Abstract
Purpose
Obesity is one of the main predisposing factors for obstructive sleep apnea (OSA) hypopnea syndrome. It has been described that body mass index (BMI) influences the accuracy of oxygen desaturation index (ODI) for the diagnosis of OSA by polysomnography (PSG). We analyzed the relationship between traditional indicators: apnea-hypopnea index (AHI) and ODI in a population at high risk for OSA, by respiratory polygraphy (RP) and PSG.
Methods
This is a retrospective study of 1898 patients with suspicion of OSA, from which 1053 underwent RP and 582 underwent PSG with OSA. We compared results considering gender, age, and degree of obesity.
Results
This study included 1333 records of patients with OSA—more than 80 % of whom were overweight or obese. We observed that AHI and ODI increased progressively with obesity grade and said increase was associated with BMI only in men. The evaluation of the agreement between AHI and ODI found a difference between normal weight and obese patients, regardless of gender.
Conclusions
Study findings contribute to understand the role of oximetry in the diagnosis of OSA in obese patients. Our results were observed using full PSG and a simplified home method. The correlation between these indicators could improve our clinical interpretation of OSA severity among obese patients when abbreviated tests are used.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are multiple risk factors and comorbidities that increase the risk for sleep apnea, such as overweight, obesity, alcohol consumption, smoking, nasal congestion, menopause, large neck circumference, craniofacial abnormalities, hypothyroidism, and acromegaly [1].
During the last decades, there has been a rapid growth in the incidence of obesity [2], being today one of the most important predisposing factors. This condition increases the risk for hypertension, dyslipidemia, diabetes, cardiovascular events, and respiratory complications [1, 3]. Approximately, one billion individuals worldwide are overweight, and overweight prevails by more than 20 % in western countries [4]. Data from surveys examining risk factors in Argentina indicate that more than half of the Argentine population (53.4 %) has some degree of excess weight [5].
Patients with obstructive sleep apnea (OSA) show high levels of hypoxia which could trigger inflammation and oxidative stress mechanisms, favoring autonomic deregulation and increasing the risk for metabolic syndrome or cardiopulmonary diseases [6]. This could be related to the abnormal metabolism of visceral fat in obese individuals [7].
Although it has been proved that moderate changes in body weight are associated to an increase in OSA severity, this relationship varies widely among patients with a body mass index (BMI) over 40 [8, 9]. Serafini et al. described that severely obese patients referred for bariatric surgery showed no correlation between BMI and worsening of OSA as determined by an increase in the apnea-hypopnea index (AHI) [10]. Furthermore, Lings et al. stated that the sensitivity of the oxygen desaturation index (ODI) increases as BMI increases, while its sensitivity is rather poor when it comes to detecting sleep disorders in normal weight patients [11].
OSA prevalence has been described to be higher in men, with a ratio of men to women of 3.2:1. This could be related to the effect of sex hormones, changes in body fat distribution, neck circumference, increased airway collapsibility, and central ventilatory control [12]. However, prevalence of sleep disorders in women could be influenced by menopause-related hormonal changes. Block et al. observed more cases of OSA among post-menopausal women [13].
Even though severely obese patients are at a high risk for OSA, the degree of correlation between traditional indicators to assess disease progression or severity (AHI and ODI) is still poorly understood using simplify methods (respiratory polygraphy (RP)).
The aim of the study was to evaluate the relationship between the apnea/hypopnea index and ODI according to different degrees of obesity.
Materials and methods
Design
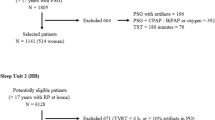
A retrospective analytical study was conducted using a database that systematically collected data from patients referred to the sleep disorders unit of two tertiary care and university hospitals in Buenos Aires City: the British Hospital and the German Hospital. The former collected information from RP and the latter from full polysomnography (PSG). Patient identity was kept confidential, and no personal data were disclosed.
Study population
Consecutive patients above 18 years of age, both men and women, were included in the study. Patients who required oxygen therapy or noninvasive ventilation during RP or PSG were excluded, as well as patients with a diagnosis of COPD, chronic heart failure, neuromuscular disease, insomnia, clinical symptoms of parasomnia, periodic limb movement, and narcolepsy. The protocol was approved by the Ethics and Review Committee at “Hospital Británico de Buenos Aires and Hospital Alemán” in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Questionnaires and collection of anthropometric data
During distribution of RP instruments or PSG procedure, the following data were collected: demographic data (age and gender), anthropometric data (e.g., BMI (kg/m2), and clinical data on subjective sleepiness.
According to the definition of the World Health Organization (WHO), patients were classified into the following groups: normal weight (BMI <25), overweight (BMI between 25.0 and 29.9 kg/m2), obese (BMI between 30 and 39.9), and morbidly obese (BMI ≥40) [4].
All patients completed the Epworth Sleepiness Scale (ESS), a validated version translated into Spanish [14].
