Abstract
Purpose
Myocardial injury induced by ischemia–reperfusion is the main pathological contributing factor of cardiovascular disease (CVD) including heart failure. Individually, both moderate-intensity aerobic exercise and curcumin supplementation have anti-amyloidogenic effects, however, the concurrent effects are unclear. We, therefore, investigated the effects of 10 weeks of moderate-intensity aerobic exercise with and without curcumin supplementation in rats.
Methods
Male Wistar rats (6–8 weeks old) were randomly assigned to one of five groups (N = 10): (a) sedentary control; (b) sedentary ischemia–reperfusion; (c) exercise (15–45 min at 12–24 m/min) with ischemia–reperfusion; (d) curcumin (50 mg/kg/day) + ischemia–reperfusion; and (e) both exercise and curcumin + ischemia–reperfusion. Infarct size, gene expression of amyloid precursor protein, enzymes involved in the cleavage of amyloid precursor protein (β-secretase-1, presenilin-1 and -2) and neprilysin, a marker of degradation of β-amyloid peptide in the left ventricle of rats were investigated.
Results
A reduction in mRNA expression of amyloid precursor protein, β-secretase-1, presenilin-1 and -2 and infarct size, and an increase in gene expression of neprilysin in the myocardium occurred in both the exercise- and the curcumin-treated rats with no further benefit with concurrent treatments.
Conclusion
Exercise and curcumin individually provided cardioprotective effects against ischemia–reperfusion-induced injury which appears to be associated with an attenuation in mRNA expression of β-amyloid peptide precursor in addition to processing enzymes and an increase in mRNA expression of neprilysin. There were no further benefits with concurrent treatments (exercise with curcumin) compared to either treatment alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure, or congestive heart failure, is a progressive cardiovascular disease (CVD) leading to high rates of morbidity and mortality, with a large economic burden [1, 2]. Myocardial injury induced by ischemia–reperfusion is the primary pathological contributing factor of heart failure [3]. Ischemia–reperfusion-induced injury reduces nutrient and oxygen delivery subsequently causing hyperemia, inflammation, and oxidative stress. These metabolic and inflammatory responses result in adverse myocardial remodeling and an increased risk of progression to heart failure [4, 5]. Adverse myocardial remodeling is defined as “a group of molecular, cellular and interstitial changes that clinically manifest as changes in size, shape, and function of the heart resulting from cardiac injury” [6]. At the molecular level, functional alterations of several proteins and cardiac gene expression influence myocardial remodeling of the left ventricle [2].
β-Secretase-1 (BACE1) is a rate-limiting enzyme for the synthesis of β-amyloid peptide (Aβ), which is also involved in the pathogenesis of Alzheimer’s disease [7]. Aβ 1–40 and 1–42 are the most common isoforms of Aβ. Importantly, age-related diseases such as Alzheimer’s and CVD have common environmental and genetic risk factors but also share several molecular mechanisms [8]. For example, plasma Aβ 1–40 is significantly increased in CVD and is associated with a higher risk of CVD mortality [2]. Further, BACE1 increases in response to ischemia, hypoxia, and cellular stress and Aβ 1–40 peptides are produced by proteolytic cleavage of amyloid precursor protein (APP) activated by BACE1 and γ-secretase [8]. BACE1 cleaves APP to produce soluble APPβ (sAPPβ) and a membrane-associated C-terminal fragment of APP (C99). C99 undergoes a series of cleavage events induced by proteolytic components of γ-secretase (presenilin-1 and -2 [PS-1 and PS-2]) to yield Aβ [7, 9, 10]. Moreover, there are enzymes that degrade extracellular peptides. Neprilysin (NEP), a zinc metalloendopeptidase has been identified as a degradation factor for catabolism of Aβ. There is a negative correlation between NEP levels and Aβ production [11].
Expression of BACE1 and Aβ proteins is upregulated in the left ventricle of heart failure patients, suggesting a pervasive role [2]. Further, Aβ has a toxic and pro-apoptotic effect on endothelial cells as well as cardiomyocytes in both humans and mice [2]. Aβ triggers a cascade of pro-inflammatory events in cells and macrophages causing oxidative stress and CVD [8]. Therefore, strategies to mitigate Aβ production through controlling Aβ precursor (APP) and/or the enzymes involved in the cleavage of APP (BACE1, PS-1, and PS-2), or via altering the expression of NEP are warranted.
