Abstract
Purpose
There is limited data regarding the sleep quality in survivors of critical illness, while the time course of the sleep abnormalities observed after ICU discharge is not known. The aim of this study was to assess sleep quality and the time course of sleep abnormalities in survivors of critical illness.
Methods
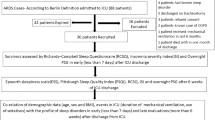
Eligible survivors of critical illness without hypercapnia and hypoxemia were evaluated within 10 days (1st evaluation, n = 36) and at 6 months after hospital discharge (2nd evaluation, n = 29). At each visit, all patients underwent an overnight full polysomnography and completed health-related quality of life questionnaires (HRQL). Lung function and electro-diagnostic tests (ED) were performed in 24 and 11 patients, respectively.
Results
At 1st evaluation, sleep quality and HRQL were poor. Sleep was characterised by high percentages of N1, low of N3 and REM stages, and high apnea–hypopnea index (AHI, events/h). Twenty-two out of 36 patients (61%) exhibited AHI ≥ 15 (21 obstructive, 1 central). None of the patients’ characteristics, including HRQL and lung function, predicted the occurrence of AHI ≥ 15. At 6 months, although sleep quality remained poor (high percentages of N1 and low of REM), sleep architecture had improved as indicated by the significant increase in N3 [4.2% (0–12.5) vs. 9.8% (3.0–20.4)] and decrease in AHI [21.5 (6.5–29.4) vs. 12.8 (4.7–20.4)]. HRQL improved slightly but significantly at 6 months. Neither the changes in HRQL nor in lung function tests were related to these of sleep architecture. Six out of eight patients with abnormal ED at 1st evaluation continued to exhibit abnormal results at 6 months.
Conclusions
Survivors of critical illness exhibited a high prevalence of obstructive sleep-disordered breathing and poor sleep architecture at hospital discharge, which slightly improved 6 months later, indicating that reversible factors are partly responsible for these abnormalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Sleep abnormalities are extremely common in critically ill patients [1]. These patients exhibit a reduction in rapid eye movement (REM) and N3 stages of sleep (slow-wave sleep), sleep disruption and numerous hypopneas or apneas (sleep-disordered breathing, SDB). Thus, although the total sleep time may be normal or even increased, the quality of sleep is poor [2]. Several factors such as the intensive care unit (ICU) environment, illness severity, acute illness that precipitated the ICU admission and the process of mechanical ventilation may contribute to these sleep abnormalities [3,4,5].
Studies have shown that ICU survivors exhibit long-term neurocognitive impairment and a perceived reduction in the quality of life after ICU discharge [6,7,8,9]. Persistent sleep abnormalities acquired during the course of the critical illness may contribute to this poor quality of life. Although sleep quality after ICU discharge has been evaluated by several studies, the results are rather conflicting [10,11,12,13,14]. This is probably due to patients’ selection bias [10,11,12] and the use of methods other than a full polysomnography to assess sleep [13, 14]. It is worth mentioning that none of these studies have examined the time course of the sleep abnormalities observed after ICU discharge.
The primary aim of our study was to assess sleep-disordered breathing and sleep architecture in survivors of critical illness without hypercapnia and hypoxemia within a 10-day time frame, and at 6 months after hospital discharge. A secondary aim was to examine if sleep abnormalities influence quality of life. For this purpose, health-related quality of life (HRQL) was reported during these periods using standard questionnaires.
Materials and methods
Patients
The study was performed in the sleep laboratory of the Department of Intensive Care Medicine of the University Hospital of Heraklion. Survivors of critical illness were screened for eligibility. Inclusion criteria were (1) age > 18 years and < 80 years, (2) the necessity of intubation and mechanical ventilation to support the respiratory system for at least 48 h, (3) Glasgow coma scale of 15 at the time of hospital discharge and (4) life expectancy > 1 year. Patients with a history of sleep-disordered breathing (i.e. sleep apnea syndrome), chronic neuromuscular disorder, chronic restrictive lung disease (i.e. lung fibrosis) and/or psychiatric disease, as well as patients with hypercapnia (PaCO2 ≥ 45 mmHg) and/or hypoxemia (PaO2 ≤ 60 mmHg) on room air at hospital discharge, were excluded. The study was approved by the Human Studies Subcommittee and informed consent was obtained from patients.
