Abstract
We studied the effects of soil temperature (7, 15, and 25°C) on the growth and photosynthesis of seedlings of the Japanese larch (Larix kaempferi) and its hybrid larch (L. gmelinii × L. kaempferi) to simulate early stages of regeneration after disturbance. At a soil temperature of 7°C, the root length per unit root biomass, chlorophyll concentration, and photosynthetic nitrogen-use efficiency (PNUE) were markedly lower in the Japanese larch than in the hybrid larch, which may indicate that the hybrid larch is better at acquiring water and nutrients. At ambient temperatures of 17–25°C, the light-saturated photosynthesis rate (P sat) of both seedlings grown at a soil temperature of 7°C was lower than at 15 or 25°C. By the 16th week, the needle area, root area, and biomass in seedlings of both types were lower at a soil temperature of 7°C than at soil temperatures of 15 or 25°C. At a soil temperature of 25°C, P sat and nitrogen uptake were lower in both larch species than at 15°C. The growth of the Japanese larch declined sharply from 15 to 25°C; however, the growth of the hybrid larch decreased only slightly from 15 to 25°C. We conclude that an increased soil temperature may retard larch growth in cold regions, especially in the case of the Japanese larch.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The larch is a typical light-demanding deciduous conifer in cool temperate and boreal forests in the northern hemisphere (Gower and Richards 1990) that is widely used for revegetation and afforestation (Ryu et al. 2009). In northern Japan, a hybrid larch was developed (Gmelin larch, Larix gmelinii, crossed with Japanese larch, L. kaempferi) to better withstand biological stresses and to increase the success of regeneration in bare areas after disturbances (Igarashi et al. 1987; Koike et al. 2000). When both larches reach reproductive age, competition occurs between the Japanese larch and the hybrid larch in regeneration zones since these larches thrive under similar environmental conditions (Qu et al. 2003, 2004a, b, 2005). It is unclear which larch species will dominate in the early stages of regeneration in cool climates.
The regenerated seedlings of the Gmelin larch commence root development at around 5°C, even in the permafrost region of central Siberia (Abaimov et al. 2000; Prokushkin et al. 2002; Korotkii et al. 2002). Thus, we expect that the hybrid larch will acclimatize better and will be more successful than the Japanese larch under similar conditions but with lower soil temperatures. Temperature at the rhizosphere strongly regulates photosynthetic activity (e.g., Strand et al. 2002) through processes such as carbon allocation (Qu et al. 2004a) and root growth (Halter et al. 1997; Häsler et al. 1999; Qu et al. 2003; Wan et al. 1999). The temperature also affects the uptake of nutrients by roots (Fitter et al. 1998). Even in permafrost regions, the Gmelin larch is a dominant tree species (Abaimov et al. 2000; Koike et al. 2000). Larches usually regenerate after forest fires in Russia (Makoto et al. 2007), encountering surface temperatures on the regenerated sites that often reach 30–35°C (Korotkii et al. 2002; Qu et al. 2004b). Both Japanese larch and Gmelin larch can survive soil temperatures of approximately 30°C. However, which species of larch seedling will acclimate to the environment of forest gaps where the soil temperature undergoes large fluctuations remains to be determined.
We studied in detail how below-ground temperatures affect the growth and root–shoot communication of the Japanese larch and the hybrid larch through patterns of carbon allocation and photosynthetic performance. Our work involved experiments to focus on the early stages of regeneration of larch species.
Materials and methods
Plant materials and experimental design
Seeds of the Japanese larch (Larix kaempferi) and its hybrid larch (L. gmelinii × L. kaempferi) were obtained from the Uryu Experimental Forest (44°3′N, 142°1′E) of Hokkaido University. The Japanese larch originated from central Japan, and it has since been planted extensively in northern Japan for timber production because of its rapid growth rate. However, the Japanese larch suffers from shoot-blight disease and is susceptible to grazing damage by voles. The hybrid larch, F1, was therefore developed to provide increased resistance to these problems (Koike et al. 2000; Ryu et al. 2009). Its mother tree, the Gmelin larch (L. gmelinii), originated from the Kuriles and is distributed at the northern extremes of the eastern part of the Eurasian continent (for further information, see Schulze et al. 1995).
