Abstract
Introduction
The objective was to evaluate the feasibility of sonographic evaluation of functional tongue motion as a tool to evaluate postoperative outcomes in human subjects using breathing-synchronized stimulation of the hypoglossal nerve—a novel therapy option for patients with obstructive sleep apnea (OSA).
Material and methods
Sixteen patients with OSA (n = 16, age 60.4 ± 10.2, BMI 28.7 ± 2.4, AHI 35.0 ± 11.8) underwent sonographic evaluation of tongue motion after initiation of therapy with the Inspire II Upper Airway Stimulation system. Sonographic examination was performed in four different planes (A = floor of the mouth frontal, B = base of the tongue horizontal, C = floor of the mouth parallel to mandible, and D = floor of the mouth median sagittal) in an attempt to visualize tongue surface, tongue and hyoid motion, and the distance of protrusion.
Results
Identification of the tongue surface was achieved in all cases in planes B, C, and D and 81 % of patients in plane A. Tongue motion was evident on the right (implant) side in 63 % in plane A and 75 % in plane B. Distance of protrusion was measured in plane B at 1.04 cm (±0.51), in plane C at 1.08 cm (±0.47), and in plane D at 0.96 cm (±0.45). Hyoid protrusion was measured in plane C or D and was 0.57 cm (±0.39). Significant correlations among the three planes were observed, but there was no correlation to the reduction of apnea-hypopnea index.
Conclusion
The results indicate feasibility of sonography to identify tongue and hyoid motions during upper airway stimulation. Useful sonographic planes and landmarks, which allow visualization of dynamic effects of upper airway stimulation, could be established. The evaluation of the tongue in a horizontal (B) and in a sagittal plane (D) appears to be superior to the other investigated planes. The approximate tongue protrusion needed to generate a significant reduction of AHI and ODI was 1 cm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder with a rising prevalence of 6 % in women and 13 % in men in the USA [1–4]. OSA is characterized by recurrent upper airway (UAW) narrowing and collapse during sleep, resulting in intermittent oxyhemoglobin desaturation and sympathetic activation [5]. As a consequence, excessive daytime sleepiness and impaired quality of life occur [6].
A growing body of evidence shows the association of OSA with significant comorbidities, such as hypertension, ischemic heart disease, stroke, congestive heart failure, metabolic syndrome, diabetes, as well as an increased risk of motor vehicle accidents [7–15]. Treatment with continuous positive airway pressure (CPAP) represents the gold standard in OSA treatment and effectively improves UAW obstruction. Consequent use of CPAP can improve the documented adverse health consequences [16]. Despite its efficacy, multiple studies uniformly demonstrate that CPAP is limited by patient non-adherence, with only 68 % of patients continuing treatment after 5 years [17, 18]. Alternative treatments to CPAP include conservative methods, such as oral appliance therapy or sleep positional training and a variety of UAW surgeries that modify soft tissues surrounding the pharynx either by tissue reduction or tissue stabilization and advancement [19]. The acceptance varies for many of the mentioned procedures, due to side effects and lack of high-quality data on effectiveness [20]. Hence, there is a demand for an alternative, preferably functional surgical approach to treat OSA in patients with CPAP non-adherence.
The cause of UAW obstruction is related to reduced activity of UAW dilatator muscles during sleep, mainly the genioglossus muscle, which is not addressed by current therapies [21, 22]. Unilateral selective stimulation of the hypoglossal nerve, causing tongue protrusion, has been developed recently and various multicenter trials demonstrate its beneficial effect in the treatment of selected patients with OSA [6, 23]. The response rate may be improved by excluding patients who exhibit complete concentric collapse at the level of the soft palate during a drug-induced sleep endoscopy (DISE) [23–26].
Tongue muscles can be visualized by different imaging modalities. In general, computed tomography represents the diagnostic standard in the imaging of head and neck pathologies, unless a more detailed exposure of soft tissue, such as the tongue, is required. Here, magnetic resonance imaging is the superior modality. Sonography combines the property of a high-resolution visualization of soft tissues with the possibility of dynamic imaging and a ubiquitous availability.
Recently, different tongue motions were detected in patients with hypoglossal nerve stimulation during surgery and postoperatively, which result in different outcomes [22]. Superficial tongue movement can be visualized directly or with nasal endoscopy. In addition, there is the need for a better method to detect tongue motion during stimulation toward eventually developing a validated, non-invasive predictive marker for outcomes [22]. Sonography, as a non-invasive and non-irradiating imaging modality with the ability to obtain high-resolution images in neck structures and the ability to visualize tongue movement in various planes, might be valuable to fulfill this need. Additionally, sonography enables visualization not only of the tongue surface but also of intrinsic structures, such as single muscles or the hyoid bone. As upper airway stimulation represents a novel treatment alternative in OSA, further information on therapy titration using different evaluation methods is desirable.