Study design
Eligible patients were approached and informed about the study’s design well before hospital discharge. If the patients agreed to participate in the study, they would be evaluated in an ambulatory sleep laboratory within 10 days (1st evaluation) and at 6 months (2nd evaluation) after hospital discharge. At each evaluation, the patients underwent clinical examinations and completed HRQL questionnaires, followed by an overnight full polysomnography (PSG). After the first 12 patients, the protocol was modified by performing complete lung function tests. Moreover, since upper airways muscle function plays a key role in sleep quality [15], a subgroup of patients underwent electro-diagnostic (ED) tests with the aim of examining the presence of critical illness myopathy and/or polyneuropathy.
Measurements
The HRQL questionnaires were (1) the 36-Item Short Form Health Survey (SF-36) to measure medical outcomes and (2) Epworth sleepiness scale (ESS). The SF-36 survey has 36 questions and generates a health profile of 8 subscale scores: physical functioning, role limitations caused by physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems and mental health. The scores on all subscales are transformed to a scale ranging from 0 (the worst score) to 100 (best score). The ESS is used to determine the level of daytime sleepiness. A score greater than 9 indicates significant daytime sleepiness. Both questionnaires have been validated for the Greek population [16, 17].
PSG studies were performed using current standard recording (Alice; Respironics, Pittsburgh, PA, USA) and scoring techniques (see Online Resource 1) [18, 19]. The lung function tests included measurements of static and dynamic lung volumes, diffusion lung capacity and maximum inspiratory and expiratory mouth pressures (MasterLab; 2.12, Jaeger, Wuerzburg, Germany) according to the ATS/ERS Pulmonary Function Tests Task Force Standardisation guidelines [20, 21]. ED tests, consisting of nerve conduction studies (NCS) and electromyography (EMG), were performed according to standard techniques (Nihon Kohden Neuropack 2 MEM-7102) (see Additional file 1) [22]. ED tests were carried out within 10 days of hospital discharge and repeated 6 months later, if the first ED tests were pathological [23].
Statistical analysis
Continuous variables are reported as medians and interquartile ranges (IQR). Categorical variables are presented as percentages and compared using Fisher’s exact test. Continuous variables were compared with Wilcoxon and Man-Whitney tests, as appropriate. Regression analysis was performed using the least square method. Univariate and multivariate logistic regression analyses were used to evaluate relationships between independent variables and indices of sleep quality. In addition, univariate and multivariate linear regression analyses were performed with AHI as the dependent variable. A two-tailed p value < 0.05 was considered significant. For the statistical analysis, IBM SPSS-Statistics for Windowsv.22 (Armonk, NY, USA) was used.
Results
Thirty-six patients were prospectively studied within 10 days of hospital discharge. After the 1st evaluation, seven patients refused to come for follow-up and thus the 2nd evaluation, performed at 6 months after hospital discharge, pertains to 29 patients. The majority of the patients (75%) had acute respiratory distress syndrome (ARDS) either as admission diagnosis or during the ICU stay. Patients’ characteristics are shown in Table 1 (see also Table S1, Online Resource 1).
First evaluation (n = 36)
At the 1st evaluation, sleep architecture was characterised by normal sleep efficiency, high percentages of N1, low percentages of N3 and REM stages, and high arousal (sleep fragmentation, SFI) and apnea–hypopnea (AHI) indices (Table 2).
Twenty-nine out of 36 patients (80.6%) exhibited AHI more than five events per hour of sleep, while in 22 of them, AHI of ≥ 15events/h was observed (Fig. S1, Online Resource 1). Mixed or pure obstructive events occurred in 28 patients, while in one patient, only central apneas were observed (no. 6 in Table S1, Online Resource 1). In patients with AHI ≥ 15 events/h, SFI was significantly higher than in patients with AHI < 15 events/h (Table 2). Also in patients with AHI ≥ 15 events/h, the sleep time with SaO2 < 90% was significantly longer and minimum SaO2 was lower than the corresponding values in patients with AHI < 15 events/h (Table 2). Baseline patients’ characteristics, days on mechanical ventilation, length of stay in ICU and hospital, and lung function tests did not differ between patients with AHI ≥ 15 and < 15 events/h (Table 3). Multiple regression analysis revealed Acute Physiology and Chronic Health Evaluation II (APACHE II) as the only variable that was negatively related to sleep efficiency. Logistic regression analysis showed that none of the patients’ characteristics predicted the occurrence of AHI ≥ 15 events/h within 10 days of hospital discharge.
