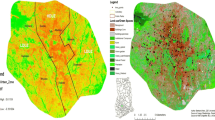
The study sites comprised a Japanese garden at the Saihoji Temple in Kyoto, Japan, and its surrounding area (Figs. 1, 2a). The climate in Kyoto is characterized by large differences in temperature between summer and winter, owing to the city’s location in the Kyoto basin. The average temperature is 15.9 °C, ranging from 28.2 °C in August to 4.6 °C in February (Japan Meteorological Agency 2017). Saihoji Temple (Fig. 2b) has one of the most famous moss gardens in Japan, called “the Moss Temple” (Iwatuski and Kodama 1961). This garden is located in the urban fringe, and is surrounded by residential areas and large forest patches (Fig. 2c, d). Seventeen 1-m-radius circular plots, 30 m apart, were established along one transect spanning the length and another spanning the width of each site, from the garden center to the surrounding areas (residential and forest areas) (Fig. 2a). When circular plots could not be set at the planned installation location (e.g., areas where installation was not permitted), these plots were established close to the planned location. Eleven, two, and four study plots were located in garden, residential, and forest areas, respectively. In these plots, bryophyte species richness and cover, landscape elements, and microclimates were recorded. Bryophyte cover was measured using grids of two sizes (5 × 5 cm and 1 × 1 cm). The definition of endangered species followed that given in the Red Data Book of Kyoto Prefecture (Kyoto Prefecture 2015). To characterize the bryophyte flora at each site, drought-sensitive bryophytes were classified according to their preference for humid habitats following Iwatsuki (2001) and Oishi (2015). Landscape elements recorded were vegetation cover [evergreen tree canopy (Can_E), deciduous tree canopy (Can_D), understory evergreen trees (Und_E), understory deciduous trees (Und_D), bushes, and grasses], substrate coverage [rocky areas (Rock), logs, buildings (Building), streams, and ponds], geographical features [slope inclination (Inc), maximum difference in elevation (Ele)], and presence/absence of daily maintenance (Maint). The microclimate variables measured were temperature (Temp) and relative humidity (RH). Vapor pressure deficit (VPD), which is an indicator of the evaporation potential of air, an important factor for bryophyte diversity (Gotsch et al. 2017), was also calculated on the basis of Temp and RH using the following equation:
where SVP is the saturated vapor pressure, calculated using the following equation (Monteith and Unsworth 2008):
Location and climate of the study sites in Kyoto City (Japan). a Location of the study sites. b Monthly temperature and precipitation in Kyoto City (Japan Meteorological Agency 2017)
Measurements of Temp and RH were taken every 15 min using temperature/humidity data loggers [Hygrochron™ iButton (DS1923); Maxim Integrated, San Jose, CA, USA]. Loggers were placed within a pipe wrapped in aluminum foil to prevent temperature increases caused by sunlight and then set on the ground, at the center of each circular plot. However, because sunlight influence could not be completely excluded, only measurements taken between sunset and sunrise (18:00–06:00) were used in the analyses. These measurements were obtained from October 19 to November 29, 2012. This period was considered suitable for the examination of drought stress on mosses, because the influences of UHIs on microclimates are clearly observed during spring and autumn (Japan Meteorological Agency 2014). In addition, mid-autumn climate is marked by a relatively small amount of precipitation (Fig. 1b) and large differences in diurnal temperature range (calculated by the Japan Meteorological Agency 2017). This climate can cause severe drought stress, enhancing the importance of the water supplied by morning dews and fogs for bryophytes.