The aim of the study was to analyze the feasibility of sonography in the evaluation of tongue motion during upper airway stimulation for OSA and to identify the ideal sonography planes and landmarks for this purpose.
Material and methods
Patient selection
Patients with moderate-to-severe OSA (15/h < apnea-hypopnea index (AHI) < 65/h), who received an implant for selective upper airway stimulation (UAS) were enrolled. Screening included inpatient polysomnography, clinical examination, and DISE to characterize the pattern of UAW obstruction according to the VOTE classification, and to rule out complete concentric collapse at the level of the soft palate [27]. The Epworth Sleepiness Scale (ESS) was used for evaluation of daytime sleepiness [28]. Patients were excluded if a body mass index (BMI) above 35 kg/m2 was present. Patients were also excluded if pronounced anatomical abnormalities preventing the effective use of UAS were identified during clinical examination (e.g., enlarged tonsils). Further exclusion criteria were a diagnosis of chronic obstructive pulmonary disease, New York Heart Association class III or IV heart failure, neuromuscular disease, hypoglossal nerve palsy, recent myocardial infarction or severe cardiac arrhythmias, persistent uncontrolled hypertension despite medication use, active psychiatric disease, and the foreseeable requirement of magnetic resonance imaging. The in- and exclusion criteria were adapted from the established criteria used in the STAR trial [6]. Informed consent was obtained for each patient. The study was approved by the local ethics committee (Fakultät für Medizin, Ethikkommission, Technische Universität München, Germany).
Upper airway stimulation system
Qualified participants underwent surgical implantation of the UAS system (Inspire II Upper Airway Stimulation System, Inspire Medical Systems, Maple Grove, Minnesota, USA). The UAS system was implanted on the patient’s right side under general anesthesia. Three surgical incisions are required for the placement of the components of the UAS system. The stimulation lead is placed around selected hypoglossal nerve fibers responsible for tongue protrusion via a horizontal upper neck incision. A second incision is required inferior to the clavicle to create a pocket superficial to the pectoralis major muscle to accommodate the implanted pulse generator (IPG). The sensing lead, enabling detection of breathing efforts to generate synchronized hypoglossal nerve stimulation, is placed via a third incision on the right lateral chest wall within a passage between the external and internal intercostal muscles. Both leads are connected to the IPG, using a subcutaneous tunneling device. Proper functioning of the complete system was ascertained prior to closure. The device was activated approximately 1 month after implantation, and patients were instructed in use of the patient sleep remote used to initiate and terminate the therapy for nighttime home use, and to self-titrate stimulation intensity within a physician-programmed range. After 1 month of nocturnal acclimatization, the UAS therapy was further titrated during inpatient polysomnography [19, 21–23, 29].
Sonographic examination
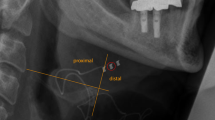
Sonographic examinations were performed using an Acuson S2000 (Siemens Healthcare, Erlangen, Germany) equipped with a linear transducer (9L4, Siemens Medical Solutions, Germany). Time of investigation was 2 months postimplantation, which equates to 1 month after initiation of therapy and after polysomnography-controlled titration of UAS. The stimulation amplitude, which was titrated during the inpatient polysomnography 2 months postimplantation, was used for sonographic examination. Therefore, this time was chosen for the examination. Sonographic examinations were performed in four different planes: A = floor of the mouth frontal, B = base of the tongue horizontal, C = floor of the mouth parallel to the mandible, and D = floor of the mouth sagittal, each of which is illustrated in Fig. 1. Exposure of the tongue surface, tongue motion, and distance of the tongue and hyoid bone protrusion were each measured during stimulation of the hypoglossal nerve. Examinations were recorded for further analysis. ShowCase software (Trillium Technology, Ann Arbor, MN, USA) was used for those analyses.
The four different planes of sonographic examination during stimulation of the upper airway: a floor of the mouth frontal, b base of the tongue horizontal, c floor of the mouth parallel to the mandible, and d floor of the mouth sagittal. The position of the ultrasound probe and the ultrasound scan are illustrated
Statistical analysis
Version 23.0 of the Statistical Package for the Social Sciences software (SPSS, Chicago, IL, USA) was used. Descriptive statistics were calculated for demographic variables and sonographic measurements of tongue motion. A paired t test was used to compare baseline and postimplantation values. Spearman’s rank correlation was used for analysis of correlations. Data are given as mean ± standard deviation. P values ≤0.05 were considered statistically significant.
