Abstract
Purpose
Different types of physical activity can induce different hormonal and physiological responses. In this study, we examined the testosterone, cortisol, creatine kinase (CK) and lactate dehydrogenase (LDH) response to acute intermittent (IE) and continuous (CE) aerobic exercise in sedentary men.
Methods
In this single-blinded randomised crossover study, eleven sedentary healthy males completed protocols (CE and IE) on two different days separated by a 1-week washout period. CE comprised 40 min of running on a treadmill at 60% of reserve heart rate. IE consisted of 40 min of running on a treadmill with intensity alternating between 50% (2 min) and 80% (1 min) of reserve heart rate. Blood samples were taken before and immediately after each exercise session.
Results
Serum testosterone concentrations increased significantly after IE (+8.0%, P = 0.021) and decreased non-significantly after CE (−5.8%, P = 0.409). The IE response was greater than the CE response (P = 0.01). Cortisol concentration decreased in both IE and CE exercise (P = 0.001 and P = 0.016, respectively), by −33.6 and −34.6%, respectively. The testosterone to cortisol ratio increased significantly after both forms of exercise (IE: P = 0.003; CE: P = 0.041). CK concentrations significantly increased from PRE to POST (IE: +20.6%, P = 0.001; CE: +26.5%, P = 0.046). Despite the increase in concentrations of LDH, the changes were not significant (F (3, 30) = 1.01, P = 0.402; IE: +11.4% and CE: +23.1%).
Conclusions
In summary, it seems that intermittent exercise can be more useful in the development of body anabolic processes in sedentary men due to pronounced increases in testosterone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sedentary lifestyle is an important factor in the increased prevalence of cardiovascular disease, which has become a large and growing problem in modern society [1]. The fact that long-term regular physical activity has beneficial effects on health promotion and improves the human body’s physiological function is obvious [2]. However, different forms of physical activity can induce different hormonal and physiological responses [3]. The physical activity recommendation for public health is participation in moderate- to vigorous-intensity aerobic activity on most days of the week [4]. However, studies in recent decades have shown that intermittent aerobic exercise with alternating bouts of high-intensity activity combined with active recovery can induce the same or greater effects on the body’s performance and physiological responses as continuous exercise at constant intensity [3, 5]. In addition, since intermittent activities may cause faster metabolic adaptations in skeletal muscle, they have been taken into consideration [6].
Testosterone is an important sex hormone that is produced and secreted by testicular Leydig cells in the male testes and has various physiological functions. One of its functions is anabolic effects on stimulating tissue growth and development. Testosterone stimulates nitrogen retention and protein synthesis, and is required in order to maintain the structural protein anabolism [7]. By contrast, cortisol is a catabolic hormone. Cortisol is a glucocorticoid that is secreted by the adrenal cortex of the adrenal glands [8] and one of its functions is protein degradation [7]. It is also indicated that this hormone is able to prevent protein synthesis and increase muscle mass [8]. Thus, reducing the concentration of testosterone and increasing the concentration of cortisol reflects a disorder in the anabolic-catabolic balance [9]. On the other hand, in exercise physiology the ratio of testosterone to cortisol is used to assess the balance between anabolic and catabolic processes. This ratio can be reduced in relation to the intensity and duration of exercise, and represents the physiological strain imposed by the exercise [10].
Various exercises can affect the concentration of these steroids, but the exact pattern of response of these hormones to different exercise protocols is not clear and increases [3] and decreases [11, 12] have been reported by various studies. It has also been stated that intermittent aerobic exercise, compared to continuous exercise, causes more damage and a significant increase in circulating testosterone levels after exercise in endurance-trained men because the muscle contractile filaments are subjected to greater stress and strain [3]. Vuorimaa et al. [13] demonstrated that in runners (middle- vs. long-distance) the muscle performance and muscle activation responses to intermittent and continuous type of running are related to their training background. Thus, it seems that training background is effective in respect of the response of these steroids to intermittent and continuous exercise [14].
In this study, we therefore compared the acute effects of continuous and intermittent aerobic exercise on the concentrations of testosterone, cortisol, CK and LDH, and also the testosterone to cortisol ratio as an indicator of the body’s anabolic-catabolic state in sedentary men in order to determine which type of exercise has more anabolic benefits in sedentary men, assuming a constant cardiac workload?