Cardioprotective effects of exercise have been well recognized and physical activity has been recommended for CVD prevention and treatment globally [12]. Beyond exercise, dietary strategies including herbs are gaining popularity in the management of CVD [13]. One purported herb is curcumin, which is a natural polyphenol extracted from the root of Curcuma longa which has anti-apoptosis, antioxidant and anti-inflammatory effects and accumulating research has revealed a role in alleviating myocardial apoptosis and improved cardiac function [14,15,16]. Curcumin attenuates ischemic injury by decreasing the expression of apoptotic genes in rats [3, 15, 16].
Currently, there is limited research examining the cardioprotective effects of exercise with curcumin supplementation [17, 18], however, there is a lack of research investigating the effects on Aβ precursors and related enzymes induced by ischemia–reperfusion. Accordingly, the aim of the present study was to investigate the effects of 10-weeks of moderate-intensity aerobic exercise along with curcumin supplementation on gene expression. We hypothesized, exercise and curcumin concurrently will further decrease ischemia–reperfusion-induced infarct size and gene expression of APP, BACE1, PS-1, and PS-2 and will increase gene expression of NEP in rats compared to either strategy individually or controls.
Materials and methods
Subjects
Fifty male Wistar rats (6–8 weeks old, 200–250 g) were used in this experiment. The rats were housed in rodent cages under standard conditions (temperature 21–24 °C, humidity 40–50%, and a 12:12 light/dark cycle) in a well-ventilated and clean room. All animals had free access to a standard diet and water. The present study was approved by the Animal Ethics Committee of Hamedan University of Medical Sciences (No. IR. BASU. REC. 1398.044), and all experiments were conducted in accordance with the Institutional Animal Care and Use Committee guidelines.
Exercise protocol
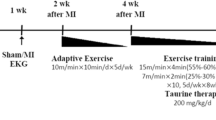
Rats were randomly assigned into five groups (each group had n = 10): sedentary-control (Sed-Con), sedentary ischemia–reperfusion (Sed-IR), exercise with ischemia–reperfusion (Ex-IR), curcumin with ischemia–reperfusion (Cu-IR), and both exercise and curcumin with ischemia–reperfusion (Ex-Cu-IR). The sample size was determined a priori using G*Power software [19]. One week prior to experiment initiation, the rats in the Ex-IR and Ex-Cu-IR groups completed a familiarization-training session on a motor-driven horizontal treadmill (BIOSEB, Vitrolles, France) which included 10-min of running at 10 m/min. Animals were then forced to run five sessions a week for 10 weeks. The treadmill running program began at 12 m/min for 15 min/day on week 1 and progressively increased to 24 m/min for 45 min/day (representing around 55–60% of V̇O2max) until week 7, and thereafter remained constant (Fig. 1). Exercise intensity during each week was performed based on previously reported protocols [20, 21].
Curcumin supplementation
The Cu-IR and Ex-Cu-IR (1 h before exercise) [3, 14] groups ingested daily oral gavage of 50 mg/kg of curcumin extract (8.20354.0010.Merck KGaA, Germany) dissolved in olive oil five times a week for 10 weeks.
Surgical preparation and tissue processing
Two days after the last session of training or curcumin treatment, the animals were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg) and xylazine (8 mg/kg). The thoracic area was shaved, and the cervical region accurately positioned to facilitate endotracheal intubation. Intubated rats were then connected to a small animal ventilator (Harvard Model 683, USA) and mechanically ventilated with a respiratory rate of 60–70 breaths/min and a tidal volume of 1.5 cc/kg. Subsequently, an incision was made on the chest to expose the heart while avoiding any cardiac damage. Next, the pericardium was gently cut, and a 6–0 silk suture was carefully passed beneath the left anterior descending artery (LAD). Ischemia was induced by retracting and complete ligation by the suture with a tourniquet. To detect laboratory-induced infarction in the rats, lead II electrocardiogram (EKG) recordings (HARVARD-USA) were captured using subcutaneous needle electrodes. Ischemia induction was confirmed by ST segment changes on EKG and impaired myocardial contraction. Following 30 min of ischemia, myocardial reperfusion was resumed by releasing the ligature. Upon reperfusion, the chest and its associated muscle layers were sutured closed, and tetracycline ointment was topically applied onto the surgical site to prevent postoperative infections and contribute to the wound healing process. After extubation, the rats were oxygenated and kept warm until full recovery from anesthesia. Then, they were caged, provided with food and water, and transferred to the animal house. Five days after myocardial reperfusion, the rats underwent a second surgery to collect tissue specimens from their myocardium. Following thoracotomy under anesthesia with an intraperitoneal injection of ketamine (60 mg/kg) and xylazine (8 mg/kg), the heart was excised, rinsed with normal saline, and weighed on the digital scale after the atria and great vessels were dissected away. Then, the left ventricular myocardium was harvested from the heart, immediately frozen in liquid nitrogen, and stored in an RNase-free microtube at − 80 °C for later analysis of gene expression changes. The control group had a similar surgery except for the LAD ligation. The hearts used to determine the infarct size were the same as those used to extract RNA. Further, the myocardial samples were always taken in the same area in all the hearts, and this corresponded with the left ventricle. Two subjects did not survive the surgery and were excluded from the analyses.