Differences in microclimates
To examine the significance of differences in microclimates between the garden and the other study sites, state space models using a Bayesian framework were constructed. This modeling procedure relied on the hypotheses that each site has original factors that affect its microclimates, and that differences among microclimates can be expressed by a constant term. Based on these assumptions, state (1) and observation (2) equations for microclimates were expressed as follows:
where μ [t] is a true value of the microclimate, Yk [t] is an observed value of the microclimate in study site (k), t is the time point (1 point/15 min), \( \sigma_{\mu }^{2} \) reflects the strength of the autocorrelation of microclimates between time points, \( \sigma_{\gamma }^{2} \) is the common variance of microclimates at study sites (because data were recorded in the same manner), and Ck is the original factor (constant). A likelihood function was constructed for these parameters, and then the significance of Ck values with Bayesian 95% credible intervals was examined using Markov chain Monte Carlo (MCMC) methods. In this modeling system, \( \sigma_{\mu }^{2} \) and \( \sigma_{\gamma }^{2} \) were constrained above zero. To avoid the arbitrary selection of prior distributions, a uniform distribution was selected for the parameters. This calculation was performed using Stan (Carpenter et al. 2017; Stan Development Team 2017), via the RStan interface on R (R Core Team 2017). In cases where the 95% credible interval (quantiles between 2.5% and 97.5%) overlapped between study sites, the parameter was regarded as insignificant. Conversely, when this interval did not overlap, these parameters were considered negatively or positively significant. Four MCMC chains were used in the analysis, with a warm-up period of 2000 samples, followed by 1000 saved iterations. To facilitate comparisons, the value for the original factor at the garden was set to zero. Posterior estimates were visually examined for chain convergence with trace plots and the potential scale reduction factor (Gelman and Rubin 1992).
Landscape elements and microclimates
To reveal the influence of landscape elements on Temp and RH, a random forest (RF) approach was used. Although this method is designed to deal with symmetrical error distribution (Lopatin et al. 2016), it can handle nonlinear relationships and a huge number of predictors without deleting them (Naghibi et al. 2016). In RF analysis, the importance of a variable is evaluated on the basis of the percentage increase of mean squared error (% Inc MSE) and on the increase in node purity (Inc Node Purity). Higher values of % Inc MSE and Inc Node Purity indicate a higher accuracy of the variable for the prediction of a dependent variable. Three types of Temp variables [Temps; average Temp (Temp_ave), Temp at 06:00 (Temp_06), and Temp at 18:00 (Temp_18)] and RH variables [RHs; average RH (RH_ave), RH at 06:00 (RH_06), and RH at 18:00 (RH_18)] were used as dependent variables, whereas landscape elements were employed as explanatory variables. In the RF model for RHs, the Temps corresponding to the RHs (e.g., Temp_06 corresponds to RH_06) were added as explanatory variables, because Temp theoretically determines RH.
Bryophyte diversity, landscape elements, and microclimates
The influence of garden environments on bryophytes was examined using generalized linear models (GLM). These models were developed to deal with both symmetrical and asymmetrical error distributions (Lopatin et al. 2016). In the present study, species richness and total cover were used as dependent variables, and landscape elements and microclimates were adopted as explanatory variables. Three VPD variables [VPDs; average VPD (VPD_ave), VPD at 06:00 (VPD_06), and VPD at 18:00 (VPD_18)] were also calculated and were included as explanatory variables in the GLMs. A Poisson error distribution and a log-link function were assigned for modeling species richness, whereas Gaussian distribution and identity link function were used for modeling coverage. Only explanatory variables that were correlated with each dependent variable (r > 0.3) were used. The best-fit models were identified by the Akaike information criterion (AIC) function. Multicollinearity was examined on the basis of the variance inflation factor (< 10). The levels of significance of GLM model fits were tested using analysis of deviance with a χ2 distribution. All data were analyzed using R (R Core Team 2017).
Results
Bryophyte diversity
The assessment of bryophyte diversity in the circular plots revealed 67 species (44 mosses and 23 liverworts), including four endangered species (Sphagnum palustre L., Leucobryum bowringii Mitt., Leptolejeunea elliptica (Lehm. & Lindenb.) Schiffner, and Riccia fluitans L.) that were only found in garden plots. Species recorded at the study sites are listed in Supplementary Material 1. Garden, residential, and forest plots had 59 (40 mosses and 19 liverworts), 15 (12 mosses and 3 liverworts), and 5 (3 mosses and 2 liverworts) species, respectively. Thirty of the 44 moss species were only found in garden plots (68.2% of moss species), and 18 of the 23 liverworts were also limited to garden plots (78.3% of liverwort species). Major species found in garden plots were Atrichum rhystophyllum (Müll. Hal.) Paris, Dicranum nipponense Besch., Leucobryum juniperoideum (Brid.) Müll. Hal., and Polytrichastrum formosum (Hedw.) G.L. Sm. Among them, A. rhystophyllum was also dominant in residential area plots. In forest plots, mosses were scarce and Pseudotaxiphyllum pohliaecarpum (Sull. & Lesq.) Z. Iwats. formed the largest patches. Twelve drought-sensitive species were found in the study sites: 11 species were found in garden plots and one in residential area plots.
