Abstract
Introduction and objectives
Obstructive sleep apnea (OSA) is a prevalent disorder among military veterans. The goal of this study is to compare the polysomnographic patterns of OSA in military veterans who have a history of post-traumatic stress disorder (PTSD) with those of veterans who have not PTSD.
Materials and methods
Seventy-two Iranian military male veterans were classified into two groups: those with PTSD (40 cases) and those without PTSD (32 cases). Each participant was diagnosed with OSA using an overnight polysomnography, during which sleep-related parameters such as sleep efficiency (SE) and apnea-related events were detected. The body mass index (BMI) and Epworth Sleepiness Scale (ESS) were also assessed.
Results
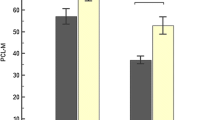
For the PTSD group, mean age was 53.83 ± 7.3 years, elapsed time since they participated in war was 28.3 ± 3.4 years, apnea-hypopnea index (AHI) was 41.2 ± 27, SE was 77.7 ± 17.55%, ESS was 7.93 ± 2.04, BMI was 26.5 ± 5.7, and PLM index was 12.725 ± 8.64. The above respective parameters for the non-PTSD group were 51.33 ± 5.9 years, 28.3 ± 3.4 years, 30.33 ± 14.7, 82.4 ± 15.65%, 10.08 ± 3.02, 31.5 ± 6.7, and 8.8 ± 3.54. The relationships of AHI with ESS and BMI were not significant in PTSD group.
Conclusion
OSA in military veterans suffering from PTSD presents more often with insomnia than obesity or increased daytime sleepiness. These findings are different from those typically seen in non-PTSD veterans with OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Military veterans with post-traumatic stress disorder (PTSD) frequently complain of sleep disturbances. The most common sleep-related complaints are difficulty in sleep initiation and maintenance, restless nights, and nightmares [1]. Poor sleep quality may exacerbate psychological disturbances, obesity, diabetes mellitus, and cardiovascular disorders in the military veterans [2, 3]. Moreover, obstructive sleep apnea (OSA) seems to be more prevalent among military veterans than among the general population [1, 3].
Obstructive sleep apnea is described as recurrent episodes of upper airway obstruction during sleep [4]. The pathophysiology of OSA is not completely clear [5]. OSA may result in serious consequences including arterial and pulmonary hypertension, cardiovascular diseases [6], and impaired cognition [7, 8]. These conditions are also prevalent among veterans [9]. Interestingly, continuous positive airway pressure (CPAP), which is an effective treatment for OSA, has been shown to relieve symptoms of PTSD in veterans with OSA [10].
In this study, we examined the polysomnographic parameters of Iranian military veterans with and without PTSD. To the best of our knowledge, this is the first study on sleep laboratory findings among a population of Middle East veterans.
Materials and methods
Seventy-two military veterans with OSA participated in a cross-sectional study. The veterans were diagnosed with OSA at the sleep laboratory of Ebn-e-Sina Hospital, Mashhad, Iran, between September 2014 and May 2015. The Ethics Committee of the Mashhad University of Medical Sciences (registered number 921368) approved the study. Written informed consent was taken from all participants. Only those people who were officially registered as military veterans with the Foundation of Martyrs and Veterans (a national foundation in charge of Veterans Affairs) were included in this study. Those veterans with a history of respiratory or cardiovascular disorders, opium consumption during the past month, or head trauma and those who had been victims of chemical exposure were excluded from the study.
Medical histories, focused on sleep-related complaints, were collected from each participant by a physician. Demographic data (including weight, height, and BMI), duration of PTSD symptoms, smoking, substance abuse, and drug history were all recorded and registered according to a check-list.
The Epworth Sleepiness Scale (ESS) scoring was applied to evaluate daytime sleepiness. The ESS, which includes eight items rated on a 4-point scale (0–3), evaluates the usual chances of dozing off or falling asleep while engaged in eight different activities. A total score of 9 or higher indicates excessive sleepiness in the responder. The validity and reliability of this questionnaire have been verified in the Iranian population [11].