Materials and methods
Participants
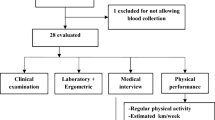
Eleven sedentary healthy males (N = 11) aged 20–26 years were recruited to this study and provided signed written informed consent after the purpose of the study and potential risks had been clarified. Their physical and physiological characteristics were as follows (mean ± SD): age = 22.3 ± 1.9 years; height = 176.7 ± 5.8 cm; body mass = 67.8 ± 7.9 kg; body mass index = 21.7 ± 2.1 kg/m2; body fat percentage = 18.3 ± 3.9%; resting heart rate = 74.0 ± 9.2 bpm; maximum heart rate = 197.6 ± 6.9 bpm; and maximal oxygen consumption (VO2max) = 39.7 ± 5.0 mL kg-1 min−1. The main inclusion criterion was being sedentary, defined as VO2max of 35–45 mL kg−1 min−1 and non-participation in regular exercise. Exclusion criteria were: abnormal blood pressure and electrocardiogram (ECG); cardiovascular, renal or pulmonary problems; diabetes; taking any medication; alcohol consumption; smoking; and chronic metabolic, physical or psychological disorders. The trial protocol was approved by the Research Ethics Committee of the Sport Sciences Research Institute of Iran (Approval code: IR.SSRI.REC.1395.114) and carried out in accordance with the Declaration of Helsinki.
Study design
In order to participate in the study, the subjects completed a medical history questionnaire and physical activity readiness questionnaire (PAR-Q). In order to assess cardiac hypertrophy, myocardial infarction and ischemia, a resting standard 12-lead electrocardiogram (Cardisuny α 1000, Fukuda, Tokyo, Japan) was taken for all subjects [15, 16]. Individuals who had abnormal ECG or heart disease based on the medical history questionnaire or a family history of cardiovascular disease were excluded from the study. The subjects’ demographic characteristics were then measured, including height, weight, BMI and blood pressure. We used a body composition analyzer (Olympia 3.3, Jawon Medical, Korea) to measure body mass index and an automated and clinically validated upper-arm BP monitor (M2, Omron Healthcare, Kyoto, Japan) to measure blood pressure in the sitting position, according to the World Health Organization’s guidance on physical measurements (http://www.who.int/chp/steps/manual/en/index3.html, 2008), at rest as well as pre and post exercise. In order to calculate the rate pressure product (RPP) we used HR and systolic BP (RPP = (HR × systolic BP)/100) pre and post exercise [17]. Two weeks before the first test day, all subjects completed the standard Bruce protocol to calculate VO2max and also to achieve maximum heart rate in order to be able to determine and control individual workload during the different exercise protocols. VO2max was estimated using the prediction equation of Foster et al. [18] for active and sedentary men. The study was designed as a single-blinded randomised crossover trial performed on two different test days separated by a 1-week washout period. The subjects were divided into two groups and randomly performed continuous or intermittent exercise in each training session in a way that eventually both exercise protocols were performed once by all subjects. The subjects were asked to refrain from any vigorous physical activity between sessions and to not consume caffeinated beverages for 24 h before test days.
Exercise training protocol
Each session consisted of 40 min of either CE or IE on a treadmill. Exercise intensity was determined as the workload achieved during the maximal graded exercise test (standard Bruce protocol) and was calculated to promote the same cardiovascular workload for both CE and IE. CE comprised 40 min of running on a treadmill at 60% of reserve heart rate. IE consisted of 40 min of running on a treadmill with intensity alternating between 50% (2 min) and 80% (1 min) of reserve heart rate, resulting in a mean workload of 60% ([(50% × 2) + 80%]/3) [19]. Reserve heart rate was calculated using the Karvonen method [20]: HRtarget = % Intensity (HRmax − HRrest) + HRrest.
Resting and maximum heart rate were obtained during the standard Bruce protocol. The heart rate of subjects was monitored during all exercise sessions using a heart rate monitor (FT4, Polar Eletro Oy, China). Each session started with a 5-min warm-up and ended with a 5-min cool-down at 30% of reserve heart rate. The subjects carried out all exercise sessions in the morning and at an environmental temperature of 24 °C and 50% humidity.