Seeds were held at 4°C for 10 days as a cold treatment and were then germinated in a soil medium (clay-loam soil, peat moss, and vermiculite in a 2:2:1 volume ratio). Under natural conditions, needle flushing of the hybrid larch takes place approximately 10 days earlier than in the Japanese larch. The formation of terminal buds and needle coloration also begins 2 weeks earlier in the hybrid larch (Koike et al. 2000).
The present experiment was performed using three temperature regulators for rhizosphere (ADVANTEC LF-680, Toyo Instruments, Chiba, Japan) in a greenhouse at the Field Science Center of Hokkaido University (at 43°W, 130°E). Because of the limited number of regulators, we switched plant materials between the treatments at intervals of approximately 1 week so as to reduce errors among regulators.
A single seedling was planted 2 weeks after germination in each rhizobox (cavity volume about 287 cm3, Eiken Instruments, Tokyo, Japan), which was then wrapped in aluminum foil. Each plastic tray held 40 rhizoboxes; two plastic trays were placed in each temperature regime and rotated at 2- or 3-day intervals to eliminate position effects. The temperatures of the chambers (7 ± 0.2, 15 ± 0.2, and 25 ± 0.2°C) were accurately controlled by a circulating water system. The ambient temperature was 17–25°C, the relative humidity was 40–60%, and the photoperiod was 16 h/day. The photoperiod was adjusted using plant culture lamps (FC4011 GL, Orderic, Tokyo, Japan), so as to give a photosynthetic photon flux (PPF) of approximately 300–350 μmol m−1 s−1, which is typical of the value at the forest edge or in forest gaps of larch plantations (Kitaoka and Koike 2005). We watered the seedlings once a week and did not administer fertilizer during the culture period.
Plant harvesting and measurements
Soil temperature treatments were maintained for 16 weeks. The seedlings were harvested at 4-week intervals. There were six replications for each treatment. At the final harvest, the photosynthetic rate of the seedlings was determined using an open-system infrared gas analyzer (IRGA) (LI-6400, LiCor, Lincoln, NE, USA). The light-saturated rate of CO2 assimilation (P sat) was measured at a CO2 concentration of 360 ppmV. We preset the PPF to 1000, 500, 200, 100, and 0 μmol m−2 s−1, with an LI-6400-02B red (680 nm)/blue (430 nm) light source.
We then calculated the initial slope of the light curves to determine the apparent quantum yield (Φ680), which represents the efficiency of light (photon) fixation capacity. We also determined the convexity of the light–photosynthesis curve (θ), which indicates the sharpness at the transition between the light limitation range and the biochemical limitation range in the photosynthetic light curve (Leverenz et al. 1990; Larcher 2003) as in the formula (Leverenz et al. 1990):
The parameters of this equation are as follows: I, incident light intensity; Φ, initial slope of light curve; θ, convexity; P max, the asymptote or light-saturated rate of photosynthesis; and R d, dark respiration rate. We also evaluated the stomata function using the C i/C a ratio.
All seedlings harvested were categorized as needles, stems, and roots. Root respiration was measured using an LI-6400 and an LI-conifer chamber, which was covered with aluminum foil. The needle area and root area of the fresh needles and roots were determined with an area-measuring system (Hongu, A., MYKA. Lab. ver. 1.01, 1995). Specific leaf area (leaf area per day mass; cm2 g−1) was measured using the projected area of dried needles with an image scanner (FB636U, Canon). The roots were scanned by making photocopies, and root lengths were measured with a digital curvi-meter (Koizumi Sokki, Tokyo, Japan).
Finally, the component parts of the seedlings were dried to a constant mass at 60°C and weighed. Subsequently, the specific root length (i.e., the root length per unit root biomass, or SRL) was calculated. All samples were milled and homogenized. The N concentration in the shoots and roots was determined using an NC analyzer (Shimadzu NC900, Kyoto, Japan). Total soluble sugar and starch contents (concentration, in mg g−1) were determined using the modified phenol–sulfuric acid method and the perchloric acid method (Rose et al. 1991).
Statistical analysis
Statistical tests were performed using the mixed model from SAS (SAS Institute 1996). Individual seedlings within the same temperature treatments were considered to be replicates. A two-way ANOVA was used, and variables included the two species, the three temperature treatments, and the four harvesting dates. Soil temperature was used as a grouping factor, and the date of measurement was a within-factor variable. To test the development of larch species under different soil temperature conditions, we compared the responses of larches across these treatments.