SF-36 revealed that the health profile of eight subscale scores were considerably lower than those of the normal population (Fig. S2, Online Resource 1). The physical and mental composite score of SF-36 (average of the 4 subscale score of the physical and mental components, respectively) were lower in patients with AHI ≥ 15 events/h than in patients with AHI < 15 events/h, the difference being significant only for physical component (Table 2). No relationship was observed between oxygenation sleep indices (SaO2 < 90% and minimum SaO2) and physical and mental composite score of SF-36.
ED tests were performed in 11 patients and in eight of them (73%) findings consistent with polyneuropathy and/or myopathy were observed (Table S2, Online Resource 1). The genioglossus muscle was examined only in five patients because the other six had a relative contraindication for the test, and one of them was found to be abnormal (20%).
Second evaluation (n = 29)
Compared to the 1st sleep study, 6 months after hospital discharge, sleep architecture had improved, as indicated by the significant increase in N3 stage and the decrease in AHI (Figs. 1 and 2, Table S3 Online Resource 1). Although oxygenation indices improved, the difference was not significant. However, similar to the 1st evaluation, sleep was characterised by relatively high N1 and low REM stages. SE and SFI did not change between the two sleep studies (Table S3, Online Resource 1). There was a highly significant linear relationship between AHI in the 1st and 2nd sleep study (Fig. 3). The patient who exhibited central apneas during the 1st sleep study was considered as normal at 6 months after hospital discharge. From 20 out of 29 patients (69.0%) who exhibited AHI ≥ 15 events/h in the 1st sleep study, nine were classified as normal, or with mild SDB, 6 months later (p = 0.001) (Fig. S3 Additional file 1). From nine patients who had mild SDB (AHI < 15 events/h) in the 1st evaluation, seven were classified as normal (AHI < 5 events/h) in the 2nd evaluation (p = 0.015) (Fig. S3, Online Resource 1). All patients who exhibited ESS ≥ 10 in the 1st evaluation scored less than 10 in the 2nd (Table S3, Fig. S4, Online Resource 1). However, there was no relationship between the change in sleep variables and that of ESS.
Individual data of apnea–hypopnea index (AHI) in the 1st and 2nd sleep study. Each coloured line denotes an individual patient. Median group values are shown by black squares. *Significant difference from the 1st sleep study. Arrow indicates one patient with very low sleep efficiency during the 2nd sleep study (6.8% of TST, 28 min) making the calculation of AHI in this patient unreliable
Relationship between apnea–hypopnea index (AHI) obtained in the 1st and 2nd sleep studies. Black circles denote patients in whom body mass index increased from 1st to 2nd sleep study [the numbers show the increase in body mass index in each patient, median increase 3 (2–5) kg/m2]. Solid line—regression line. Dashed line—line of identity. Arrow indicates one patient with very low sleep efficiency during the 2nd sleep study (6.8% of TST, 28 min) making the calculation of AHI in this patient unreliable
Although quality of life improved significantly, it remained below normal in all aspects (Table S4, Online Resource 1). There was no relationship between the change in quality of life from the 1st to the 2nd evaluation and that of sleep architecture, including sleep oxygenation indices.
Lung function tests were evaluated in 19 out of 24 patients at 6 months. Compared to the 1st evaluation, maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) had increased significantly at 6 months, remaining, however, lower than the predicted normal (Table S5, Online Resource 1). Overall, at 6 months, lung volume had increased, with the change being noted as significant only for total lung capacity (TLC) (Table S5, Online Resource 1). There was no relationship between the change in variables of lung function tests and that of sleep architecture.
In one out of eight patients who had abnormal ED tests at 1st evaluation, follow-up examination was not performed (lost to follow-up) (Table S2, Online Resource 1). At the 2nd evaluation the ED tests continued to be abnormal in six out of seven patients studied, while in one patient, the abnormalities were completely restored. Although full recovery of the genioglossus muscle function was observed in the patient deemed abnormal during the 1st examination, AHI remained above 15 events/h (Table S2, Online Resource 1). This patient increased body mass index (BMI) by 8 kg/m2, meeting the criteria for morbid obesity at the 2nd evaluation.