We also assessed the participants with the Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI). The BAI is a 3-point scale measuring the severity of anxiety [12], with each question being scored as 0 (not at all), 1 (it did not bother me much), 2 (moderately, it was not pleasant at times), and 3 (severely, it bothered me a lot) [12]. The BDI assesses the severity of depression in children and adults. The original BDI was designed in 1961 [13] and is rated on a 4-point scale ranging from 0 to 3 based on the severity of each item; the maximum total score is 63. The standard cut-off scores are 0–9 (minimal depression), 10–18 (mild depression), 19–29 (moderate depression), and 30–63 (severe depression) [13].
After all baseline data were gathered, the participants underwent an overnight polysomnography (PSG). Sleep-related parameters including sleep onset latency, sleep efficiency (SE), wake after sleep onset (WASO), number of awakenings during the record, total sleep time (TST), and rapid eye movement (REM) latency were recorded. The apnea-hypopnea index (AHI), sleep-related movements, snoring index, arousals, and blood-oxygen desaturations were detected while the participants were monitored by a surveillance camera during sleep. Sleep efficiency was ascertained as total sleep time divided by time spent in bed expressed as a percentage. Sleep onset latency (SOL) is time in minutes from the beginning of the study to the first epoch of sleep [14]. According to the instructions of AASM, the following definitions were also given for PSG regarding the diagnosis of sleep apnea: (1) apnea occurs after 10 s of airflow cessation, (2) hypopnea happens after 10 s of a 30% decrease of air flow with brainwave arousal or a drop of more than 3% in arterial oxygen saturation, (3) the apnea-hypopnea index (AHI) is the average frequency of apnea and hypopnea in 1 h of sleep, and (4) the arousal index denotes the frequency of arousals during sleeping hours [15].
Finally, patients were referred to a psychiatrist to confirm the diagnosis of PTSD. Accordingly, the participants were divided into two groups: participants with PTSD (n = 40) and participants without PTSD (n = 32).
Statistical analysis
Descriptive statistics were used to calculate mean and standard deviation. To analyze the data, the independent samples’ t test and Pearson product-moment correlation were calculated using SPSS, version 16. A P value of < 0.05 was considered as statistically significant.
Results
In the PTSD group, the mean age was 53.8 ± 7.3 years, and the mean time of combat exposure was 28.3 ± 3.4 years. Average duration of PTSD symptoms was 25.7 ± 8.5 years. The mean age of the non-PTSD group was 51.33 ± 5.9 years, and in average, combat exposure was 27.8 ± 4.1 years (Table 1). Descriptive statistics of sleep structure (Table 2) indicated different results between the two groups for the parameters of sleep architecture. For the PTSD group, mean SE was 77.7 ± 17.56% [normal figure, > 85% [14]] and mean SOL was 32.28 ± 20.43 min [normal range, < 21 min [14]]. For the non-PTSD group, mean SE was 82.40 ± 15.65% and mean SOL time was 14.45 ± 6.2 min (P value < 0.05).
In the PTSD group, the most frequent complaint was chronic insomnia (24 sufferers; 60%); data were gathered through clinical evaluation, based on the ICSD-3 criteria for insomnia [15]. The majority of PTSD patients (n = 20) had difficulty maintaining sleep. Mean sleep efficiency in these patients was less than 75%. Other common complaints were diurnal aggression (24; 55%), morning headache (18, 45%), choking during sleep (12; 40%), and fatigue (10; 25%). All participants in the PTSD group were taking medications for insomnia, PTSD, or both. The medications most frequently taken were benzodiazepines (clonazepam, lorazepam, or alprazolam), SSRIs or SSNRIs (sertraline, citalopram, fluoxetine, or venlafaxine), gabapentin, tricyclic antidepressants, and quetiapine, see Table 1 for a complete listing. None of the participants were alcohol consumer. Thirty patients were regular smokers (more than five cigarettes per day).