Results
Growth response of seedlings to below-ground temperature
There was a significant difference in the height of shoots between the Japanese larch and its hybrid larch (data not shown; P < 0.05). Specifically, the height of Japanese larch seedlings at below-ground temperatures of 7°C at 16 weeks was about 65 and 59% of the values for Japanese larch seedlings grown at temperatures of 15 and 25°C, respectively; for the hybrid larch, the corresponding figures were 72 and 65%, respectively.
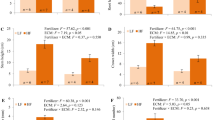
Root areas (cm2) did not differ significantly between the Japanese larch and the hybrid larch at 16 weeks (P > 0.05 data not shown). In the Japanese larch, root areas at 7°C soil temperatures (5.6 ± 1.0) differed significantly (P < 0.01) from values at 15°C (16.7 ± 2.1) and 25°C (15.2 ± 4.0). A similar response to temperature was found in the hybrid larch, for which the root area was higher at 15°C (10.6 ± 2.8) and 25°C (10.8 ± 2.8) than at 7°C (4.4 ± 0.5). Consequently, the SRL (i.e., the root length per unit root biomass, cm mg−1; Fig. 1) at 7°C was markedly lower in the Japanese larch than in the hybrid larch throughout the growing season. The SRL values of the Japanese larch were 2.58, 2.80, and 3.51 at below-ground temperatures of 7, 15, and 25°C, respectively. The SRL values of the hybrid larch were 2.85, 2.45, and 2.90 at temperatures of 7, 15, and 25°C, respectively. There was also a decline in the SRL from the beginning to the end of the season in both species.
Specific root length of Japanese larch (JL) and hybrid larch (HL) seedlings grown at soil temperatures of 7, 15, and 25°C for 16 weeks. Different letters indicate an intraspecific statistical difference in means (P = 0.05). An asterisk indicates a significant difference (P < 0.05) between the two species under the same temperature conditions
Japanese larch seedlings grown at a below-ground temperature of 7°C had smaller needle areas (5.0 ± 0.8 cm2) than seedlings grown at temperatures of 15°C (12.6 ± 2.0 cm2) or 25°C (11.9 ± 2.6 cm2; data not shown). At the end of the growing season, the needle areas (cm2) of the hybrid larch seedlings were 3.3 ± 0.2, 7.0 ± 2.1, and 7.0 ± 2.4 cm2 at soil temperatures of 7, 15, and 25°C, respectively.
Biomass partitioning
Root mass (mg plant−1) of the Japanese larch at 7°C increased by a factor of 5.6 from its initial value, whereas the hybrid larch increased 8.0 times (P < 0.01; Fig. 2). At 15°C, the root mass of the Japanese larch increased 13.2 times, and the hybrid larch increased 21.4 times from initial values. At 25°C, the root masses of the Japanese larch and the hybrid larch increased 12.3 times and 22.2 times from initial values, respectively.
Shoot biomass and root biomass of two larch seedlings grown at below-ground temperatures of 7, 15, and 25°C, for 4, 8, 12, and 16 weeks. Values are means ± SE of six seedlings. Data within a series marked by the same letter do not differ significantly (P < 0.05) between the Japanese larch (JL) or hybrid larch (HL)
In the Japanese larch, the shoot dry mass (mg plant−1) was significantly higher at a soil temperature of 15°C (147.9 ± 25.1) than at 7°C (58.1 ± 3.5) or 25°C (123.7 ± 48.7) (P < 0.05). For the hybrid larch, the shoot dry mass values were 46.0 ± 3.2, 124.1 ± 38.9, and 119.3 ± 38.0, respectively, at soil temperatures of 7, 15, and 25°C (Fig. 2). The Japanese larch had a higher SLA than the hybrid larch at both temperatures (Table 1). In both species, the highest SLA values were observed at 7°C (124.20 ± 0.28 cm2 g−1 in the Japanese larch and 114.45 ± 0.11 cm2 g−1 in the hybrid larch), and the lowest SLA values were recorded at 15°C (109.10 ± 0.08 cm2 g−1 in the Japanese larch, 78.68 ± 0.07 cm2 g−1 in the hybrid larch) (data not shown).
Photosynthesis and respiration of root and shoot
Root respiration rate (μmol m−2 s−1) was strongly influenced by the soil temperature (Fig. 3). With increasing soil temperature, the root respiration rates increased significantly from approximately 0.46 and 0.26 at 7°C, through approximately 0.56 and 0.40 at 15°C, to approximately 0.85 and 0.51 at 25°C in the Japanese larch and the hybrid larch, respectively.