Blood sampling and biochemical analysis
Blood samples were taken from the antecubital vein of subjects by venous puncture before (PRE) and immediately after (POST) each exercise session in a sitting position. Initially, blood samples were allowed to clot at room temperature and then serum from the blood sample was separated by centrifugation (3500 g for 20 min). The serum was drawn off and stored at −70 °C for use in subsequent analysis of testosterone, cortisol, creatine kinase (CK) and lactate dehydrogenase (LDH). Serum testosterone concentrations were determined quantitatively by a special kit (Lot No. 18786105) based on electrochemiluminescence immunoassay (ECLIA) using a cobas e411 analyzer (Roche Diagnostics, Mannheim, Germany). This assay has a measuring range of 0.087–52.0 nmol/L and the limit of quantitation was 0.416 nmol/L (intermediate precision: 2.8–8.4%).
Serum cortisol concentrations were measured using a human enzyme-linked immunosorbent assay kit (Diagnostics Biochem Canada Inc, Ontario, Canada, and Lot No. 161380). The sensitivity of the assay was 0.4 µg/dL (calibrator range: 0.5–60 µg/dL).
To measure the concentrations of serum CK and LDH, we used commercial kits (MAN Co, Tehran, Iran) and analyzed with a Selectra XL chemistry analyzer (Vital Scientific N.V., Netherlands) based on photometric assay. The detectable range of this assay was 10–1700 U/L and 50–1200 U/L for CK (Lot No. CK2798S6) and LDH (Lot No. Ld12696), respectively.
Statistical analysis
SPSS software version 17.0 (SPSS Inc., IBM, Chicago, IL, USA) was used for statistical analysis. Before further analysis, the normality of the data was examined by applying the Shapiro–Wilk test. This test showed that only the testosterone to cortisol ratio (T/C ratio) data had skewed distribution, so we used the nonparametric Friedman’s test to compare the T/C ratio data across the various points and then used the Wilcoxon signed-rank test for pair comparison. Other variables were analyzed using a repeated-measures analysis and, if necessary, an LSD post hoc test was applied. Relationships between variables were assessed with a Pearson correlation coefficient. In all the statistical analyses, the level of statistical significance was set at P < 0.05.
Results
Descriptive results of the dependent variables for the pre- and post-exercise samples are shown in Table 1. The results showed that serum testosterone concentrations (F (3, 30) = 3.38, P = 0.031) had increased significantly after IE (+8.0%, P = 0.021). However, a non-significant decrease in testosterone concentrations was observed after CE (−5.8%, P = 0.409). The IE response was greater than the CE response (P = 0.01) (Fig. 1a).
Testosterone, cortisol and T/C ratio before (PRE) and immediately after (POST) different exercise protocols. Open bars = intermittent exercise (IE); solid bars = continuous exercise (CE). * Significant difference between pre and post exercise values (P < 0.05). # Significant difference between post-exercise values (P < 0.05). T/C ratio = testosterone to cortisol ratio
After exercise, the cortisol concentration had decreased for both IE and CE (P = 0.001 and P = 0.016, respectively), by −33.6 and −34.6%, respectively (F (3, 30) = 5.3, P = 0.005). There was no significant difference between the two types of exercise in post-exercise mean serum cortisol concentrations (P = 0.778) (Fig. 1b).
The T/C ratio had increased significantly after both forms of exercise (IE: P = 0.003; CE: P = 0.041), but there was no difference between the two exercise protocols in this ratio (P = 0.534) (Fig. 1c).
Statistical analysis of CK and LDH as indirect markers of muscle damage revealed that CK concentrations significantly increased from PRE to POST in both exercise protocols (F (2.08, 20.88) = 4.37, P = 0.025; IE: +20.6%, P = 0.001; CE: +26.5%, P = 0.046) (Fig. 2a). However, despite the increase in concentrations of LDH, the changes were not significant (F (3, 30) = 1.01, P = 0.402; IE: +11.4% and CE: +23.1%) (Fig. 2b). These responses were not significantly different between the two exercise sessions (P > 0.05).