Results
Patient characteristics and clinical outcomes
The study population consisted of 16 participants (100 % men), with a mean age of 60.4 years (SD 10.2) and a mean BMI of 28.7 kg/m2 (SD 2.4). All participants had a history of non-adherence to CPAP therapy. The mean AHI before implantation was 35.0/h (SD 11.8), and the mean oxygen desaturation index (ODI) was 32.8/h (SD 14.6). The mean ESS before implantation was 11.7 (SD 6.4). The UAS system was successfully implanted in all patients. The system was activated for the first time 1 month after implantation, with a mean functional threshold of 0.53 V (SD 0.22). The functional threshold is defined as the level of voltage at which bulk tongue motion is achieved. After 1 month of further nocturnal acclimatization and self up-titration at home (increments of 0.1 V, within patient control range established by physician), the mean incoming stimulation amplitude was 1.57 V (SD 0.36). Further titration during polysomnography resulted in a mean stimulation amplitude of 1.94 V (SD 0.46). Mean AHI with UAS was 3.4/h (SD 2.9, p < 0.001) and mean ODI was 5.2/h (SD 3.7, p = 0.004). The mean ESS was reduced to a value of 6.8 (SD 2.8, p = 0.004, Table 1), examined after inpatient overnight polysomnographic titration.
Sonographic examination
Visualization of tongue movement via a frontal plane of the floor of the mouth (plane A) enabled exposure of the tongue surface in 81 % of cases. Tongue protrusion could be described as predominantly right-sided in 63 % of cases. Horizontal sonography of the base of the tongue (plane B) enabled exposure of the tongue surface in 100 % of cases. Tongue motion was described as predominantly right-sided in 75 % of cases. The mean tongue protrusion in this plane was 1.04 cm (SD 0.51, Fig. 2). Examination of the floor of the mouth with a plane parallel to the mandible (plane C) enabled exposure of the tongue surface in 100 % of cases. The mean tongue protrusion in this plane was 1.08 cm (SD 0.47, Fig. 3). Tongue protrusion in plane C correlated with the titrated threshold (r = 0.696, p = 0.006). Bilateral tongue motion in plane A correlated with the amount of tongue protrusion in plane C (r = 0.789, p = 0.001). Sagittal sonography of the floor of the mouth (plane D) enabled exposure of the tongue surface in 100 % of cases. Mean tongue protrusion in this plane was 0.96 cm (SD 0.45, Fig. 4), and the mean protrusion of the hyoid bone was 0.57 cm (SD 0.39, Fig. 5). Results are summarized in Table 2. In addition to the protrusion of the hyoid bone, activation of the geniohyoid muscle by stimulation could be evaluated. Hyoid protrusion showed correlation with tongue protrusion in plane B (r = 0.735, p = 0.007). No significant correlation between tongue or hyoid protrusion and absolute or relative AHI or ODI reduction was found.
Sonography of the floor of the mouth with a plane parallel to the mandible (plane C). On the left side, the hyoid bone is visible with the attached geniohyoid muscle without stimulation. On the right side, the contraction of the geniohyoid muscle is visible during stimulation causing protrusion of the hyoid bone (distance 1.91 cm)
Discussion
Useful sonographic planes for the visualization of essential landmarks of UAS were established. The use of sonographic evaluation of various factors in tongue and hyoid motion during UAS for obstructive sleep apnea was demonstrated for the first time. The effect of UAS in this clinical cohort was consistent with other reports, showing a beneficial effect of UAS on AHI and ODI as well as health-related quality of life in patients with moderate-to-severe OSA. Comparable results have already been published as early as 2001, namely by Schwartz et al. in a pilot study on UAS [21].
To our knowledge, there is only one other study, published by Goding et al. that used imaging to investigate airway changes during hypoglossal nerve stimulation. In this study, fluoroscopy was used in patients who received another stimulation system (Apnex Medical Hypoglossal Nerve Stimulation), which has been taken off the market [30]. Changes in the anteroposterior pharyngeal airway as well as position of the tongue base and the hyoid bone were recorded in this cohort. Anterior tongue base movements and increased airway dimensions of the pharynx were demonstrated independent of BMI. In contrast to sonography, fluoroscopy allows visualization of the posterior pharyngeal wall, which cannot be achieved with ultrasound, and therefore, accurate measurement of the airway diameter is possible. However, this modality requires radiation, and visualization of single muscle groups of the tongue is not possible. These limitations can be overcome with sonography. Until now, no other investigations have been published on any other imaging modality for evaluating the UAS therapy.