Root respiration rates of seedlings of the Japanese larch (JL) and its hybrid larch (HL) grown at soil temperatures of 7, 15, and 25°C for 16 weeks. Different letters indicate an intraspecific statistical difference in means (P = 0.05). An asterisk indicates a significant difference (P < 0.05) between the two species under the same temperature conditions. Vertical bars represent the SE (n = 6)
By the 16th week, no interaction effect was observed in species according to the soil temperature, and the P sat value for the seedlings was lower at 7°C than at 15 or 25°C in both larch species (Table 2). Japanese larch and hybrid larch seedlings grown at a below-ground temperature of 15°C exhibited a higher photosynthetic rate than seedlings grown at 7 or 25°C. At a soil temperature of 25°C, the P sat values of seedlings of both larch species decreased compared with those at a below-ground temperature of 15°C. However, this tendency was more pronounced in Japanese larch seedlings. The P sat value of the hybrid seedlings at a soil temperature of 15°C was slightly lower than that of seedlings in the 25°C treatment group (Table 2).
Generally, the quantum yield (Φ680 nm) of the Japanese larch under the three different temperature scenarios was slightly larger than that of the hybrid larch (P < 0.01). With an increasing soil temperature, Φ680 nm also increased in both larches. Φ680 nm was about three times larger, independent of species, between 7 and 25°C. Significant differences in the θ value (convexity; dimensionless) were not observed within each larch species at the different temperatures, while interspecies differences were observed: the θ value of the Japanese larch was larger than that of the hybrid larch at 15°C (P < 0.05). No difference was found in the two species in the 7 and 25°C treatments. There was no difference in the ratio of C i/C a (μmol μmol−1) across either species or treatment temperatures. However, the C i/C a of the Japanese larch at 25°C exhibited the smallest value of 0.19, and all others were in the range of 0.32–0.63.
Nitrogen concentration, chlorophyll, and photosynthetic nitrogen-use efficiency
There were significant differences in the N content of needles, stems, and roots in both larch species according to soil temperature as shown in Table 1 (P < 0.05). The N content was lower in the stem than in needles or roots. In Japanese larch seedlings, the N content of needles at a soil temperature of 7°C was significantly lower than at 25°C. N contents of roots were significantly lower at 25°C compared to other treatments for both species. However, there was no significant difference in the root N content in seedlings of either larch species at soil temperatures of 7 and 15°C. The N content of needles was lowest at a soil temperature of 25°C in the hybrid larch seedlings (Table 1).
The photosynthetic nitrogen-use efficiency (PNUE) also exhibited differing patterns in the two larch species (Fig. 4). The hybrid larch had a higher PNUE than the Japanese larch across all temperature treatments. The greatest PNUE of seedlings was found at a below-ground temperature of 25°C in the hybrid larch and at 15°C in the Japanese larch.
At the end of the growing season, the chlorophyll concentration at a below-ground temperature of 7°C was lower than at 15 or 25°C for the Japanese larch (Fig. 5). By contrast, the chlorophyll concentration of hybrid larch seedlings was lowest at a soil temperature of 25°C. Moreover, the chlorophyll concentration of the Japanese larch at a below-ground temperature of 7°C was significantly lower that of the hybrid larch (Fig. 5).
Chlorophyll a + b (Ca + b) (mg mm−2) of Japanese larch (JL) and hybrid larch (HL) seedlings grown at soil temperatures of 7, 15, and 25°C for 16 weeks. Different letters indicate an intraspecific statistical difference in the means (P = 0.05). An asterisk indicates a significant difference (P < 0.05) between the two species under the same temperature conditions
Soluble sugar and starch concentration
The concentration of soluble sugar in Japanese larch seedlings grown at a below-ground temperature of 7°C was 9.1% of the dry mass, significantly higher than the values of 5.0% observed at 15°C and of 4.6% observed at 25°C (Table 3; P < 0.01). In contrast to the Japanese larch, the concentrations of soluble sugar in seedlings of the hybrid larch displayed no significant differences between soil temperatures. The soluble sugar concentration in hybrid larch needles grown at the below-ground temperatures of 7, 15, and 25°C were 10.2, 10.5, and 10.1%, respectively.