The comparison of species richness and cover among the study sites is shown in Fig. 3. Average species richness and cover across all plots were 11.6 ± 7.9 species and 230,214.8 ± 226,137.9 cm2, respectively (mean ± SD). The highest species richness and cover were recorded in garden plots; significant differences were detected between garden and surrounding areas (residential and forest areas; species richness, t = 2.93, df = 15, p = 0.01; cover, t = 3.80, df = 15, p < 0.01; t test). However, when values were compared among the three land use types, only differences in cover between garden and residential area plots were significant (p = 0.0187; multiple comparison).
Landscape elements
Landscape elements were compared among study sites (Table 1), and significant differences based on multiple comparison tests were observed in Ele, Inc, Can_E, Building, and Maint. Garden plots had the highest values for Maint, whereas forest plots had the highest values for Ele, Inc, and Can_E. Garden plots also showed the highest values for Can_D, Und_D, Stream, Pond, and Rock, although differences of these elements were not significant among the sites.
Temperature and RH
Temperature and RH were measured during October–November 2012 (Fig. 4). All types of study sites showed similar trends in the variation of these microclimate parameters. While Temp gradually decreased from 18:00 to 06:00, being lowest at 06:00, RH showed the opposite trend, being highest at 06:00. The average Temp was lowest in the garden plots; in contrast, RH was highest in them.
Temperature and relative humidity among the study sites. Values are averages during the measuring period. All differences in temperature and relative humidity across study sites were significant on the basis of Bayesian 95% credible intervals, except for relative humidity between residential areas and forests
To examine differences in microclimates between the garden and the other land use types, statistical models using a Bayesian framework were employed. The Ck values obtained from Eq. (2) and the Bayesian 95% credible intervals are listed in Table 2. None of the Ck values overlapped within these intervals, except for the RH value between residential areas and forests, indicating that the differences in microclimates between garden and other land use types were significant (p < 0.05).
Next, using RF models, the influence of landscape elements on microclimates (Temp_ave, Temp_06, and Temp_18) was examined (Fig. 5). In RF models for Temps (Temp_ave, Temp_06, and Temp_18), the variances explained were − 20.7, − 29.2, and − 21.7, respectively. However, all the explanatory variables (i.e., landscape elements) demonstrated % Inc MSE values lower than 10%. In the RF models for RHs, the % Inc MSE values of the landscape elements were also smaller than 10% and could not sufficiently explain the models (Fig. 5). On the other hand, because the RF models for RHs included both Temps and landscape elements as explanatory variables, Temps could explain part of these RF models, and the % Inc MSE values of Temps were larger than 10%.
Random forest (RF) analysis for microclimates. The values of percentage increase in mean squared error (% Inc MSE) of explanatory variables are shown. Temp_ave, average temperature; Temp_06, temperature at 06:00; Temp_18, temperature at 18:00; RH_ave, average relative humidity; RH_06, relative humidity at 06:00; RH_18, relative humidity at 18:00; Maint, maintenance; Ele, maximum differences in elevation; Inc, inclination; Can_E, canopy cover of evergreen trees; Can_D, canopy cover of deciduous trees; Und_E, understory cover of evergreen trees; Und_D, understory cover of deciduous trees
Generalized linear models
Bryophyte diversity based on landscape elements and microclimates was explained using GLMs (Table 3). As strong correlations (r > 0.9) were found among Temps and among RHs and VPDs, GLMs were constructed using Temp_ave values as representative of Temps and RH_ave values as representative of RHs and VPDs. Species richness was positively affected by Can_D and Rock, and negatively affected by Temp_ave and Building. Bryophyte cover showed similar trends; however, RH_ave and Maint also had significantly positive influence. All of these models were significant (p < 0.01).
Discussion
The results of this study showed that the species richness and cover of bryophytes were higher in garden plots than in other study sites; in addition, garden microclimates significantly differed from those of surrounding areas. Although RF models indicated that landscape elements did not account for the characteristics of these microclimates, GLMs showed that microclimates significantly affected bryophyte diversity.