Discussion
The main findings of our study are as follows: (1) in survivors of critical illness without hypercapnia and hypoxemia, within 10 days of hospital discharge, sleep quality was poor characterised by severe disruption of sleep architecture and excessive sleep-disordered breathing, mainly of the obstructive type which in 61% of patients was classified as moderate or severe (AHI ≥ 15 events/h). (2) Although at 6 months after hospital discharge sleep quality remained relatively poor, compared to the 1st sleep study, significant improvement in N3 stage and AHI was observed, resulting in significantly more patients being classified as normal or with mild SDB. (3) Even though quality of life improved at 6 months, continued to be lower than normal. (4) There was no relationship between the change in quality of life and that of sleep disturbances.
Poor sleep quality at hospital discharge in our patients presents many similarities to that observed in non-sedated critically ill patients, the main difference being the lower sleep efficiency and the abolition of the circadian rhythm during critical illness [24, 25]. Although at 6 months after hospital discharge, N3 stage was significantly increased, sleep quality remained poor with a high percentage of N1 and low percentages of REM stages. It follows that survivors of critical illness exhibit sustained long-term poor sleep quality. Since REM is the most restorative sleep stage responsible, along with other high brain functions, for memory formation [26], poor sleep quality might contribute to long-term cognitive dysfunction observed in these patients.
Our finding that the vast majority of survivors of critical illness who needed intubation and mechanical ventilation to support the respiratory system for at least 48 h during the ICU stay exhibit obstructive SDB within 10 days of hospital discharge, although novel, is not unexpected. Current studies have shown that four pathophysiologic mechanisms, operating alone or in combination, lead to obstructive events [27, 28]. These are the following: (1) increased upper airway collapsibility (increased passive critical closing pressure of upper airways) [29], (2) high chemical loop gain (increased ventilatory sensitivity to chemical stimuli) [28], (3) low arousal threshold (arousal occurs at low inspiratory effort) [30] and (4) poor responsiveness of pharyngeal dilators (mainly the genioglossus muscle) to negative pharyngeal pressure [31]. Critical illness may adversely affect all these pathophysiologic mechanisms for various reasons. Firstly, the morphology and passive properties of upper airways may be modified due to endotracheal intubation, tissue edema and low lung volumes. Secondly, stimulation of various lung receptors due to the repair process of the lung injury, combined with interstitial lung edema and low lung volumes may increase the chemical loop gain causing unstable breathing, a well-known mechanism for obstructive and central sleep-related events. Thirdly, poor sleep quality in our patients characterised by very high percentages of N1 stage, could affect the arousal threshold. Studies have shown that the arousal threshold is 37% lower in N1 compared with N2 or N3 stages, and this decrease might contribute to the increased incidence of SDB [32]. Finally, the occurrence of ICU-acquired weakness may affect the pharyngeal dilators, particularly the genioglossus, by reducing the muscles responsiveness to negative intraluminal airway pressure [33]. We observed that in one out of the five patients tested, genioglossus contraction was reduced, while in eight out of 11 patients, findings consistent with polyneuromyopathy were evident. In addition, the reduced MIP and MEP also indicate ICU-acquired muscle weakness. Although the number of patients in whom ED tests were performed is small for a meaningful statistical analysis, at 1st evaluation, five out of eight patients with findings consistent with polyneuromyopathy exhibited moderate (n = 2) or severe (n = 3) obstructive SDB. On the other hand, three patients with normal ED tests were classified as normal (n = 2) or with mild SDB (n = 1). The activity of the dilators may also be adversely affected by the low arousal threshold. It has been shown in patients with obstructive sleep apnea syndrome (OSAS) that cortical arousals inhibit the sustained activation beyond the obstructive phase of the genioglossus muscle (referred to as after-discharge), thereby potentially promoting recurrence of obstruction [34]. Since recovery from ICU-acquired weakness may be incomplete even 5 years after ICU discharge, this mechanism may explain why several patients continued to exhibit obstructive SDB at 6 months after hospital discharge [35]. Specific quantitative studies examining the function of dilators of upper airways may clarify the role of ICU-acquired muscle weakness on sleep. Finally, bilateral or unilateral hypoglossal nerve damage following intubation has been reported and this might affect SDB [36]. Further studies, using a specific methodological approach [27], are needed to clarify the extent to which these mechanisms contribute to SDB.