In the hybrid larch, although the soluble sugar concentration in the roots was highest at a soil temperature of 7°C, no difference was found for this parameter at temperatures of 15 and 25°C. However, hybrid larch seedlings had a significantly higher starch concentration at a below-ground temperature of 7°C than at 15 and 25°C (Table 3).
Discussion
The hybrid larch did not reduce its growth to the same extent as the Japanese larch at a soil temperature of 25°C. However, a declining growth trend, especially evident in the chlorophyll values, was observed in the hybrid larch at a 25°C soil temperature. Although all P sat values were lower, as we expected because of the low cultivation PPF (300–350 μmol m−2 s−1) that simulated the light condition of intermediate-sized forest gaps (Qu et al. 2005), P sat values of both larch seedlings were also reduced at a soil temperature of 7°C (Table 2). The chlorophyll content of needles of the Japanese larch at a below-ground temperature of 7°C was significantly lower than at temperatures of 15 and 25°C, due to a reduced photosynthetic rate given the low soil temperature and the stressful environment (e.g., Körner 1999; Larcher 2003; Qu et al. 2005).
Even though larches can acclimate to cool climatic conditions (Gower and Richards 1990), the biomass of the two larch seedlings increased markedly with increasing temperature (Fig. 1). However, the P sat of Japanese larch seedlings at a below-ground temperature of 15°C was higher than that of seedlings grown at a 25°C soil temperature. As a result, their size and biomass were larger at a below-ground temperature of 15°C than at 25°C, which may be attributed to the suppressed root respiration at lower temperatures.
The Japanese larch accumulated about twice as much soluble sugar in shoot and root biomass at a below-ground temperature of 7°C than at temperatures of 15 or 25°C. At a soil temperature of 7°C, the hybrid larch seedlings exhibited a significantly higher starch concentration in roots than at temperatures of 15 or 25°C (Table 3). In general, the observed increase in soluble carbohydrates, particularly starch, at low temperatures indicates that P sat persisted even at lower temperatures but that growth did not. Thus, the unused carbohydrates may be stored as starch to increase frost hardiness (Domisch et al. 2001; Farrar 1998; Korotkii et al. 2002; Larcher 2003). Our data indicate that both larch seedlings can survive at a below-ground temperature of 7°C with a high accumulation of carbohydrates. However, their growth was impaired by the low soil temperature.
At a soil temperature of 7°C, the root masses of both larch seedlings were significantly lower than at 15 or 25°C (Fig. 2). This fact clearly indicates that exposure to low soil temperature reduces root extension through low photosynthetic capacity as found in several plants (Domisch et al. 2001; Halter et al. 1997; Koike et al. 2003; Larcher 2003; Landhäusser et al. 2001; Korotkii et al. 2002; Qu et al. 2004b). Moreover, at a soil temperature of 7°C, the Japanese larch had a smaller SRL than the hybrid larch (Fig. 1). SRL reflects the ability of a plant to compete for resources such as nutrients and water (Qu et al. 2003). Plants can increase their capacity for nutrient and water uptake by producing a longer root for a given root mass, i.e., by increasing their SRL (Kroon and Visser 2003). The higher SRL of the hybrid larch at a lower soil temperature suggests that this species is superior to the Japanese larch in acquiring water and nutrients at an early stage of regeneration. In fact, the high SLA value in the hybrid larch may save the carbon allocation to needles under low soil temperature of 7°C. The acquisition of water and nutrients may increase due to a low cost of root respiration energy for the hybrid larch as compared with the Japanese larch.
In terms of PNUE characteristics, the PNUE of the hybrid larch was higher at low soil temperatures. In our results, at a soil temperature of 7°C, the hybrid larch exhibited low photosynthetic rates, but interestingly, the mass-based PNUE of the hybrid larch was higher than that of the Japanese larch at all of the three studied soil temperatures, including 7°C. It is unclear why higher PNUE values were observed in the hybrid larch even at low soil temperatures.