In the non-PTSD group, the most common complaint was tiredness (16 individuals; 50%) followed by insomnia (nine individuals; 28.12%) and snoring (six individuals; 18.7%). Sleeping pills were taken by eight patients. Half of them (16 patients) were smokers, and none of them consumed alcohol.
The BAI scores showed that 37 PTSD veterans (92.5%) suffered from anxiety, 22 (55%) of whom had severe anxiety. Based on BDI scores, 29 PTSD veterans (72.5%) showed depression symptoms; in 13 (32.5%), the symptoms were severe. These figures were lower in the control group (Table 1). Eighteen PTSD veterans (45%) and 12 non-PTSD veterans (36.1%) had hypertension, and eight PTSD veterans (17.4) and six non-PTSD patients (18.6) had a history of heart disease.
For the PTSD group, mean BMI was 26.5 ± 5.7 kg/m2 (BMI range, 19–44 kg/m2), mean ESS was 7.93 ± 2.04, and mean AHI was 41.2 ± 27.2. The results are summarized in Table 2. Among these patients, 16 had severe sleep apnea, 15 moderate, and nine mild. We also investigated for other sleep disorders. According to ICSD-3 criteria [15], two participants in the PTSD group were diagnosed with REM sleep behavior disorder (RBD), who had REM sleep without atonia accompanied with sleep-related vocalizations. Periodic limb movement of sleep (PLMS) with an index of more than 15 was detected in eight (20%) participants. Four had a history of diabetes mellitus, and one was on imipramine. None of them abused opium. The mean PLM index was 12.725 ± 8.64 in the PTSD group. We did not find other sleep-related disorders such as bruxism or confusional arousals in either group.
In non-PTSD veterans, mean BMI was 31.5 ± 6.7, mean AHI was 30.33 ± 14.7, mean ESS was 10.08 ± 3.02, and mean PLM index was 8.8 ± 3.54. RBD was not found in this group and PLMS with an index of more than 15 was detected in eight patients (25%). All parameters are presented in Table 2.
We examined the correlation between daytime sleepiness, as evaluated by ESS, and AHI (r = 0.109), BMI (r = 0.335), SE (r = − 0.104), SpO2 (r = 0.095), and DES (r = 229) in the PTSD group. These results show that there is no significant correlation between the ESS score and either OSA severity or any other parameters. According to the independent samples test, there was no significant relationship between AHI and headache (P = 0.66), aggression (P = 0.987), insomnia (P = 0.159), tiredness (P = 0.104), or choking (P = 0.714). In other words, there was no relationship between these symptoms and the severity of sleep apnea in PTSD veterans.
Discussion
The current study was conducted on 72 Iranian male veterans with and without PTSD, all of whom had been diagnosed with OSA. Some studies suggest that the frequency of OSA is higher in military veterans with PTSD than in the general population [3, 16,17,18,19]. Male gender, age, and obesity are major risk factors for OSA [20, 21]. A high BMI is strongly associated with the risk of OSA in middle age [14], while this is not the case for young patients [21]. In our study, all OSA patients were male and most were middle-aged, but their BMI, especially in the PTSD group, was not consistent with obesity. Thus, our study suggests that high BMI is not a reliable predictor for OSA in veterans with PTSD. This is similar to Langton et al.’s study, which did not find a relationship between enlistment body mass index and the development of obstructive sleep apnea in the US military [22]. Another study by Colvonen et al. indicated that a classic presentation of OSA, such as obesity and older age, may not be seen in Iraq and Afghanistan war veterans [23]. We proposed that war-related PTSD itself might be a risk factor for OSA regardless of weight. Factors like serotonin-related mechanisms, which may be involved in the pathophysiology of PTSD [24], could play a role in developing OSA. The latter also has been shown to be associated with the presence of serotonin receptors in the brainstem centers responsible for ventilation control and in the hypoglossal neurons [25].