The role of sympathetic activity as a potential contributing factor to poor sleep quality deserves some comments. Critical illness is a potent stimulus of the sympathetic nervous system [37] and this might contribute to reduced N3 stage [38, 39] at hospital discharge. The significant increase in N3 stage 6 months later could be partly due to decreased sympathetic nerve activity. Nevertheless, sympathetic activity was not measured in our study and thus the role of this factor in our findings is unknown.
Similar to previous studies [6, 8, 9], we observed that survivors of critical illness expressed long-term low quality of life. Nevertheless, contrary to other studies [6], neither the physical nor the mental composite score of SF-36 was related to sleep disturbances. Also, the magnitude of improvement of the quality of life at 6 months after hospital discharge was not related to the observed sleep quality. Although different study designs might explain the discrepancy among studies, the subjective nature of SF-36 could be responsible for these variable results. Similar findings were obtained with ESS, indicating that at least in these patients, SDB may not be associated with subjective sleepiness.
To our knowledge, this is the first study to prospectively collect data regarding the sleep quality of survivors of critical illness at hospital discharge and 6 months later. The high incidence of SDB at these two time points is in discordance with the studies of Lee et al. and Dhooria et al., demonstrating a low incidence of SDB in ARDS survivors studied within 6 months and at 1 month after ICU discharge, respectively [10, 11]. Selection of the studied population may explain these differences. Lee et al. studied patients who reported sleeping difficulties, while Dhooria et al. studied young patients [median age 24 (IQR 22–28)] with very few or no comorbidities, normal/low BMI and acute tropical illness with multiple system organ failure as the cause of ARDS. On the other hand, a recent preliminary study prospectively examined the prevalence of sleep apnea at 3 months of hospital discharge in 35 patients admitted in ICU with acute hypercapnic respiratory failure. Twenty percent of these patients had known underlying diagnosis of OSA, and 17% were already on home non-invasive ventilation (NIV) [40]. However, from this group, only 16 patients were studied and the prevalence of severe sleep apnea (≥ 30 events/h) was 56% [40]. Because we studied only patients without hypercapnia and hypoxemia on room air, a direct comparison between studies is not feasible. Solverson et al., using actigraphy and questionnaires, found reduced sleep quality following critical illness, which was associated with poor health-related quality of life [13]. Obviously in this study, sleep architecture and SDB were not examined.
Our study has some limitations. Firstly, although we studied patients without hypercapnia and hypoxemia and we excluded patients with pre-existing sleep disorders or diseases that are associated with high incidences of sleep abnormalities (lung fibrosis, neuromuscular disorders etc.), it is possible that some of our patients had undiagnosed obstructive sleep apnea syndrome, given the high incidence of this disease in ageing populations, particularly those with increased BMI. The prevalence of AHI ≥ 15 events/h (moderate to severe SDB) in the general population ranges between 5 to 16% in men and 1.2 to 20% in women [see ref. [41] for review]. In our study, severe SDB was observed in 70% of men and 50% of women, much higher than in the general population. Nevertheless, in our study, the severity of SDB was not related to either age or to BMI. Furthermore, the significant decrease of AHI observed at 6 months after hospital discharge clearly points out that reversible factors related to critical illness, such as ICU-acquired muscle weakness, alteration in lung volumes, tissue edema of upper airways and modulation of ventilatory control mechanisms, might be involved in sleep-related breathing disorders. Secondly, the contribution of each of the four pathophysiologic mechanisms to obstructive sleep apnea in our patients was not examined. Thirdly, ED tests were only performed on 11 patients, due to different factors (unavailability of the specific test when scheduled, patients’ unwillingness). However, we believe that due to the high incidence of abnormal tests observed in examined patients (eight out of 11), the role of ICU-acquired muscle weakness in SDB should be specifically investigated in this patient population. Finally, there was not a non-ICU hospitalised group to allow for comparisons.
Conclusions
In conclusion, we have demonstrated that the majority of survivors of critical illness who needed intubation and mechanical ventilation to support the respiratory system for at least 48 h during ICU stay, exhibited poor sleep quality and moderate to severe obstructive sleep-disordered breathing at hospital discharge. Although, at 6 months, sleep quality remained poor, sleep architecture improved, indicating that reversible factors linked to critical illness are partly responsible for sleep abnormalities. Future research is required to investigate the pathophysiological mechanisms leading to sleep disturbances following critical illness.