Sonography is feasible to provide alternative imaging for intra- and postoperative evaluation of tongue motion. Multiple planes can reveal substantial information about various tongue deformations in real time [31]. Horizontal planes enable the differentiation between unilateral and bilateral movements (plane A and B in this study), whereupon horizontal exposure of the base of the tongue just above the hyoid bone provides additional information on the distance of tongue protrusion (plane B). This plane seems to be more relevant since successful UAS therapy results in dilatation of the UAW at the level of the tongue base. Sagittal planes enable visualization of most of the length of the tongue (plane C and D in this study). In plane C, with sagittal paramedian projection on the right side parallel to the mandible, reliable exposure of the tongue surface and measurement of tongue protrusion were possible. Additionally, contraction of the horizontal and oblique neuromuscular compartments of the genioglossus muscle could be observed. Demonstration of the tongue surface and tongue motion in plane D is possible in a more standardized manner, as this plane is less prone to variation. While visualization of the genioglossus muscle is limited in this plane (as the midline septum of the tongue should be displayed in this plane), other details come into focus including the hyoid bone and geniohyoid muscle. Accordingly, contractions of the geniohyoid muscle with resulting anterior displacement of the hyoid bone can be evaluated. Sonographic examinations could be further used as a tool to evaluate efficacy of upper airway stimulation. In the presented cohort, a significant reduction of AHI and ODI down to normal ranges was achieved by a stimulated tongue protrusion of approximately 1 cm (0.96 cm in plane D and up to 1.08 cm in plane C). This could be used as an approximate value for therapy titration and determinant of success after implantation.
Though the geniohyoid muscle is not confirmed to be a pure protrusor muscle—it moves the hyoid bone up and forward during the first phase of deglutition—its contribution to UAW dilatation and reduction of airway resistance is well established [32]. Anterior movement of the hyoid bone during UAS was demonstrated in 23 of 25 patients with fluoroscopy by Goding et al. [30]. Furthermore, stimulation of the genioglossus and geniohyoid muscles reduces the UAW resistance [1]. These results implicate the possible beneficial effect of including the innervating fibers from the first cervical nerve (C1 branch), which supply the geniohyoid muscle and travel alongside the hypoglossal nerve, into the stimulation lead’s cuff. As the C1 branch is sometimes hard to detect during surgery due to its variable pathway, sonography can be helpful in the intraoperative evaluation of stimulation of the geniohyoid muscle and in the postoperative titration of the UAS device, by enabling differentiation between active contractions and passive movement of the geniohyoid muscle.
The extent of tongue and hyoid protrusion in this study did not correlate with the improvement of AHI or ODI in this group of patients. An explanation for this is that we had no non-responder to UAS in our cohort, and therefore, only patients with significant improvements in objective parameters were included. Hence, tongue motions as observed here were appropriate for a response. It is conceivable that non-responders to UAS, i.e., patients who did not show an adequate improvement of AHI or ODI during UAS, present with less tongue or hyoid protrusion in sonographic examination. Another explanation for the absence of correlation between sonographic parameters and clinical improvement is that UAS activation only used the titrated stimulation amplitude. During this study, the sonographic examinations were performed 2 months after implantation, using effective stimulation amplitude that was defined in overnight titration. Further investigations could focus on the relationship between different stimulation amplitudes and the extent of tongue and hyoid protrusion, as well as its predictive value in acute postoperative determination of treatment response.
In conclusion, useful sonographic planes and landmarks, which enable the visualization of dynamic effects of upper airway stimulation, were established. The evaluation of the tongue in a horizontal (B) and in a sagittal plane (D) appears to be superior to the other investigated planes. The average tongue protrusion needed to generate a significant reduction of AHI and ODI was approximately 1 cm. The value of sonography during placement of the stimulation lead or the postoperative therapy titration requires further investigation and is part of ongoing research. A matter of particular interest is the hyoid protrusion caused by recruitment of the geniohyoid muscle. Further investigations will concentrate on the relationship between the extent of tongue and hyoid protrusion with stimulation amplitude.