Garden landscapes and microclimates
When microclimates were compared, garden plots showed significantly lower Temp and higher RH values than surrounding sites. However, contrary to the expectations, these differences in microclimates were not explained well by local landscape elements within the circular plots (Fig. 5), although the characteristics of garden landscapes as a whole (large vegetation areas and water surfaces; Table 1) correspond to elements that have been suggested to contribute to lower Temp and higher RH values (Saaroni and Ziv 2003; Georgi and Zafiriadis 2006; Liu et al. 2009). These results suggest that whole landscapes across sites, not local landscapes within the circular plots, would affect local microclimates, forming cool and humid conditions within the garden. Conversely, the lowest levels of vegetation cover and water surface areas in the residential area probably contributed to its highest Temp and lowest RH values.
Garden landscapes and bryophyte diversity
The garden showed higher bryophyte species richness and cover than the surrounding areas. How then, did the garden landscape contribute to this increased diversity? In the GLMs, Rock, Can_D, and Maint were selected as positive factors for bryophyte species richness and/or cover. The higher values of these elements in the garden than in surrounding areas (Table 1) suggest that they enhance bryophyte diversity by providing suitable habitats, which is consistent with the results of a previous study (Oishi 2012). Rocky areas are important for bryophyte diversity, owing to the growth preferences of certain species (Oishi 2012). Moreover, as rocky areas are unlikely to be covered with weeds, they can also increase the richness of small species. The other two factors, Can_D and Maint, might contribute to bryophyte diversity by promoting suitable light condition. The brighter light conditions under deciduous forests than under evergreens enable bryophytes to perform photosynthesis effectively, and daily maintenance, such as clearing fallen leaves and weeding, protects bryophytes from being covered by leaves and weeds (Oishi 2012).
In contrast, the GLM results showed that Building had a negative effect on species richness and cover. This negative influence is explained by the unfavorable substrate for bryophytes (e.g., plastic materials) and intensive disturbance (e.g., trampling).
Garden microclimates and bryophyte diversity
According to the GLM models, Temp_ave and RH_ave had significant influences on species richness and cover. This might be explained by the fact that high Temp often reduces bryophyte diversity through a reduction in net photosynthesis (Frahm 1990), whereas low RH inhibits photosynthesis as bryophytes cannot balance their water potential on their own (Karger et al. 2012). Moreover, high RH enhances the frequency of morning dews, which increases photosynthetic gain and mitigates the damage caused by drought stress (Csintalan et al. 2000). Thus, the garden microclimates characterized by lower Temp and higher RH provide suitable habitats for bryophytes, resulting in high bryophyte diversity.
Interestingly, 11 of the 12 drought-sensitive species found in all sites were exclusively present in the garden, including L. elliptica that is designated as endangered because of its high sensitivity to atmospheric drought stress (Kyoto Prefecture 2015). The presence of these species is notable as it suggests a role for Japanese gardens in the conservation of drought-sensitive species. Although the influence of RH on species richness was less pronounced than that of landscape elements in GLMs, the higher RH in gardens may still be meaningful for drought-sensitive species. In particular, the synergism between higher RH and lower Temp in gardens than in other sites can effectively mitigate the influence of drought stress on bryophytes. These effects may also be reflected in the high liverwort species richness found in the garden (Supplementary Material 1), as they are even less drought tolerant than mosses (Vitt et al. 2014).
Bryophyte diversity and its ecological function in urban areas
From an ecological viewpoint, conservation of bryophyte diversity contributes to improve urban biodiversity and for the reinforcement of species resilience, through promoting biological interactions (among bryophytes and among these and other species) and through the physiological properties of bryophytes. For example, the presence of bryophyte communities promotes the establishment of epiphytic ferns in desiccated urban areas, as bryophyte communities hold fern spores and provide sufficient moisture for their fertilization (Mizuno et al. 2015). Additionally, terrestrial bryophytes facilitate infiltration into the soil (Belnap 2006), which can be beneficial for improving biomass and chemical processes in urban soils (Scalenghe and Ajmone-Marsan 2009). The ecological function of bryophytes in relation to water storage is also remarkable; while garden elements can reduce drought stress for bryophytes, these bryophytes may in turn increase humidity within the garden. This is because bryophytes have high water holding capacity (e.g., 665–1470% of dry mass; Michel et al. 2012) and absorb large amounts of atmospheric water relative to their biomass, releasing it gradually to the surrounding environment during the drying process. These functions may contribute to the increase of ecological resilience in urban ecosystems susceptible to drought stress caused by urbanization.