Regarding their presenting symptoms, sleep maintenance insomnia was the most frequent symptom in veterans with PTSD. Their mean ESS was not in the range of excessive daytime sleepiness. The PSG variables indicated significant increased sleep onset latency and decreased sleep efficiency which were compatible with the patients’ symptoms. Krakow et al.’s and Forbus et al.’s studies also showed a high frequency of OSA among American veterans suffering from insomnia [19, 26]. Whether these findings can be attributed to a high level of anxiety or other reasons, these demand further investigation; nonetheless, these findings indicate the complex clinical presentation of OSA in PTSD veterans. In addition, we did not find a significant correlation between ESS and AHI in these veterans. In comparison, non-PTSD veterans commonly complained from excessive daytime tiredness, which is similar to the majority of OSA patients. Thus, it seems that war-related PTSD alters the clinical picture of OSA. Moreover, we failed to show a significant link between AHI and any of the symptoms of PTSD veterans. Similar findings have been noted in other studies conducted with different methods and among different ethnic groups of veterans [19, 23, 26, 27].
While anxiety and depression are common symptoms in PTSD, our study demonstrates that these symptoms also occur in the absence of PTSD. Although each condition (PTSD and OSA) can increase the anxiety and depression symptoms [19, 28], the high scores of BAI and BDI among non-PTSD veterans demonstrate the potential contribution of OSA alone. Liempt et al. have shown that OSA is correlated with the severity of PTSD and, therefore, exacerbates PTSD symptoms [1].
Sleep-related movement disorders in the form of PLM were more common among subjects with PTSD; however, their occurrence was as frequent as in ordinary individuals with OSA [29, 30]. As many patients had been on medications influencing PLM (gabapentin, clonazepam, or SSRIs), it is difficult to make a rough conclusion regarding this result. Further investigations are required to evaluate the frequency of PLMS in veterans and its effect on their clinical state. Regarding RBD and its relation with PTSD or OSA, the gathered data from our study should be interpreted with caution due to the small sample size. We found cases of RBD only in PTSD group; however, according to Mysliwiec et al.’s study, RBD has been reported in both PTSD and non-PTSD veterans as a component of trauma-associated sleep disorder [31].
Conclusion
Polysomnography records from military veterans who suffer from OSA and PTSD indicate significant lower sleep efficiency (SE), which is compatible with their most common complaint (insomnia). Unlike veterans without PTSD, this condition is not accompanied by daytime sleepiness and weight gain. Thus, there should always be high suspicions of OSA in PTSD veterans, even without its common red flags (morbid obesity or sleepiness).
References
Liempt S, Westenberg H, Arends J, Vermetten E (2011) Obstructive sleep apnea in combat-related posttraumatic stress disorder: a controlled polysomnography study. Eur J Psychtraumatol 10:3402
Vieweg W, Julius DA, Fernandez A, Tassone DM, Narla SN, Pandurangi A (2006) Posttraumatic stress disorder in male military veterans with comorbid overweight and obesity: psychotropic, antihypertensive, and metabolic medications. Prim Care Companion J Clin Psychiatry 8(1):25–31. https://doi.org/10.4088/PCC.v08n0104
Yesavage JA, Kinoshita LM, Kimball T, Zeitzer J, Friedman L, Noda A, David R, Hernandez B, Lee T, Cheng J, O'Hara R (2012) Sleep-disordered breathing in Vietnam veterans with posttraumatic stress disorder. Am J Geriatr Psychiatry 20(3):199–204. https://doi.org/10.1097/JGP.0b013e3181e446ea