RNA extraction and real-time PCR
First, 50–100 μg of the frozen left ventricle tissue was ground into a powder using a mortar and pestle with liquid nitrogen, re-suspended with 1000 μL of lysis buffer (TRIzol Reagent, Invitrogen, Waltham, MA, USA), and transferred into a microtube. Then, 300 μL of chloroform was added to the mixture, which was subsequently vortexed to become milky in color. After 15 min of incubation on ice, the homogenate was centrifuged at 12,000g for 15 min at 4 °C, and the supernatant was completely collected and transferred to a new microtube, to which 500 μL of isopropanol was added, followed by pipetting only once. Afterward, the solution was incubated on ice for 10 min and centrifuged at 12,000g for 15 min at 4 °C. Next, the supernatant was discarded, and the RNA pellet was washed with 1000 μL of 70% ethanol and subjected to centrifugation at 7500g for 10 min at 4 °C. Once the alcohol was poured off, the final RNA pellet was air-dried for 10 min, dissolved in 30–40 μL of DEPC-treated water, placed on ice for 5 min, and stored at − 80 °C. RNA concentration was checked using a Nanodrop reader (Thermo Fisher Scientific Inc, Waltham, MA), with a concentration of 1000 mg/μL selected as the reference value. That is, if the concentration of RNA was 1000 mg/μL, 1 μL of the purified RNA sample was used to generate cDNA. For cDNA synthesis using the Easy™ cDNA Synthesis kit (Parstous, Mashhad, Iran), 0.5–2 μL of total RNA (depending on its concentration) was mixed with 10 μL of Buffer Mix (2×), 2 μL of enzyme mix, and DEPC-treated water to reach a final volume of 20 μL. Subsequently, the entire mixture was incubated in the thermocycler as follows: 25 °C for 10 min, 47 °C for 60 min (cDNA synthesis by the enzyme reverse transcriptase), and 85 °C for 5 min (RT inactivation). Finally, the samples were frozen and stored at − 20 °C. To quantify the gene expression of APP, BACE1, PS1, PS2, and NEP, real-time PCR was performed using the 2× real-time PCR Master Mix kit (including SYBR® Green I) (BioFACT™, South Korea) on a Rotor-Gene 3000 system under the following conditions: initial denaturation at 95 °C for 15 min, denaturation at 95 °C for 20 s, and elongation at 60 °C for 40 s (the last two steps were repeated 30–50 times). Primer sequences for the genes APP, PS1, PS2, BACE1, NEP, and β-actin were designed as follows: APP (F: 5′-GCTGCTGACCGAGGACTGAC-3′; R: 5′-GATGGCACCTTTGTTTGAACCC-3′), BACE1 (F: 5′-CGCCGTCTCACAGTCATCCAC-3′; R: 5′-CCGCCGTCCTGAACTCATCG-3′), PS1 (F: 5′-TCTACAGTGTTCTGGTTGGTAAGG-3′; R: 5′-AAATGAGCCCGAAGGTGATGG-3′), PS2 (F:5′-CGGAGGAGGAGGAGGAAAGG-3′; R: 5′-TAGACACAAGCCAATGAGGATG-3′), NEP (F: 5′-AGATGGAGACCTCGTTGACTGG-3′; R: 5′-TTC TGA TAGGCTCTGTATGCTTGG-3′), and β-actin (F: 5′-CCACACTTTCTACAATGAGC-3′; R: 5′-ATACAGGGACAACACAGC-3′). Besides, changes in the relative gene expression levels were calculated using the 2−ΔΔCt method.
Determination of infarct size
The frozen heart was sliced transversely into slices of 3 mm width using a heart matrix and stained with 1% 2, 3, 5-triphenyltetrazolium chloride solution (TTC; in 0.1 M phosphate buffer; pH = 7.4) (TTC, Sigma, St. Louis, MO, USA) for 15 min at 37 °C to visualize the infarct zone and were subsequently fixed in 10% formalin for 24 h. Both surfaces of each section were photographed with a digital camera connected to a simple microscope to quantify the infarct area (pale pink or white) and non-infarct area (red) via Adobe Photoshop software (version 7.0, Adobe Systems). Finally, myocardial infarct size was reported as a percentage of the left ventricular area (Fig. 2).