Conclusions
This study showed that the high bryophyte diversity found in a Japanese garden was attributed to its environment. In particular, the microclimates formed by garden landscapes were beneficial for the conservation of drought-sensitive species, which might be explained by the combined influence of garden elements (e.g., large vegetation and water surface areas). Considering that drought stress is a major threat to bryophytes in urban areas, Japanese gardens characterized by these elements can function as conservation sites for drought-sensitive species, increasing urban bryophyte diversity. Such conservation may contribute to the reinforcement of the functional diversity and ecological resilience of urban biodiversity owing to the biological interactions and physiological properties of bryophytes.
References
Aragón G, Abuja L, Belinchón R, Martinez I (2015) Edge type determines the intensity of forest edge effect on epiphytic communities. Eur J For Res 134:443–451
Belnap J (2006) The potential roles of biological soil crusts in dryland hydrology cycles. Hydrol Process 20:3159–3178
Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A (2017) Stan: a probabilistic programming language. J Stat Softw. https://doi.org/10.18637/jss.v076.i01
Csintalan Z, Takács Z, Proctor M, Nagy Z, Tuba Z (2000) Early morning photosynthesis of the moss Tortula ruralis following summer dew fall in a Hungarian temperate dry sandy grassland. Plant Ecol 151:51–54
Daniels GD, Kirkpatrick JB (2006) Does variation in garden characteristics influence the conservation of birds in suburbia? Biol Conserv 133:326–335
Davies ZG, Fuller RA, Loram A, Irvine KN, Sims V, Gaston KJ (2009) A national scale inventory of resource provision for biodiversity within domestic gardens. Biol Conserv 142:761–771
Frahm J-R (1990) Bryophyte phytomass in tropical ecosystems. Bot J Linn Soc 104:23–33
Gelman A, Rubin D (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–511
Georgi NJ, Zafiriadis K (2006) The impact of park trees on microclimate in urban areas. Urban Ecosyst 9:195–209
Goddard MA, Dougil AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 26:90–98
Goddard MA, Dougill AJ, Benton TG (2013) Why garden for wildlife? Social and ecological drivers, motivations and barriers for biodiversity management in residential landscapes. Ecol Econ 86:258–273
Gotsch SG, Davidson K, Murray JG, Duarte VJ, Draguljić D (2017) Vapor pressure deficit predicts epiphyte abundance across an elevational gradient in a tropical montane region. Am J Bot 104:1790–1801
Hasegawa J (2002) Bryophyte flora in Saihoji Temple (Koke-dera). In: Kyoto prefecture (ed) Red data book of Kyoto prefecture 2002, vol 2. Landforms, Geology, and Natural Communities, Kyoto prefectre, Kyoto, pp 292–297 (in Japanese)
Iida Y, Imanishi J, Oishi Y, Morimoto Y (2010) Consideration on suitable microclimate of moss garden based on turf surface moisture dynamics of Polytrichum commune Hedw. J Jpn Inst Landscape Archit 73:407–412 (in Japanese)
Iwatsuki Z (2001) Mosses and liverworts of Japan. Heibonsha, Tokyo (in Japanese)
Iwatuski Z, Kodama T (1961) Mosses in Japanese gardens. Econ Bot 14:264–269
Japan Meteorological Agency (2014) Monitoring reports on heat island. http://www.data.jma.go.jp/cpdinfo/himr/2014/himr2014.pdf. Accessed 11 Jan 2018 (in Japanese)
Japan Meteorological Agency (2017) Past meteorological data. http://www.jma.go.jp/jma/index.html. Accessed 11 Jan 2018 (in Japanese)
Karger DN, Kluge J, Abrahamczyk S, Salazar L, Homeier J, Lehnert M, Amoroso VB, Kessler M (2012) Bryophyte cover on trees as proxy for air humidity in the tropics. Ecol Indic 20:277–281
Knapp S, Kühn I, Wittig R, Ozinga WA, Poschlod P, Klotz S (2008) Urbanization causes shifts in species’ trait state frequencies. Preslia 80:375–388
Kyoto Prefecture (2015) Red data book of Kyoto prefecture. http://www.pref.kyoto.jp/kankyo/rdb/index.html. Accessed 11 Jan 2018 (in Japanese)