In comparison with the Japanese larch, root growth of the hybrid larch at 7°C was relatively low, which may suggest the suppression of metabolism through root respiration and absorption of nitrogen. Accordingly, the hybrid larch maintained a higher PNUE than the Japanese larch across all three temperature ranges. It has been widely reported that an increase in growth is usually accompanied by increasing respiration to produce more energy (Field and Mooney 1986; Lambers et al.1998; Larcher 2003). It has been suggested that the hybrid larch may efficiently allocate nitrogen to photosynthetic organs to maintain a high PNUE by reducing the respiratory consumption of roots in the case of low soil temperature until more favorable conditions arise. The hybrid larch may compensate for its low Φ680 nm and θ of the light-photosynthetic curve at a soil temperature of 7°C (Table 2). The chlorophyll content of the hybrid larch was significantly higher than that of the Japanese larch (Fig. 5). Increasing the chlorophyll content is one of the most efficient means of nitrogen allocation under low light conditions (Kitaoka and Koike 2004), and the hybrid larch and the Japanese larch show similar responses to low irradiance by acclimating to better utilize available nitrogen resources. From these findings, we would expect the hybrid larch to be more competitive than Japanese larch in dry and infertile soil conditions (Qu et al. 2003), even in the context of low soil temperatures.
In conclusion, the growth of the Japanese larch and hybrid larch was inhibited by low soil temperature through a lower photosynthetic rate and biomass production, as compared to results from a below-ground temperature of 15°C. Both larch seedlings survived at a soil temperature of 7°C with a high accumulation of carbohydrates. However, the higher SRL, chlorophyll concentration, and PNUE of the hybrid larch at lower soil temperatures may suggest that it is more efficient than the Japanese larch at acquiring water and nutrients. The growth of the Japanese larch was significantly impaired at a below-ground temperature of 25°C, while the growth of the hybrid larch was not noticeably altered. Thus, an increase in soil temperature from 3 to 5°C would significantly retard the growth of larches in cold regions by increasing root respiration, especially in the Japanese larch, although its natural distribution is farther south than that of the Gmelin larch. In this sense, the hybrid larch may benefit from increased growth at early stages of regeneration at increasing temperatures.
References
Abaimov AP, Ayryanova OA, Prokushkin SG, Koike T, Matsuura Y (2000) Forest ecosystems of the cryolithic zone of Siberia: regional features, mechanisms of stability and pyrogenic changes. Eurasian J For Res 1:1–10
Domisch T, Finér L, Lehto T (2001) Effects of soil temperature on biomass and carbohydrate allocation in Scots pine (Pinus sylvestris) seedlings at the beginning of the growing season. Tree Physiol 21:465–472
Farrar JF (1998) Temperature and the partitioning and the translocation of carbon. Symp Exp Biol 42:203–236
Field C, Mooney HA (1986) The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
Fitter AH, Graves JD, Self GK, Brown TK, Bogie DS, Taylor K (1998) Root production, turnover and respiration under two grassland types along an altitudinal gradient: influence of temperature and solar radiation. Oecologia 114:20–30
Gower ST, Richards JH (1990) Larches: deciduous conifers in an evergreen world. Bioscience 40:818–826
Halter R, Sands R, Ashton DH, Nambiar EKS (1997) Root growth of subalpine and montane Eucalyptus seedlings at low soil temperature. Trees 12:35–41
Häsler R, Streule A, Turner H (1999) Shoot and root growth of young Larix decidua in contrasting microenvironments near the alpine timberline. Phyton 39:47–52
Igarashi T, Yajima T, Matsuda K, Natsume S, Takikawa S (1987) Natural regeneration in Japanese larch plantation. Res Bull Res For Hokkaido Univ 44:1019–1040
Kitaoka S, Koike T (2004) Invasion of broadleaf tree species into a larch plantation: seasonal light environment, photosynthesis, and nitrogen allocation. Physiol Plant 121:604–611
Kitaoka S, Koike T (2005) Seasonal and yearly variations in light use and nitrogen use by seedlings of four deciduous broad-leaved tree species invading larch plantations. Tree Physiol 25:467–475