Percentage of infarct size in the experimental groups. SED sedentary, IR ischemia–reperfusion, EX exercise, CU curcumin. †Indicates significant difference compared to Sed + IR group. *Indicates significant difference compared to Cu + IR group. Yellow arrow indicates infarct area (pale pink or white) and red arrow indicates non-infarct area (red). Values are means with SEM represented by vertical bars
Statistical analyses
All data are reported as mean ± SEM. The Shapiro–Wilk test was used to test for normality. Group differences were determined by one-way analyses of variance (ANOVA) followed by Tukey’s honestly significant difference post hoc test when a significant F-ratio was observed. Comparison of percentages of infarct size were made using Chi-square test. Statistical analyses were performed using IBM SPSS Statistics 20 for Windows (IBM Corp., Chicago, IL) with significance set at an α level < 0.05.
Results
Characteristics of the experimental groups
Post-experiment characteristic of the experimental groups is shown in Table 1. Following the experiment, no significant differences were observed between groups for body weight and heart-to-body weight ratio (g/g) × 102 (P = 0.9, and 0.09, respectively). A significant difference (F = 7.72, P = 0.0001) was found for heart weight among groups. Heart weight (g) was significantly heavier in the Ex-Cu-IR group compared to the Sed-Con, Sed-IR, and Cu-IR groups (P = 0.002, 0.004, and 0.002, respectively).
Infarct size
After the 10-week training program, a significant between-group difference (F = 6.31, P = 0.0001) was found in infarct size. Infarct size was significantly lower (P = 0.0001) in Ex-IR (15.26 ± 0.77%), Cu-IR (20.65 ± 1.35%), and Ex-Cu-IR (15.62 ± 0.85%) groups when compared to that of the Sed-IR (43.28 ± 2.71%) group. Also, Ex-IR and Ex-Cu-IR groups showed lower infarct size compared to Cu-IR (P = 0.035 and 0.046, respectively).
mRNA expression of APP, BACE1, PS-1, PS-2, and NEP
Gene expression of APP, BACE1, PS-1, PS-2, and NEP are shown in Fig. 3A–E. A significant interaction was found in APP (F = 7.46, P < 0.0001), BACE1 (F = 8.98, P < 0.0001), PS-1 (F = 6.61, P < 0.0001), PS-2 (F = 4.70, P < 0.0001), and NEP (F = 6.31, P < 0.0001). Post-hoc analyses revealed that mRNA levels of APP was significantly greater in Sed-IR (2.69-fold) and Cu-IR (1.82-fold) groups when compared to the Sed-Con group (P < 0.0001, and P < 0.004, respectively). Cu-IR (1.82 ± 0.28), Ex-IR (1.01 ± 0.19) and Ex-Cu-IR (1.08 ± 0.42) were significantly lower (P < 0.002, P < 0.0001, and P < 0.0001, respectively) compared to Sed-IR (2.69 ± 0.55). Also, this value in Ex-IR and Ex-Cu-IR groups was significantly lower (P < 0.004 and P < 0.01, respectively) than Cu-IR.
mRNA expression of amyloid precursor protein (APP). SED sedentary, CON control, IR ischemia–reperfusion, EX exercise, CU curcumin. §Indicates significant difference compared to SED-CON group. ‡Indicates significant difference compared to SED-IR group. *Indicates significant difference compared to CUR-IR group (P ≤ 0.05). Values are means, with SEM represented by vertical bars
mRNA expression of BACE1 was significantly greater in Sed-IR (3.97-fold) and Cu-IR (2.75-fold) groups compared to Sed-Con (P < 0.0001 and P < 0.001, respectively). Cu-IR (2.75 ± 0.57), Ex-IR (1.75 ± 0.42) and Ex-Cu-IR (1.88 ± 0.26) were significantly (P < 0.028, P < 0.0001, and P < 0.0001, respectively) lower in mRNA expression of BACE1 when compared to Sed-IR (3.97 ± 1.21).