Duran J, Esnaola S, Rubio R (2001) Obstructive sleep apnea-hypopnea and related clinical features in a population based sample of subjects aged 30 to 70 years. Am J Respir Crit Care Med 163(3):685–689. https://doi.org/10.1164/ajrccm.163.3.2005065
Edwards BA, Eckert DJ, Jordan AS (2016 (Epub ahead of print)) Obstructive sleep apnoea pathogenesis from mild to severe: is that all the same? Respirology 22(1):33–42. https://doi.org/10.1111/resp.12913
McnNicholas WT, Bonsignore MR, the Management Committee of EU COST ACTION B26 (2007) Sleep apnea as independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29:156–178
Hrubos-Strøm H, Nordhus IH, Einvik G, Randby A, Omland T, Sundet K, Moum T, Dammen T (2012) Obstructive sleep apnea, verbal memory, and executive function in a community-based high-risk population identified by the Berlin Questionnaire Akershus Sleep Apnea Project. Sleep Breathing 16(1):223–231. https://doi.org/10.1007/s11325-011-0493-1
Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, Bozzali M, Fasano F, Giulietti G, Djonlagic I, Malhotra A, Marciani MG, Guttmann CRG (2011) Cognitive profile and brain morphological changes in obstructive sleep apnea. NeuroImage 54(2):787–793. https://doi.org/10.1016/j.neuroimage.2010.09.065
Kibler JL, Tursich M, Ma Malcom L, Greeanbarg RM (2014) Metabolic, autonomic and immune markers for cardiovascular disease in posttraumatic stress disorder. World J Cardiol 6(6):455–461. https://doi.org/10.4330/wjc.v6.i6.455
Orr JE, Smales C, Alexander TH, Stepnowsky C, Pillar G, Malhotra A, Sarmiento KF (2016) Treatment of OSA with CPAP is associated with improvement in PTSD symptoms among veterans. J Clin Sleep Med 29:140–116
Haghighi KS, Montazeri A, Mehrizi AK, Aminian O, Golkhandan AR, Saraei M et al (2013) The Epworth Sleepiness Scale: translation and validation study of the Iranian version. Sleep Breathing 17(1):419–426. https://doi.org/10.1007/s11325-012-0646-x
Asghari A, Mohammadi F, Kamrava SK, Tavakoli S, Farhadi M (2012) Severity of depression and anxiety in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 269(12):2549–2553. https://doi.org/10.1007/s00405-012-1942-6
Kaviani H, Mousavi AS (2005) Psychometric properties of the Persian version of Beck anxiety inventory. Tehran Univ Med J 65:136–140
Kryger MH, Roth T, Dement WC (2017) Principles and practice of sleep medicine. 6th ed. USA: Philadelphia Elsivier St. Louis 640
American Academy of Sleep Medicine (2014) International classification of sleep disorders, 3rd edn. American Academy of Sleep Medicine, Darien
Ocasio-Tascón ME, Alicea-Colón E, Torres-Palacios A, Rodríguez-Cintrón W (2006) The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath 10(2):70–75. https://doi.org/10.1007/s11325-005-0043-9
Webber MP, Lee R, Soo J, Gustave J, Hall CB, Kelly K, Prezant D (2011) Prevalence and incidence of high risk for obstructive sleep apnea in World Trade Center-exposed rescue/recovery workers. Sleep Breath 15(3):283–294. https://doi.org/10.1007/s11325-010-0379-7
Youakim JM, Doghramji K, Schutte SL (1998) Posttraumatic stress disorder and obstructive sleep apnea syndrome. Psychosomatics 39(2):168–171. https://doi.org/10.1016/S0033-3182(98)71365-9
Forbus L, Kelly UA (2015) Screening for obstructive sleep apnea in veterans seeking treatment of posttraumatic stress disorder. Adv Nurs Sci 38(4):298–305. https://doi.org/10.1097/ANS.0000000000000091
Wu WT, Tsai SS, Shih TS, Lin MH, Chou TC, Ting H, et al. (2015) The association between obstructive sleep apnea and metabolic markers and lipid profiles. PLoS One 10(6)
Lettieri CJ, Eliasson AH, Andrada T, Khramtsov A, Raphaelson M, Kristo DA (2005) Obstructive sleep apnea syndrome: are we missing an at-risk population? Chest J 128(4_MeetingAbstracts:133S-b-S