Respiratory polygraphy
Records were taken from patients recruited in the British Hospital using Apnea Link Plus (ResMed, Australia), a type III portable monitoring device. Monitored aspects included the following: nasal pressure sensing (linearized to obtain a flow-time curve), respiratory effort with single qualitative belt, oximetry for information on pulse saturometry (SO2) with average time of signal <1 s, and heart rate measured with a thimble-shaped sensor (XPod, Nonin, USA).
Patients were guided by hospital qualified staff on the use and assembly of RP instruments the same day that overnight records were taken using the self-administered technique at home. Demonstrations included mock installation and operation of the polygraph device. Records were downloaded, edited, and analyzed using Apnea Link® 9.0 software. Manual editing of automatically recorded data was performed by a team of pulmonologists trained in the guidelines of the American Academy of Sleep Medicine (AASM) [15].
Polysomnography
All the subjects who underwent PSG were recruited by the German Hospital and tested using a computerized polysomnographic system (NEUROTRACE or MINI-PC; Akonic, Buenos Aires, Argentina) that included electroencephalogram (F4/A2, C4/A2, and O2/A2), bilateral electrooculogram, submental electromyogram, bilateral leg electromyogram, and electrocardiogram. A nasal cannula, thoracic and abdominal piezoelectric belts, and a finger probe (Nonin, OEM III) were used to assess airflow, respiratory effort, and SO2, respectively.
PSG reading was performed manually by a widely experienced medical staff. The sleep stages and the arousals, analysis of apneas, hypopneas (identical criteria to RP scoring), and respiratory effort-related arousals (RERAs) were in agreement with international criteria [15].
Criteria used
Only edited records with more than 4 h of good-quality signal were accepted. A respiratory event was considered apnea when there was a drop in nasal airflow by <90 % and hypopnea when nasal airflow decreased by ≥50 % associated with a drop in oxygen saturation of ≥3 %, of at least 10 s, in both cases (Chicago criterion). Flow reduction ≥50 % associated with arousal was scored as hypopnea when PSG was used.
AHI was defined as the number of respiratory events (apneas or hypopneas) over total recording time (TRT) during RP and total sleep time (TST) during PSG.
Patients were classified as normal (AHI <5/h), mild (AHI between 6 and 14.9 events per hour), moderate (AHI between 15 and 29.9/h), and severe (AHI >30/h). The oxygen desaturation index (ODI) was calculated as drop in oxygen saturation ≥3 % in comparison with immediately preceding basal value, and it has been calculated over percentage of TRT valid for analysis (manually edited pages) when we analyzed the RP record.
Statistical analysis
Results were presented as percentage of categorical variables or as mean and standard error for numerical variables. The Mann-Whitney test was used to compare differences between two groups, and the nonparametric Kruskall-Wallis test together with Dunn’s multiple comparison test was used to compare results of three or more groups. The statistical analysis was carried out with Prism 5 software (GraphPad, La Jolla, CA) and MedCalc Statistical Software version 14 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014).
Results
Characteristics of studied population
During the 3-year term of the study, 1898 records were collected from patients who underwent RP or PSG for having high clinical probability of sleep apnea. Forty-six patients were excluded from the study for having a diagnosis of COPD, 27 for interstitial pulmonary disease, and 13 for neuromuscular diseases. In addition, 169 patients were excluded for presenting records lasting less than 4 h and 8 due to incomplete data.
Thus, 1053 RP and 582 PSG records from patients with a suspicion of OSA were finally analyzed.
Out of all studied records, 1333 (81.5 %) had an AHI >5 events per hour. As depicted in Table 1, patients with OSA presented more advanced age and higher BMI than patients without OSA.
Classification of subjects studied according to BMI
In the population studied by RP, only 11.5 % had normal BMI (<25). Out of these 121 patients, 78 had sleep apneas and represented 9.2 % of the population with OSA. They were mostly men (67.1 %) with a mean age of 56.4 ± 1.9 years.
The 21.8 % of the patients studied by PSG had normal weight. Out of these, 88 patients (18.0 % of the total population studied) had OSA. Of them, 56 % were men with a mean age of 52.9 ± 1.8 years old.
When the patients were classified by their BMI, we observed that more than 80 % of the analyzed population was either overweight or obese. While most normal weight, overweight, and obese patients were men, the group of morbidly obese patients studied by RP was mainly made up of female and younger patients, 45.5 % of whom were enrolled from a bariatric surgery program. We observed in both groups that obese patients were more symptomatic compared with normal weight or morbidly obese subjects; however, these results did not reach statistically significant differences (Table 2(A and C)). We also analyzed BMI in the population grouped by age; nevertheless, we did not find significant differences (Table 2(B and D)).
Influence of gender, age, and BMI on respiratory sleep disorders
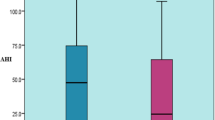
Obese men showed a significant increase in AHI (events/h) as compared to normal weight patients (28.5 ± 1.22 [obese]; 36.9 ± 2.7 [morbidly obese] versus 14.3 ± 1.4; p < 0.001) using data of RP (Fig. 1a) and (39.1 ± 2.1 and 66.3 ± 7.9 versus 22.1 ± 2.2, respectively; p < 0.001) using data of PSG record (Fig. 1c). ODI (events/h) using 3 % threshold showed similar results: (32.1 ± 1.2 [obese]; 41.8 ± 2.3 [morbidly obese] versus 15.8 ± 1.4; p < 0.001) (Fig. 2a) and (29.7 ± 2.2 and 65.6 ± 8.1, respectively; versus 15.5 ± 2.2; p < 0.001) (Fig. 2c). However, these findings were not observed in women (Fig. 2b, d).