Expression of PS-1 in Sed-IR (3.52-fold) and Ex-IR (2.57-fold) groups was significantly greater compared to Sed-Con (P < 0.0001). Cu-IR (1.33 ± 0.36), Ex-IR (2.57 ± 0.38), and Ex-Cu-IR (1.14 ± 0.6) groups showed significantly (P < 0.0001, P < 0.04, and P < 0.0001, respectively) lower values compared to Sed-IR (3.52 ± 0.85). Also, the Ex-IR group showed significantly greater mRNA expression of PS-1 compared to Cu-IR and Ex-Cu-IR (P < 0.003 and P < 0.001, respectively).
mRNA expression of PS-2 in Sed-IR (2.68-fold) and Cu-IR (1.83-fold) were significantly greater compared to Sed-Con (P < 0.0001, and P < 0.03, respectively). Cu-IR (1.83 ± 0.21), Ex-IR (1.09 ± 0.08), and Ex-Cu-IR (1.34 ± 0.25) significantly decreased (P < 0.028, P < 0.0001, and P < 0.0001, respectively) levels of mRNA expression compared to Sed-IR (2.68 ± 0.93).
Expression of NEP were significantly lower in the Sed-IR (0.29-fold), Cu-IR (0.57-fold) and Ex-Cu-IR (0.57-fold) groups compared to Sed-Con (P < 0.0001, P < 0.0001, and P < 0.003, respectively). Also, NEP mRNA levels were significantly greater in Cu-IR (0.595 ± 0.09), Ex-IR (0.803 ± 0.12), and Ex-Cu-IR (0.574 ± 0.11) compared to Sed-IR (0.274 ± 0.02) with no significant differences between Cu-IR, Ex-IR, and Ex-Cu-IR (Figs. 4, 5, 6, 7).
mRNA expression of β-secretase-1 (BACE1). SED sedentary, CON control, IR ischemia–reperfusion, EX exercise, CU curcumin. §Indicates significant difference compared to SED-CON group. ‡Indicates significant difference compared to SED-IR group (P ≤ 0.05). Values are means, with SEM represented by vertical bars
mRNA expression of presenilin-1 (PS-1). SED sedentary, CON control, IR ischemia–reperfusion, EX exercise, CU curcumin. §Indicates significant difference compared to SED-CON group. ‡Indicates significant difference compared to SED-IR group. *Indicates significant difference compared to CUR-IR group. €Indicates significant difference compared to CUR-IR group (P ≤ 0.05). Values are means, with SEM represented by vertical bars
mRNA expression of presenilin-2 (PS-2). SED sedentary, CON control, IR ischemia–reperfusion, EX exercise, CU curcumin. §Indicates significant difference compared to SED-CON group. ‡Indicates significant difference compared to SED-IR group (P ≤ 0.05). Values are means, with SEM represented by vertical bars
mRNA expression of neprilysin (NEP). SED sedentary, CON control, IR ischemia–reperfusion, EX exercise, CU curcumin. §Indicates significant difference compared to SED-CON group. ‡Indicates significant difference compared to SED-IR group (P ≤ 0.05). Values are means, with SEM represented by vertical bars
Discussion
This study was the first study to investigate concurrent moderate-intensity aerobic exercise and curcumin supplementation following ischemia–reperfusion-induced myocardial infraction on gene expression of APP, enzymes involved in the APP processing, and degradation enzyme for catabolism of Aβ peptide in the left ventricle of rats. Further, mRNA expression of proteolytic components of γ-secretase (presenilin-1 and -2 [PS-1 and PS-2]) in the infarcted myocardium was also assessed. The major findings were that 10 weeks of moderate-intensity aerobic exercise alone or with curcumin supplementation significantly decreased ischemia–reperfusion-induced infarct size in the myocardium of rats. Moreover, exercise and curcumin attenuated gene expression of the Aβ precursor and its cleaving enzymes and improved mRNA expression of catabolic enzyme involved in Aβ degradation and clearance.