Laurance WF, Nascimento HEM, Laurance SG, Andrade A, Ewers RM, Harms KE et al (2007) Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS One 2:e1017
Liu W, You H, Dou J (2009) Urban–rural humidity and temperature differences in the Beijing area. Theor Appl Climatol 96:201–207
Lopatin J, Dolos K, Hernández J, Galleguillos M, Fassnacht FE (2016) Comparing generalized linear models and random forest to model vascular plant species richness using LiDAR data in a natural forest in central Chile. Remote Sens Environ 173:200–210
Loram A, Tratalos J, Warren PH, Gaston KJ (2007) Urban domestic gardens (X): the extent and structure of the resource in five major cities. Landscape Ecol 22:601–615
Michel P, Lee WG, During HJ, Cornelissen JHC (2012) Species traits and their non-additive interactions control the water economy of bryophyte cushions. J Ecol 100(1):222–231
Mizuno T, Momohara A, Okitsu S (2015) The effects of bryophyte communities on the establishment and survival of an epiphytic fern. Folia Geobot 50:331–337
Mizutani M (1975) How to make nice moss-carpets. Proc Bryol Soc Japan 1:134–136 (in Japanese)
Monteith JL, Unsworth MH (2008) Principles of environmental physics, 3rd edn. Academic, Amsterdam
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Naghibi SA, Pourghasemi HR, Dixon B (2016) GIS-based groundwater potential mapping using boosted regression tree, classification and regression tree, and random forest machine learning models in Iran. Environ Monit Assess 188:44
Oishi Y (2009) A survey method for evaluating drought-sensitive bryophytes in fragmented forests: a bryophyte life-form based approach. Biol Conserv 142:2854–2861
Oishi Y (2012) Influence of urban green spaces on the conservation of bryophyte diversity: the special role of Japanese gardens. Landscape Urban Plan 106:6–11
Oishi Y (2015) Kokezanmai. Iwanami Shoten, Tokyo (in Japanese)
Oishi Y, Morimoto Y (2016) Identifying indicator species for bryophyte conservation in fragmented forests. Land Ecol Eng 12:107–114
Pauchard A, Alaback PB (2006) Edge type defines alien plant species invasions along Pinus contorta burned, highway and clearcut forest edges. Forest Ecol Manag 223:327–335
Plascencia M, Philpott SM (2017) Floral abundance, richness, and spatial distribution drive urban garden bee communities. Bull Entomol Res 107:658–667
R Core Team (2017) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.org/. Accessed 25 Nov 2017
Ryu Y-H, Baik J-J (2012) Quantitative analysis of factors contributing to urban heat island intensity. J Appl Meteorol Climatol 51(5):842–854
Saaroni H, Ziv B (2003) The impact of a small lake on heat stress in a Mediterranean urban park: the case of Tel Aviv, Israel. Int J Biometeoro 47:156–165
Scalenghe R, Ajmone-Marsan F (2009) The anthropogenic sealing of soils in urban areas. Landscape Urban Plan 90:1–10
Stan Development Team (2017) RStan: the R interface to Stan. R package version 2.16.2. http://mc-stan.org. Accessed 25 Nov 2017
Vitt DH, Crandall-Stotler B, Wood A (2014) Bryophytes: survival in a dry world through tolerance and avoidance. In: Rajakaruna N, Boyd RS, Harris BT (eds) Plant ecology and evolution in harsh environments. Nova Science, New York, pp 267–295
Zartman CE (2003) Habitat fragmentation impacts on epiphyllous bryophyte communities in Central Amazonia. Ecology 84:949–954
Acknowledgements
I would like to thank Mr. Shugaku Fujita, a chief priest in Saihoji, and the Japan Broadcasting Corporation (NHK) for their cooperation with this study. This study was funded by the Special research funds (C2) from Fukui Prefectural University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oishi, Y. The influence of microclimate on bryophyte diversity in an urban Japanese garden landscape. Landscape Ecol Eng 15, 167–176 (2019). https://doi.org/10.1007/s11355-018-0354-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11355-018-0354-1