CK and LDH serum concentrations before (PRE) and immediately after (POST) different exercise protocols. Open bars = intermittent exercise (IE); solid bars = continuous exercise (CE). * Significant difference between pre and post exercise values (P < 0.05). CK creatine kinase, LDH lactate dehydrogenase
The results also reveal that RPP increased significantly after both exercise protocols (IE: +49.4%, P = 0.001; CE: +38.5%, P = 0.001) and there was no significant difference between IE and CE (P = 0.2), indicating that the same cardiac workload was applied by the two exercise protocols.
Discussion
The results of this study showed that testosterone concentration was significantly increased only after intermittent exercise, while the average concentration of this hormone showed a non-significant decrease after continuous exercise. There was a difference between the effects of intermittent and continuous exercise on concentrations of this hormone; testosterone levels after intermittent exercise were significantly higher than after continuous exercise. Many studies have reported an increase in testosterone concentrations after acute exercise [3, 14, 21], but there are also studies that observed a significant decrease in testosterone concentrations after acute exercise [22, 23]. In this regard, Karkoulias et al. [23] reported a decrease in testosterone after endurance exercise in amateur athletes. It is not clear what causes a reduction in testosterone after endurance exercise. It is possible that exercise with an influence on the hypothalamus or a direct effect on testicular secretion causes changes in the concentrations of this hormone. Karkoulias et al. [23] stated that marathon running, by creating acute disorder in testicular secretion, led to a decrease in testosterone concentration after the exercise. In the present study, the decrease in testosterone was observed only after continuous exercise; however, this reduction was not statistically significant. Since the testosterone plays a role in increasing muscle protein synthesis, the reduction in the concentration of this hormone can alter the pathway amino acids from protein synthesis to gluconeogenesis [24].
On the other hand, Vuorimaa et al. [14] studied the changes in serum testosterone concentration after 40 min of continuous and intermittent exercise in well-trained middle-distance runners and marathon runners. Their findings showed that testosterone increased significantly in middle-distance runners after both types of exercise, but the increase in testosterone immediately after intermittent exercise in the marathon runners was not significant. It is noteworthy that the testosterone response to intermittent exercise was greater in middle-distance runners than in marathon runners. They stated that training background can have an impact on the response of testosterone to intermittent and continuous exercise [14] because the muscle performance and muscle activation responses to intermittent and continuous type of running are different and related to runners training background (fast intermittent training vs. continuous types of running training) [13]. Hackney et al. [3] examined free testosterone changes in 15 endurance-trained males after continuous and intermittent exercise. Their findings showed that testosterone concentration significantly increased after both forms of exercise; there was a difference between the two exercise protocols, with the testosterone significantly higher after intermittent exercise. This difference in testosterone concentrations between the two types of exercise is consistent with the findings of the present study. Hackney et al. [3] stated that this difference could be due to the fact that intermittent exercise places greater demands on the anabolic processes of the tissue, because its bouts of high-intensity activity producing an additional contraction force that causes more tension and stress in the skeletal muscle. This causes further damage to the muscle structural proteins, and more hormonally mediated processes are needed for compensation and reconstruction of these proteins. Meanwhile, in the present study we measured CPK and LDH as indirect markers of muscle damage [25]. Our findings showed that CPK had increased significantly after both intermittent and continuous exercise, while the increase in LDH was non-significant. There was no significant difference between the two exercise protocols in CPK and LDH values. Hence, it can be said that both exercises equally cause muscle damage, but these markers cannot give an accurate reflection of the type of muscle cell damage [26]. So perhaps whit regardless of more structural muscle damage during the intermittent exercise, reasons such as bouts of high-intensity activity and greater concentrations of catecholamines during intermittent exercise can mention for differences in the concentrations of testosterone between two forms of exercise.
Since the intensity of the exercise is an important factor in increasing testosterone levels [27], bouts of high-intensity of intermittent exercise may result in an increase in the concentration of testosterone. In addition, a significant positive correlation between testosterone and adrenaline and noradrenaline has been reported during exercise [27]; by stimulating the Leydig cells, catecholamine plays a role in increasing testosterone secretion during acute exercise [28]. In the present study, we did not measure the concentrations of catecholamines, but several studies have shown that intermittent exercise can increase concentrations of catecholamines more than continuous exercise [29, 30]. A significant increase in testosterone after intermittent exercise and the difference between the two types of aerobic exercise in the values of this steroid may therefore be due to a greater catecholamine response to the intermittent exercise.