Our results support previous studies demonstrating a relationship between cardiovascular disease and Aβ production [2, 8, 22]. Despite, a lack of direct assessment of Aβ, several upstream factors involved in Aβ production were determined. Significantly lower mRNA expression of APP and processing enzymes were found in the experimental groups (following exercise and curcumin), indicating a positive effect of both exercise and curcumin on the attenuation of APP production. Moreover, our results suggest that although curcumin decreased the expression of APP and APP-cleaving enzymes, the combination of moderate-intensity aerobic exercise with curcumin did not significantly alter APP expression compared to moderate-intensity aerobic exercise and curcumin supplementation alone. To our knowledge, this is the first study to assess moderate-intensity aerobic exercise and curcumin supplementation on APP following ischemia–reperfusion-induced myocardium injury. Hence, we compared the present results to those of Alzheimer’s disease. In support of our findings, Zhang and colleagues [23] reported 12 weeks of moderate-intensity aerobic exercise (45 min at 5–12 m/min, 5 sessions/day) decreased Aβ levels and inhibits the amyloidogenic pathway of APP in the hippocampus of APP/PS1 transgenic mice. Alkadhi and Dao [24] revealed that 4-weeks of treadmill exercise (2–4 × 15 min at 10 m/min) attenuated increases in APP levels in the hippocampus of rats. Ehehalt and colleagues [25] demonstrated that the amyloidogenic pathway of APP metabolism occurs in structures enriched in cholesterol within the membrane termed “lipid rafts”. As Cholesterol is an essential component of lipid rafts [26], alteration in cholesterol through moderate-intensity aerobic exercise may alter the configuration of enzymes involved in APP metabolism influencing Aβ generation [27]. Importantly, APP processing may be altered by excessive adiposity, and previous research has found increases in the expression of BACE1 following high-fat feeding [28,29,30]. As a rate-limiting enzyme in the cholesterol biosynthetic pathway, hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) plays a significant role in cholesterol synthesis [31]. Inhibition of this enzyme reduces the distribution of APP in lipid rafts and may play a critical role in sequential proteolysis of APP and Aβ production [32].
Exercise [23] and curcumin [33] independently have been shown to significantly decrease HMGCR, which would reduce cholesterol synthesis. Zhang and colleagues [23] attributed the lower APP-cleaving enzymes to decreased HMGCR and attenuated cholesterol generation, which is supportive of reduced BACE1, PS-1, and PS-2 enzymes in the Cu-IR, Ex-IR, and Ex-Cu-IR groups. However, the precise mechanism(s) that regulate the content and/or activity of these enzymes are multifactorial and may be associated with impaired energy metabolism, increased inflammation, and cellular stress that may be directly altered exercise and curcumin [24, 34,35,36].
In contrast, compared to Sed-IR, NEP expression was significantly greater in the experimental groups with no between-group differences indicating no superior effects of moderate-intensity aerobic exercise with curcumin supplementation compared to moderate-intensity aerobic exercise and curcumin alone. NEP, an endothelial cell surface zinc metallopeptidase is present in cardiac myocytes. Aβ decreases the number of both cardiomyocytes and endothelial cells through apoptosis [2]. In apoptosis process, with the activation of endonucleases, DNA fragmentation occurs [37]. Both Aβ stimulation and expression of BACE1 led to fragmentation increases and activation of apoptosis pathway [2]. Accordingly, increased mRNA expression of NEP and decreased APP and its processing enzymes’ expression may in-part contribute to the lower Aβ-mediated infarction observed in the Cu-IR, Ex-IR, and Cu-Ex-IR groups compared to Sed-IR. Moreover, the antioxidant activity of curcumin through donating hydrogen ions and via neutralizing reactive oxygen intermediates may also be protective [38].
A limitation of the present study was the lack of investigation on Aβ protein level, which had been nevertheless evaluated by previous authors in the murine hippocampus following exercise [23, 24]. The purpose of the present work was that of tracking the upstream factors involved in Aβ production, to identify an effective method for reducing Aβ production or improving Aβ clearance. In any case, authors are aware of the fact that protein levels are more indicative than gene expression values and suggest that further studies should be devoted at confirming our data by assessing Aβ protein amount.
Conclusions
In conclusion, moderate aerobic exercise (15–45 min at 12–24 m/min, 5 times a week) for 10 weeks as well as curcumin supplementation (50 mg/kg) decreased ischemia–reperfusion-induced infarct size alleviated gene expression of APP and processing enzymes and increased mRNA expression of NEP in myocardium of rats. However, the combination of moderate-intensity aerobic exercise and curcumin supplementation had no superior effects compared to either treatment alone.
Data availability
Original source data is available.