Abbreviations
- HRQL:
-
health-related quality of life
- ED:
-
electro-diagnostic tests
- AHI:
-
apnea–hypopnea index
- REM:
-
rapid eye movement
- SDB:
-
sleep-disordered breathing
- ICU:
-
intensive care unit
- PSG:
-
polysomnography
- SF-36:
-
36-Item Short Form Health Survey
- ESS:
-
Epworth sleepiness scale
- NCS:
-
nerve conduction studies
- EMG:
-
electromyography
- IQR:
-
interquartile ranges
- ARDS:
-
acute respiratory distress syndrome
- SFI:
-
sleep fragmentation index
- SaO2:
-
oxygen saturation
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- MIP:
-
maximal inspiratory pressure
- MEP:
-
maximal expiratory pressure
- TLC:
-
total lung capacity
- BMI:
-
body mass index
- OSAS:
-
obstructive sleep apnea syndrome
- NIV:
-
non-invasive ventilation
- CPAP:
-
continuous positive airway pressure
References
Elliot R, McKinley S, Cistulli P, Fien M (2013) Characterisation of sleep in intensive care using 24-h polysomnography: an observational study. Crit Care 17:R46
Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse JL, Jones SF (2015) Sleep in the intensive care unit concise clinical review. Am J Respir Crit Care Med 7:731–738
Kamdar BB, Needham DM, Collop NA (2012) Sleep deprivation in critical illness: its role in physical and psychological recovery. J Intensive Care Med 27(2):97–111
Freedman NS, Kotzer N, Schwab RJ (1999) Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med 159:1155–1162
Little A, Ethier C, Ayas N, Thanachayanont T, Jiang D, Mehta S (2012) A patient survey of sleep quality in the intensive care unit. Minerva Anestesiol 78:406–414
Orwelius L, Nordlund A, Nordlund P, Edell-Gustafsson U, Sjoberg F (2008) Prevalence of sleep disturbances and long-term reduced health-related quality of life after critical care: a prospective multicenter cohort study. Crit Care 12:R97
McKinley S, Aitken LM, Alison JA, King M, Leslie G, Burmeister E, Elliot D (2012) Sleep and other factors associated with mental health and psychological distress after intensive care for critical illness. Intensive Care Med 38:627–633
Hurel D, Loirat P, Saulnier F, Nicolas F, Brivet F (1997) Quality of life 6 months after intensive care: results of a prospective multicenter study using a generic health status scale and a satisfaction scale. Intensive Care Med 23:331–337
Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM (2006) Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 32:1115–1124
Lee CM, Herridge MS, Gabor JY, Tansey CM, Andrea M, Hanly PJ (2009) Chronic sleep disorders in survivors of the acute respiratory distress syndrome. Intensive Care Med 35:314–320
Dhooria S, Seghal IS, Agrawal AK, Agarwal R, Aggarwal AN, Behera D (2016) Sleep after critical illness: study of survivors of acute respiratory distress syndrome and systematic review of literature. Indian J Crit Care Med 20(6):323–331
Adler D, Pepin JL, Dupuis-Lozeron E, Espa-Cervena K, Merlet-Violet R, Muller H, Jansenns JP, Brochard L (2017) Comorbidities and subgroups of patients surviving severe acute hypercapnic respiratory failure in the intensive care unit. Am J Respir Crit Care Med 196(2):200–207
Solverson KJ, Easton PA, Doig CJ (2016) Assessment of sleep quality post-hospital discharge in survivors of critical illness. Respir Med 114:97–102
Chishti A, Batchelor AM, Bullock RE, Fulton M, Gascoigne AD, Baudouin SV (2000) Sleep-related breathing disorders following discharge from intensive care. Intensive Care Med 26:426–433
White DP, Younes MK (2012) Obstructive sleep apnea. Compr Physiol 2(4):2541–2594
Anagnostopoulos F, Niakas D, Pappa E (2005) Construct validation of the Greek SF-36 health survey. Qual Life Res 14:1959–1965
Tsara V, Serasli E, Amfilochiou A, Constantinidis T, Christaki P (2004) Greek version of the Epworth sleepiness scale. Sleep Breath 8(2):91–95
Iber C, Ancoli-Israel S, Chesson AL, Quan SF (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine, Westchester
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson SL, Tangredi MM (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 8(5):597–619
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J (2005) General considerations for lung function testing. Eur Respir J 26:153–161