Another finding of this study was that cortisol concentrations had decreased significantly after both types of exercise. Furthermore, there was no significant difference in post-exercise cortisol concentrations between intermittent and continuous exercise. Cortisol typically increases in response to endurance exercise. In this field, Hackney et al. [3] and Vuorimaa et al. [14] reported that cortisol levels increased significantly after about 40–47 min of continuous and intermittent aerobic exercise in endurance athletes, which is not consistent with our findings. In the study by Wahl et al. [31], the continuous exercise protocol on a cycle ergometer (130 min at 55% peak power output) significantly decreased cortisol concentration, while the intermittent protocol (4 × 4 min at 90–95% PPO separated by 3 min of active recovery at 45% PPO) resulted in a non-significant increase in cortisol in 12 healthy subjects (triathletes/cyclists). It is noteworthy that both the continuous and intermittent protocols of the study significantly increased testosterone concentrations, and that a significant difference was also reported in post-exercise cortisol and testosterone values between the two types of exercise. Some findings of this study are consistent with the results obtained in the present study.
Furthermore, Hoffman et al. [32] showed that high-intensity intermittent exercise led to a significant reduction in cortisol immediately after exercise in active men. Decreases in cortisol after concurrent training in trained males [12] and after 40 min of endurance exercise at 75% of maximum heart rate in recreationally trained women [33] have also been reported. Previous findings have suggested that acute fatigue during exercise is associated with reduced cortisol concentrations [34]. In addition, cortisol is a hormone with a circadian cycle, and most studies that observed a decrease in the concentration of this hormone after exercise attributed this to the involvement of the circadian cycle [32, 33].
Lo et al. [35] examined the effect of exercise intensity on cortisol changes. Their results showed that low- and intermediate-low-intensity running led to a reduction in cortisol concentration immediately after exercise. Also, Hill et al. [36] demonstrated that exercise at an intensity higher than 60% of maximum oxygen consumption can significantly increase cortisol concentration, and it seems that low-intensity exercise is able to reduce cortisol levels. So it seems that exercise intensity is a factor that can reverse cortisol response to endurance exercise. This factor (exercise intensity) may be the reason for differences in cortisol results between our study and the studies conducted by Hackney et al. [3] and Vuorimaa et al. [14] after continuous and intermittent exercise.
In addition, the results showed that the ratio of testosterone to cortisol increased significantly after intermittent and continuous exercise, but there was no difference in this ratio between the two forms of exercise. A significant increase in this ratio has been reported after 20 min of continuous and intermittent exercise in middle-distance runners and after continuous exercise only in marathon runners [14]. In a study by Wahl et al. [31], the ratio of testosterone to cortisol increased after intermittent and continuous exercise. Also, Sgrò et al. [21] showed that the testosterone to cortisol ratio had increased at the end of recovery in the submaximal exercise and significantly decreased in the 15 min of recovery after maximal exercise, while immediately after maximal exercise and during recovery this ratio was significantly less than with submaximal exercise. The decrease in this ratio has been observed after exhaustive endurance exercise in endurance-trained males [11]. According to the results of the aforementioned studies, it seems that, unlike sub-maximal activity, high-intensity and exhaustive endurance exercise can reduce the testosterone to cortisol ratio.
Since the testosterone to cortisol ratio is mentioned as an indicator of anabolic/catabolic state [34] and represents the physiological stress induced by training [10], the post-exercise increase in this ratio in the present study may indicate anabolic conditions after the continuous and intermittent exercise. Although no significant difference was observed in the testosterone to cortisol ratio between the two forms of exercise, intermittent exercise had a better mean than continuous exercise. Although testosterone was significantly increased only after intermittent exercise and the testosterone level after continuous exercise decreased, according to the testosterone to cortisol ratio it seems that the physiological stress imposed by the two types of exercise was similar.
However, one of the major limitations of this study was the lack of sampling in successive time during recovery from exercise in order to specify changes of these steroids in the recovery period. In future studies examining continuous and intermittent aerobic exercise, it is also recommended considering catecholamine concentration changes as one of the factors affecting the secretion of testosterone.