References
Morris JH, Chen L (2019) Exercise training and heart failure: a review of the literature. Card Fail Rev 5(1):57–61. https://doi.org/10.15420/cfr.2018.31.1
Greco S, Zaccagnini G, Fuschi P, Voellenkle V, Carrara M, Sadeghi I et al (2017) Increased BACE1-AS long noncoding RNA and β-amyloid levels in heart failure. Cardiovasc Res 113(5):453–463. https://doi.org/10.1093/cvr/cvx013
Mokhtari-Zaer A, Marefati N, Atkin SL, Butler AE, Sahebkar A (2018) The protective role of curcumin in myocardial ischemia–reperfusion injury. J Cell Physiol 234(1):214–222. https://doi.org/10.1002/jcp.26848
Bugger H, Pfeil K (2020) Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim Biophys Acta Mol Basis Dis 7:165768. https://doi.org/10.1016/j.bbadis.2020.165768
Dookun E, Walaszczyk A, Redgrave R, Palmowski P, Tual-Chalot S, Suwana A et al (2020) Clearance of senescent cells during cardiac ischemia-reperfusion injury improves recovery. Aging Cell 19(10):e13249. https://doi.org/10.1111/acel.13249
Azevedo PS, Polegato BF, Minicucci MF, Paiva SAR, Zornoff LAM (2016) Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol 106(1):62–69. https://doi.org/10.5935/abc.20160005
Maia MA, Sausa E (2019) BACE-1 and γ-secretase as therapeutic targets for Alzheimer’s disease. Pharmaceuticals 12(1):41. https://doi.org/10.3390/ph12010041
Stamatelopoulos K, Sibbing D, Rallidis LS, Georgiopoulos G, Stakos D, Braun S et al (2015) Amyloid-beta (1–40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J Am Coll Cardiol 65(9):904–916. https://doi.org/10.1016/j.jacc.2014.12.035
Suzuki K, Iwata A, Iwatsubo T (2017) The past, present, and future of disease-modifying therapies for Alzheimer’s disease. Proc Jpn Acad Ser B Phys Biol Sci 93(10):757–771. https://doi.org/10.2183/pjab.93.048
Bursavich MG, Harrison BA, Blain JF (2016) Gamma secretase modulators: new Alzheimer’s drugs on the horizon? J Med Chem 59(16):7389–7409. https://doi.org/10.1021/acs.jmedchem.5b01960
Hersh LB, Rodgers DW (2008) Neprilysin and amyloid beta peptide degradation. Curr Alzheimer Res 5(2):225–231. https://doi.org/10.2174/156720508783954703
Moreira JBN, Wohlwend M, Wisløff U (2020) Exercise and cardiac health: physiological and molecular insights. Nat Metab 2(9):829–839. https://doi.org/10.1038/s42255-020-0262-1
Naveed M, Majeed F, Taleb A, Zubair HM, Shumzaid M, Farooq et al (2020) A review of medicinal plants in cardiovascular disorders: benefits and risks. Am J Chin Med 48(2):259–286. https://doi.org/10.1142/S0192415X20500147
Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang X, Cui XG et al (2020) Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med 24(21):12355–12367. https://doi.org/10.1111/jcmm.15725
Li H, Sureda A, Devkota HP, Pittalà V, Barreca D, Silva AS et al (2020) Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv 38:107343. https://doi.org/10.1016/j.biotechadv.2019.01.010
Jiang S, Han J, Li T, Xin Z, Ma Z, Di W et al (2017) Curcumin as a potential protective compound against cardiac diseases. Pharmacol Res 119:373–383. https://doi.org/10.1016/j.phrs.2017.03.001
Choi Y, Tanabe Y, Akazawa N, Zempo-Miyaki A, Maeda S (2019) Curcumin supplementation attenuates the decrease in endothelial function following eccentric exercise. J Exerc Nutr Biochem 23(2):7–12. https://doi.org/10.20463/jenb.2019.0010
Wafi AM, Hong J, Rudebush TL, Yu L, Hackfort B, Wang H et al (2019) Curcumin improves exercise performance of mice with coronary artery ligation-induced HFrEF: Nrf2 and antioxidant mechanisms in skeletal muscle. J Appl Physiol 126(2):477–486. https://doi.org/10.1152/japplphysiol.00654.2018
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191. https://doi.org/10.3758/bf03193146
Lu K, Wang L, Wang C, Yang Y, Hu D, Ding R (2015) Effects of high-intensity interval versus continuous moderate-intensity aerobic exercise on apoptosis, oxidative stress and metabolism of the infarcted myocardium in a rat model. Mol Med Rep 12:2374–2382. https://doi.org/10.3892/mmr.2015.3669
Esposito F, Ronchi R, Milano G, Margonato V, Di Tullio S, Marini M et al (2011) Myocardial tolerance to ischemia-reperfusion injury, training intensity and cessation. Eur J Appl Physiol 111(5):859–868. https://doi.org/10.1007/s00421-010-1707-0
Zhang B, Bian X, He P, Fu X, Higuchi K, Yang X et al (2014) The toxicity mechanisms of action of Ab25–35 in isolated rat cardiac myocytes. Molecules 19(8):12242–12257. https://doi.org/10.3390/molecules190812242