Koike T, Yazaki K, Funada R, Maruyama Y, Mori Y, Sasa K (2000) Forest health and vitality in northern Japan—a history of larch plantation. Res Note Fac For Univ Joensuu 92:49–60
Koike T, Kitao M, Quoreshi AM, Matsuura Y (2003) Growth characteristics of root–shoot relations of three birch seedlings raised under different water regimes. Plant Soil 255:303–310
Körner Ch (1999) Alpine plant life. Springer, Heidelberg
Korotkii T, Prokushkin SG, Matsuura Y, Koike T (2002) Effects of soil temperature on the content of nitrogen compounds in seedlings of Larix gmelinii regenerated on permafrost in central Siberia. Eurasian J For Res 5:39–48
Kroon HDE, Visser EJW (2003) Root ecology. Springer, Heidelberg
Lambers H, Champin III FS, Pons TL (1998) Physiological plant ecology. Springer, New York
Landhäusser SM, DesRochers A, Lieffers VJ (2001) A comparison of growth and physiology in Picea glauca and Populus tremuloides at different soil temperatures. Can J For Res 31:1922–1929
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional groups. Springer, Berlin
Leverenz JW, Falk S, Pilström C-M, Samuelsson G (1990) The effects of photoinhibition on the photosynthetic light-response curve of green plant cells (Chlamydomonas reinhardtii). Planta 182:161–168
Makoto K, Nemilostiv YP, Zyryanova OA, Kajimoto T, Matsuura Y, Yoshida T, Satoh F, Sasa K, Koike T (2007) Regeneration after forest fires in mixed conifer broad-leaved forests of the Amur region of Far Eastern Russia: the relationship between species specific traits against fire and recent fire regimes. Eurasian J For Res 10:51–58
Prokushkin SG, Prokushkin AM, Stasova VV, Mori S, Sakamoto Y, Quoreshi AM, Koike T (2002) Reaction of Larix gmelinii roots under low soil temperatures in northern parts of central Siberia. Eurasian J For Res 4:25–28
Qu LY, Quoreshi AM, Koike T (2003) Root growth characteristics, biomass and nutrient dynamics of seedlings of two larch species raised under different fertilization regimes. Plant Soil 255:293–302
Qu LY, Shinano T, Quoreshi AM, Tamai Y, Osaki M, Koike T (2004a) Allocation of 14C-Carbon in two species of larch seedlings infected with ectomycorrhizal fungi. Tree Physiol 24:1369–1376
Qu LY, Kayama M, Kitaoka S, Akasaka M, Sasa K, Koike T (2004b) Micro-environmental analysis of the natural regeneration of larch forest in northern Japan. Eurasian J For Res 7:3–51
Qu LY, Ji DH, Shi FC, Sasa K, Koike T (2005) Growth and photosynthetic performance of seedlings of two larch species grown in shaded conditions. Eurasian J For Res 8:43–51
Rose R, Rose CL, Omi SK, Forry KR, Durall DM, Bigg WL (1991) Starch determination by perchloric acid vs enzymes: evaluating the accuracy and precision of six colorimetric methods. J Agric Food Chem 39:2–11
Ryu K, Watanabe M, Shibata H, Takagi K, Nomura M, Koike T (2009) Ecophysiological responses of the larch species in northern Japan to environmental changes as a base of afforestation. Landscape Ecol Eng. doi:10.1007/s11355-009-0063-x
SAS Institute (1996) SAS/STAT user’s guide. SAS Institute, Cary, NC
Schulze ED, Schulze W, Kelliher FM, Vygodskaya NN, Zieger W, Kobak II, Koch H, Arneth A, Kusnetsova WA, Sogatchev A, Lsajev A, Bauer G, Hollinger DY (1995) Aboveground biomass and nitrogen nutrient in a chronosequence of pristine Dahurian Larix stand in eastern Siberia. Can J For Res 25:943–960
Strand M, Lundmark T, Söderbergh I, Mellander PE (2002) Impacts of seasonal air and soil temperatures on photosynthesis in Scots pine trees. Tree Physiol 22:839–847
Wan X, Landhäusser SM, Zwiazek JJ, Lieffers VJ (1999) Root water flow and growth of aspen (Populus tremuloides) at low root temperatures. Tree Physiol 19:879–884
Acknowledgments
We thank Dr. A. M. Quoreshi, Mr. T. Ueda, Mr. Y. Miyamoto, and Mr. A. Okuya for technical assistance and Dr. T. Hasegawa for help with statistical analyses. Financial support for L. Qu from the National Natural Science Foundation of China (No. 30700639) and from the JSPS Grant-in-Aid (Basic Research B; 20380083) for T.K. is gratefully acknowledged. This work is a contribution to the Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, L.Y., Kitaoka, S., Makoto, K. et al. Root–shoot communication of the seedlings of Japanese larch and a hybrid species grown in different soil-temperature regimes. Landscape Ecol Eng 5, 115–123 (2009). https://doi.org/10.1007/s11355-009-0071-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11355-009-0071-x