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J (2005) Interpretative strategies for lung function tests. Eur Respir J 26:948–968
Preston DC, Shapiro B (2005) Electromyography and neuromuscular disorders: clinical-electrophysiologic correlations, 2nd edn. Elsevier, Philadelphia, pp 117–160
Ameridou I Neurophysiological investigation of the innervation of the 1st dorsal interosseous muscle of the foot & hand and of the extensor digitorum brevis muscle. National Archive of PhD Theses. http://www.openarchives.gr/archive/47. Last updated 2014–07-29
Boyko Y, Holst R, Jennum P, Oerding H, Nikolic M, Toft P (2017) Melatonin secretion pattern in critically ill patients: a pilot descriptive study. Critical Care Research and Practice 2017:1–8
Korompeli A, Muurlink O, Kavrochorianou N, Katsoulas T, Fildissis G, Baltopoulos G (2017) Circadian disruption of ICU patients: a review of pathways, expression, and interventions. J Crit Care 38:269–277
Casey SJ, Solomons LC, Steier J, Kabra N, Burnside A, Pengo MF, Moxham J, Goldstein LH, Kopelman MD (2016) Slow wave and REM sleep deprivation effects on explicit and implicit memory during sleep. Neuropsychology 30(8):931–945
Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butles J, Passaglia CL, Jackson AC, Malhotra A, White DP (2013) A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol 114:911–922
Wellman A, Jordan AS, Malhotra A, Fogel AB, Katz ES, Schory K, Edwards JK, White DP (2004) Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 170(11):1225–1232
Malhotra A, Pillar G, Fogel B, Beauregard J, Edwards J, White DP (2001) Upper-airway collapsibility. measurements and sleep effects. Chest 120:156–161
Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A (2013) Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med 188:996–1004
Eckert DJ, Lo YL, Saboisky JP, Jordan AS, White DP, Malhotra A (2011) Sensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J Appl Physiol 111:1644–1653
Sforza E, Krieger J, Petiau C (1999) Arousal threshold to respiratory stimuli in OSA patients: evidence for a sleep-dependent temporal rhythm. Sleep 22(1):69–75
Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim-Yeh S, Malhotra A (2010) The effect of increased genioglossus activity and endexpiratory lung volume on pharyngeal collapse. J Appl Physiol 109:469–475
Younes M, Loewen A, Ostrowski M, Hanly P (2014) Short-term potentiation in the control of pharyngeal muscles in obstructive apnea patients. Sleep 37(11):1833–1849
Hermans G, Van den Berghe G (2015) Clinical review: intensive care unit acquired weakness. Crit Care 19:274
Shah AC, Barnes C, Spiekerman CF, Bollag LA (2015) Hypoglossal nerve palsy after airway management for general anesthesia: an analysis of 69 patients. Anesth Analg 120(1):105–120
Dünser MW, Hasibeder WR (2009) Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 24(5):293–316
Somers VK, Dyken ME, Mark AL, Abboud FM (1993) Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328:303–307
Hornyak M, Cejnar M, Elam M, Matousek M, Wallin BG (1991) Sympathetic muscle nerve activity during sleep in man. Brain 114:1281–1295
Thille AW, Cordoba-Izquierdo A, Maitre B, Boyer L, Brochard L, Drouot X (2018) High prevalence of sleep apnea syndrome in patients admitted to ICU for acute hypercapnic respiratory failure: a preliminary study. Intensive Care Med 44(2):267–269
Franklin KA, Lindberg E (2015) Obstructive sleep apnea is a common disorder in the population- a review on the epidemiology of sleep apnea. J Thorac Dis 7(8):1311–1322
Acknowledgements
The authors would like to thank the nursing teams of the sleep, lung function tests and electro-diagnostic tests laboratories of the University Hospital of Heraklion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 58 kb)
Rights and permissions
About this article
Cite this article
Alexopoulou, C., Bolaki, M., Akoumianaki, E. et al. Sleep quality in survivors of critical illness. Sleep Breath 23, 463–471 (2019). https://doi.org/10.1007/s11325-018-1701-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1701-z