References
Pengo MF, Steier J (2015) Emerging technology: electrical stimulation in obstructive sleep apnoea. J Thorac Dis 7:1286–1297
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177:1006–1014
Steier J, Martin A, Harris J, Jarrold I, Pugh D, Williams A (2014) Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision across the UK. Thorax 69:390–392
Strollo PJ, Rogers RM (1996) Obstructive sleep apnea. N Engl J Med 334:99–104
Strollo PJ, Soose RJ, Maurer JT, de Vries N, Cornelius J, et al. (2014) Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 370:139–149
Narkiewicz K, Somers VK (1999) Obstructive sleep apnea as a cause of neurogenic hypertension. Curr Hypertens Rep 1:268–273
Martinez D, Klein C, Rahmeier L, da Silva RP, Fiori CZ, et al. (2012) Sleep apnea is a stronger predictor for coronary heart disease than traditional risk factors. Sleep Breath 16:695–701
Palomäki H, Partinen M, Juvela S, Kaste M (1989) Snoring as a risk factor for sleep-related brain infarction. Stroke 20:1311–1315
Bradley TD, Rutherford R, Grossman RF, Lue F, Zamel N, et al. (1985) Role of daytime hypoxemia in the pathogenesis of right heart failure in the obstructive sleep apnea syndrome. Am Rev Respir Dis 131:835–839
Lévy P, Bonsignore MR, Eckel J (2009) Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J 34:243–260
Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL (2002) Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165:677–682
Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM (2009) Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 108:246–249
Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, et al. (2010) Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 122:352–360
Tregear S, Reston J, Schoelles K, Phillips B (2009) Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med 5:573–581
Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE (2011) A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 15:343–356
McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ (1999) Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 159:1108–1114
Stuck BA, Leitzbach S, Maurer JT (2012) Effects of continuous positive airway pressure on apnea-hypopnea index in obstructive sleep apnea based on long-term compliance. Sleep Breath 16:467–471
Woodson BT, Gillespie MB, Soose RJ, Maurer JT, de Vries N, et al. (2014) Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg 151:880–887
Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB, et al. (2010) Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 33:1396–1407
Schwartz AR, Bennett ML, Smith PL, De Backer W, Hedner J, et al. (2001) Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 127:1216–1223
Heiser C, Maurer JT, Steffen A (2015) Functional outcome of tongue motions with selective hypoglossal nerve stimulation in patients with obstructive sleep apnea. Sleep Breath 20:553–560
Van de Heyning PH, Badr MS, Baskin JZ, Cramer Bornemann MA, De Backer WA, et al. (2012) Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope 122:1626–1633
Steffen A, Frenzel H, Wollenberg B, König IR (2015) Patient selection for upper airway stimulation: is concentric collapse in sleep endoscopy predictable. Sleep Breath 19:1373–1376
Dotan Y, Golibroda T, Oliven R, Netzer A, Gaitini L, et al. (2011) Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur Respir J 38:338–347
Vanderveken OM, Maurer JT, Hohenhorst W, Hamans E, Lin HS, et al. (2013) Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 9:433–438
Kezirian EJ, Hohenhorst W, de Vries N (2011) Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol 268:1233–1236
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Maurer JT, Van de Heyning P, Lin HS, Baskin JZ, Anders C, et al. (2012) Operative technique of upper airway stimulation: an implantable treatment of obstructive sleep apnea. Oper Tech Otolaryngol Head Neck Surg 23:227–233
Goding GS, Tesfayesus W, Kezirian EJ (2012) Hypoglossal nerve stimulation and airway changes under fluoroscopy. Otolaryngol Head Neck Surg 146:1017–1022
Stone M (2005) A guide to analysing tongue motion from ultrasound images. Clin Linguist Phon 19:455–501
Bassiri Gharb B, Tadisina KK, Rampazzo A, Hashem AM, Elbey H, et al. (2015) Microsurgical anatomy of the terminal hypoglossal nerve relevant for neurostimulation in obstructive sleep apnea. Neuromodulation 18:721–728
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
Benedikt Hofauer, Andreas Knopf, Muras Bas, Markus Wirth, and Konrad Stock certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript. Kingman Strohl is the Site Principal Investigator at Case Medical Center for the Star Phase III FDA trial and for the Post-Approval Study for upper airway stimulation (Inspire Medical Systems); no other conflicts to declare. Clemens Heiser is study investigator and consultant of Inspire Medical Systems and received personal fees from Neuwirth Medical Products, Heinen & Loewenstein, Sutter Medizintechnik, outside the submitted work; no other conflicts to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments of comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hofauer, B., Strohl, K., Knopf, A. et al. Sonographic evaluation of tongue motions during upper airway stimulation for obstructive sleep apnea—a pilot study. Sleep Breath 21, 101–107 (2017). https://doi.org/10.1007/s11325-016-1383-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-016-1383-3