Zhang XL, Zhao N, Xu B, Chen XH, Li TJ (2019) Treadmill exercise inhibits amyloid-β generation in the hippocampus of APP/PS1 transgenic mice by reducing cholesterol-mediated lipid raft formation. NeuroReport 30(7):498–503. https://doi.org/10.1097/WNR.0000000000001230
Alkadhi KA, Dao AT (2018) Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Mol Cell Neurosci 86:25–29. https://doi.org/10.1016/j.mcn.2017.11.008
Ehehalt R, Keller P, Haass C, Thiele C, Simons K (2003) Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol 160(1):113–123. https://doi.org/10.1083/jcb.200207113
Michikawa M (2003) The role of cholesterol in pathogenesis of Alzheimer’s disease: dual metabolic interaction between amyloid beta-protein and cholesterol. Mol Neurobiol 27(1):1–12. https://doi.org/10.1385/MN:27:1:1
Diaz M, Fabelo N, Martin V, Ferrer I, Gomez T, Marin R (2015) Biophysical alterations in lipid rafts from human cerebral cortex associate with increased BACE1/AbetaPP interaction in early stages of Alzheimer’s disease. J Alzheimers Dis 43(4):1185–1198. https://doi.org/10.3233/JAD-141146
Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS et al (2008) High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 106(1):475–485. https://doi.org/10.1111/j.1471-4159.2008.05415.x
Wang R, Li JJ, Diao D, Kwak YD, Liu L, Zhi L et al (2013) Metabolic stress modulates Alzheimer’s β-secretase gene transcription via SIRT1-PPARγ- PGC-1 in neurons. Cell Metab 17(5):685–694. https://doi.org/10.1016/j.cmet.2013.03.016
Zhang T, Pan BS, Zhao B, Zhang LM, Huang YL, Sun FY (2009) Exacerbation of poststroke dementia by type 2 diabetes is associated with synergistic increases of beta-secretase activation and beta-amyloid generation in rat brains. Neuroscience 161(4):1045–1056. https://doi.org/10.1016/j.neuroscience.2009.04.032
Tobert JA (2003) Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat Rev Drug Discov 2(7):517–526. https://doi.org/10.1038/nrd1112
Cordle A, Landreth G (2005) 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci 25(2):299–307. https://doi.org/10.1523/JNEUROSCI.2544-04.2005
Manzoni AG, Passos DF, da Silva JLG, Bernardes VM, Bremm JM, Jantsch MH et al (2019) Rutin and curcumin reduce inflammation, triglyceride levels and ADA activity in serum and immune cells in a model of hyperlipidemia. Blood Cells Mol Dis 76:13–21. https://doi.org/10.1016/j.bcmd.2018.12.005
Ding L, Li J, Song B, Xiao X, Zhang B, Qi M et al (2016) Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol Appl Pharmacol 1(304):99–109. https://doi.org/10.1016/j.taap.2016.05.011
Zhang Y, Zeng Y (2019) Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4 /MyD88/NF-κB signal pathway. Drug Dev Res 80(3):353–359. https://doi.org/10.1002/ddr.21509
MacPherson RKE (2017) Filling the void: a role for exercise-induced BDNF and brain amyloid precursor protein processing. Am J Physiol Regul Integr Comp Physiol 313(5):R585–R593. https://doi.org/10.1152/ajpregu.00255.2017
Gregory C (1991) Apoptosis: the molecular basis of cell death. In: Tomei LD, Cope FO (eds) Curr Commun Cell Mol Biol 3. Cold Spring Harbor Laboratory, New York
Sarkar A, De R, Mukhopadhyay AK (2016) Curcumin as a potential therapeutic candidate for helicobacter pylori associated diseases. World J Gastroenterol 22(9):2736–2748. https://doi.org/10.3748/wjg.v22.i9.2736
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ZS contributed to project administration, conceptualization, methodology, visualization, and investigation. FN supervised the project and methodology. AN performed the laboratory experiments. MS and SCF contributed to interpretation, writing, reviewing, and editing of the manuscript. All authors read and approved the manuscript, and all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The present study was approved by the Animal Ethics Committee of Hamedan University of Medical Sciences (No. IR. BASU. REC. 1398.044), and all experiments were conducted in accordance with the Institutional Animal Care and Use Committee guidelines.
Informed consent
This is an animal study with no requirement for informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sayevand, Z., Nazem, F., Nazari, A. et al. Cardioprotective effects of exercise and curcumin supplementation against myocardial ischemia–reperfusion injury. Sport Sci Health 18, 1011–1019 (2022). https://doi.org/10.1007/s11332-021-00886-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-021-00886-w