Langton RS, Neyra J, Downs JW, Niebuhr DW (2016) The relationship between enlistment body mass index and the development of obstructive sleep apnea in the US military. Mil Med 181(8):913–919. https://doi.org/10.7205/MILMED-D-15-00295
Colvonen PJ, Masino T, Drummond S, Myers US, Angkaw AC, Norman SB (2015) Obstructive sleep apnea and posttraumatic stress disorder among OEF/OIF/OND veterans. J Clin Sleep Med : JCSM 11(5):513–518. https://doi.org/10.5664/jcsm.4692
Kovacic Petrovic Z, Nedic Erjavec G, Nikolas Perkovic M, Peraica T, Pivac N (2016) No association between the serotonin transporter linked polymorphic region polymorphism and severity of posttraumatic stress disorder symptoms in combat veterans with or without comorbid depression. Psychiatry Res 244:376–381. https://doi.org/10.1016/j.psychres.2016.08.017
Veasey SC (2003) Serotonin agonists and antagonists in obstructive sleep apnea: therapeutic potential. Am J Respir Med 2(1):21–29. https://doi.org/10.1007/BF03256636
Krakow B, Melendrez D, Warner TD, Clark JO, Sisley BN, Dorin R, Harper RM, Leahigh LK, Lee SA, Sklar D, Hollifield M (2006) Signs and symptoms of sleep-disordered breathing in trauma survivors: a matched comparison with classic sleep apnea patients. J Nerv Ment Dis 194(6):433–439. https://doi.org/10.1097/01.nmd.0000221286.65021.e0
Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R (2006) The psychological risks of Vietnam for US veterans: a revisit with new data and methods. Science 313(5789):979–982. https://doi.org/10.1126/science.1128944
Rezaeitalab F, Moharrari F, Saberi S, Asadpour H, Rezaeetalab F et al (2014) The correlation of anxiety and depression with obstructive sleep apnea syndrome. J Res Med Sci 19(3):205–210
MN W, Lai CL, Liu CK, Yen CW, Liou LM, Hsieh CF et al (2015 Dec) Basal sympathetic predominance in periodic limb movements in sleep with obstructive sleep apnea. J Sleep Res 24(6):722–729
Vetrugno R, Provini F, Montagna P (2006 Spring) Restless leg syndrome and periodic limb movements. Rev Neurol Dis 3(2):61–70
Mysliwiec V, Brock MS, Creamer JL, O'Reilly BM, Germain A, Roth BJ (2017) Trauma associated sleep disorder: a parasomnia induced by trauma. Sleep Med Rev 30:S1087–0792(17) 30019-9
Acknowledgements
We would like to thank the research deputy of the Mashhad University of Medical Sciences and the Foundation of Martyrs and Veterans of Iran, Khorasan Razavi Province, for their cooperation with this study.
Author information
Authors and Affiliations
Contributions
Fariborz Rezaeitalab designed the project and carried out the research, collected the data, interviewed the patients, coordinated the study, interpreted polysomnographies, participated in all of the research, and prepared the manuscript. Naghmeh Mokhber collected the data. Yalda Raavnshad analyzed and interpreted the data. Soheila Saberi participated in drafting the article and revising for important intellectual content. Fariba Rezaeetalab participated in the research and gave final approval of the version to be submitted and any other revised version. All authors have read and approved the content of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Mashhad University of Medical Sciences (registered number 921368) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
This article is part of the Topical Collection on Comorbid Insomnia and OSA (COMISA) in Veterans.
Rights and permissions
About this article
Cite this article
Rezaeitalab, F., Mokhber, N., Ravanshad, Y. et al. Different polysomnographic patterns in military veterans with obstructive sleep apnea in those with and without post-traumatic stress disorder. Sleep Breath 22, 17–22 (2018). https://doi.org/10.1007/s11325-017-1596-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1596-0