In summary, both intermittent and continuous exercise reduced cortisol, but only intermittent exercise led to a significant increase in testosterone. It seems that intermittent exercise may be more useful in the development of body anabolic processes in sedentary men due to its ability to significantly increase testosterone. However, to confirm these results more studies are needed with a larger population and long-term training to investigate the effect of adaptation to continuous and intermittent training.
References
Young DR, Hivert M-F, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, Lewis CE, Owen N, Perry CK, Siddique J (2016) Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation 134(13):e262–e279
Miller KR, McClave SA, Jampolis MB, Hurt RT, Krueger K, Landes S, Collier B (2016) The health benefits of exercise and physical activity. Curr Nutr Rep 5(3):204–212. doi:10.1007/s13668-016-0175-5
Hackney AC, Hosick K, Myer A, Rubin D, Battaglini CL (2012) Testosterone responses to intensive interval versus steady-state endurance exercise. J Endocrinol Invest 35(11):947–950
Oja P, Titze S (2011) Physical activity recommendations for public health: development and policy context. EPMA J 2(3):253–259
Guimaraes GV, Ciolac EG, Carvalho VO, D’Avila VM, Bortolotto LA, Bocchi EA (2010) Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res 33(6):627–632. doi:10.1038/hr.2010.42
Laursen PB, Jenkins DG (2002) The scientific basis for high-intensity interval training. Sports Med 32(1):53–73. doi:10.2165/00007256-200232010-00003
Winters S (2004) Male hypogonadism: basic, clinical, and therapeutic principles, 1st edn. Humana Press, New York
Esposito A, Bianchi V (2012) Cortisol: physiology, regulation and health implications. Nova Science Publishers Inc, New York
Hloogeveen A, Zonderland M (1996) Relationships between testosterone, cortisol and performance in professional cyclists. Int J Sports Med 17(6):423–428
De Luccia TPB (2016) Use of the testosterone/cortisol ratio variable in sports. Open Sports Sci J 9(1):104–113
Anderson T, Lane AR, Hackney AC (2016) Cortisol and testosterone dynamics following exhaustive endurance exercise. Eur J Appl Physiol 116(8):1503–1509. doi:10.1007/s00421-016-3406-y
Rosa G, Fortes MdSR, Mello DBd (2016) Concurrent training decreases cortisol but not zinc concentrations: effects of distinct exercise protocols. Scientifica. doi:10.1155/2016/7643016 (Article ID 7643016)
Vuorimaa T, Virlander R, Kurkilahti P, Vasankari T, Häkkinen K (2006) Acute changes in muscle activation and leg extension performance after different running exercises in elite long distance runners. Eur J Appl Physiol 96(3):282–291. doi:10.1007/s00421-005-0054-z
Vuorimaa T, Ahotupa M, Häkkinen K, Vasankari T (2008) Different hormonal response to continuous and intermittent exercise in middle-distance and marathon runners. Scand J Med Sci Sports 18(5):565–572
Sokolow M, Lyon TP (1949) The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 37(2):161–186. doi:10.1016/0002-8703(49)90562-1
Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, Wisloff U, Ingul CB, Stoylen A (2012) Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol 19(2):151–160. doi:10.1177/1741826711400512
McArdle WD, Katch FI, Katch VL (2010) Exercise physiology: nutrition, energy, and human performance, 7th edn. Lippincott Williams & Wilkins, Philadelphia
Foster C, Jackson AS, Pollock ML, Taylor MM, Hare J, Sennett SM, Rod JL, Sarwar M, Schmidt DH (1984) Generalized equations for predicting functional capacity from treadmill performance. Am Heart J 107(6):1229–1234. doi:10.1016/0002-8703(84)90282-5
Ciolac EG, Guimarães GV, Bortolotto LA, Doria EL, Bocchi EA (2009) Acute effects of continuous and interval aerobic exercise on 24-h ambulatory blood pressure in long-term treated hypertensive patients. Int J Cardiol 133(3):381–387. doi:10.1016/j.ijcard.2008.02.005
Karvonen MJ, Kentala E, Mustala O (1957) The effects of training on heart rate: a longitudinal study. Ann Med Exp Biol Fenn 35:307–315
Sgrò P, Romanelli F, Felici F, Sansone M, Bianchini S, Buzzachera C, Baldari C, Guidetti L, Pigozzi F, Lenzi A (2014) Testosterone responses to standardized short-term sub-maximal and maximal endurance exercises: issues on the dynamic adaptive role of the hypothalamic-pituitary-testicular axis. J Endocrinol Invest 37(1):13–24
Vingren JL, Budnar RG, McKenzie AL, Duplanty AA, Luk H-Y, Levitt DE, Armstrong LE (2016) The acute testosterone, growth hormone, cortisol and interleukin-6 response to 164-km road cycling in a hot environment. J Sports Sci 34(8):694–699. doi:10.1080/02640414.2015.1068440
Karkoulias K, Habeos I, Charokopos N, Tsiamita M, Mazarakis A, Pouli A, Spiropoulos K (2008) Hormonal responses to marathon running in non-elite athletes. Eur J Intern Med 19(8):598–601
Guezennec CY, Ferre P, Serrurier B, Merino D, Pesquies PC (1982) Effects of prolonged physical exercise and fasting upon plasma testosterone level in rats. Eur J Appl Physiol 49(2):159–168. doi:10.1007/bf02334064
Rodrigues P, Wassmansdorf R, Salgueirosa FM, Hernandez SG, Nascimento VB, Daros LB, Wharton L, Osiecki R (2016) Time-course of changes in indirect markers of muscle damage responses following a 130-km cycling race. Rev Brasileira de Cineantropometria Desempenho Humano 18(3):322–331
Baird MF, Graham SM, Baker JS, Bickerstaff GF (2012) Creatine-kinase-and exercise-related muscle damage implications for muscle performance and recovery. J Nutr Metab. doi:10.1155/2012/960363 (Article ID 960363)
JeŽová D, Vigaš M, Tatár P, Kvetňanský R, Nazar K, Kaciuba-UŚcilko H, Kozlowski S (1985) Plasma testosterone and catecholamine responses to physical exercise of different intensities in men. Eur J Appl Physiol 54(1):62–66. doi:10.1007/bf00426300
Derbré F, Vincent S, Maitel B, Jacob C, Delamarche P, Delamarche A, Zouhal H (2010) Androgen responses to sprint exercise in young men. Int J Sports Med 31(05):291–297
Bally L, Zueger T, Buehler T, Dokumaci AS, Speck C, Pasi N, Ciller C, Paganini D, Feller K, Loher H (2016) Metabolic and hormonal response to intermittent high-intensity and continuous moderate intensity exercise in individuals with type 1 diabetes: a randomised crossover study. Diabetologia 59(4):776–784
Peake JM, Tan SJ, Markworth JF, Broadbent JA, Skinner TL, Cameron-Smith D (2014) Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am J Physiol Endocrinol Metab 307(7):E539–E552
Wahl P, Mathes S, Köhler K, Achtzehn S, Bloch W, Mester J (2013) Acute metabolic, hormonal, and psychological responses to different endurance training protocols. Horm Metab Res 45(11):827–833
Hoffman JR, Falk B, Radom-Isaac S, Weinstein Y, Magazanik A, Wang Y, Yarom Y (1996) The effect of environmental temperature on testosterone and cortisol responses to high Intensity, intermittent exercise in humans. Eur J Appl Physiol 75(1):83–87. doi:10.1007/s004210050130
Copeland JL, Consitt LA, Tremblay MS (2002) Hormonal responses to endurance and resistance exercise in females aged 19–69 years. J Gerontol Ser A Biol Sci Med Sci 57(4):B158–B165
Viru A, Viru M (2004) Cortisol-essential adaptation hormone in exercise. Int J Sports Med 25(06):461–464
Lo C, Brown M, Contursi M, Lewis G, Lieberman D, Baggish A (2016) Characterization of cortisol kinetics at different running intensities. J Am Coll Cardiol 67(13):1635
Hill E, Zack E, Battaglini C, Viru M, Viru A, Hackney A (2008) Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest 31(7):587–591
Acknowledgements
The authors would like to thank the subjects for their committed participation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ahmadi, M.A., Zar, A., Krustrup, P. et al. Testosterone and cortisol response to acute intermittent and continuous aerobic exercise in sedentary men. Sport Sci Health 14, 53–60 (2018). https://doi.org/10.1007/s11332-017-0399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-017-0399-9