To analyze the role of age, patients were grouped as follows: less than 40, between 40 and 60, and more than 60 years of age. AHI showed no proportional increase in relation to age in patients studied by both methods (Fig. 3). Findings in men studied by PSG showed a significant increase of ODI in patients older than 60 years old (25.3 ± 1.8) compared with patients younger than 40 (22.5 ± 2.7; p < 0.05) (Fig. 4c). Besides, a proportional increase in ODI in relation to age was found in women studied by PSG: 4.8 ± 1.1 (<40 years old), 12.4 ± 1.4 (40 to 60 years old) (p < 0.05), and 15.0 ± 2.1 (>60 years old) (p < 0.001) (Fig. 4d).
Analysis of correlation between traditional indicators (differences between AHI and ODI)
We found that the difference between AHI and ODI increased progressively with obesity level, both for men and women: −1.5 ± 0.4 (normal weight), −1.5 ± 0.4 (overweight), −3.6 ± 0.4 (obese), and −5.1 ± 1.1 (morbidly obese), p < 0.001, in men and −1.3 ± 0.3, −1.7 ± 0.3, −3.6 ± 0.4, and −4.4 ± 0.5, p < 0.001, respectively, in women using data of RP (Fig. 5a, b). Similar findings were obtained when we analyzed data collected by PSG: 6.5 ± 0.9, 8.8 ± 0.8, 9.6 ± 1.3, and 12.0 ± 5.8 in men and 5.4 ± 0.8, 7.2 ± 1.5, 9.6 ± 1.3, and 10.7 ± 3.1 in women, respectively (Fig. 5c, d). We also have performed a Bland-Altman plot to show these differences. Data about men population with morbidly obesity were shown in Fig. 5e, f.
Difference between AHI versus ODI in men (a, c) and women (b, d) grouped per obesity class. Dots for extreme values (the plot showed the whiskers of the 5 to 95th centiles from confidence interval). Bland-Altman plot was performed studying men with morbid obesity: e population studied by RP and f population studied by PSG
Finally, we have analyzed the correlation between AHI and ODI. We find spearman r of 0.9 and 0.8 with p < 0.001 in patients diagnosed using RP and PSG, respectively.
No statistically significant differences were found between AHI-ODI agreement and age in patients studied by RP (Fig. 6).
Discussion
Findings in this “at-risk” population show that variables such as age, gender, and obesity could be related to sleep apnea and its consequence in the SO2. Different mechanisms have already been proposed to explain the relationship between obesity and apnea, such as decreased thoracopulmonary compliance, shortening of the trachea, reduced pulmonary volumes, abnormalities in ventilatory control [16], and hormonal changes related to adipose organ metabolism, besides age-related factors which could influence the onset of abnormal respiratory events [17]. Previous studies, using in-lab PSG, have shown that severity indicators commonly used to assess OSA behave differently, both in obese and in advanced age populations [18].
In spite of its low sensitivity, ESS is one of the validated methods to assess subjective sleepiness in patients with a clinical suspicion of OSA. ESS scores showed gender-related differences, a finding that could be explained by the lower subjective sleepiness perception described in women [19]. Our study was conducted in a population at high risk for sleep disorders; however, our patients were not very symptomatic. When symptoms were assessed with regard to the degree of obesity, a slight increase was perceived in the ESS scores of obese patients. ESS scores were lower in patients with morbid obesity. Araghi and collaborators recorded similar findings using PSG [20]. Even though, this result could be controversial—since it could be influenced by functional respiratory reserve, mean saturation during sleep, and hypopnea definition criteria—similar results have been previously described [21, 22]. These findings could attach more importance to the role of oximetry in the diagnosis of sleep respiratory disorders in subjects with a BMI >25.
Although guidelines in effect acknowledge a high correlation between AHI and ODI (>0.95) [23], our findings show that agreement between these indicators disappears progressively with the increase of BMI. An underestimation of AHI, ranging between 10 and 15 %, has been previously described, when obtained from RP as compared to PSG [24, 25]. In addition to this, another apparent limitation of RP is not being able to “see sleep” and therefore micro-arousals. Therefore, RP cannot define incomplete obstruction events known as Respiratory Effort-Related Arousals (RERAs), more frequently described in women. Moreover, it could be related with the applicability of our results, because these patients may have higher rate of RERA compared with the usual middle-aged male patient.
In those patients who underwent PSG, flow reduction ≥50 % associated with arousal was scored as hypopnea, showing that AHI was higher than the ODI. This point could be explained by the higher sensitivity of PSG to identify hypopneas.
Notwithstanding this, AHI is still the most frequently used parameter to classify disorder severity, both in PSG and in RP [26]. Values of AHI and ODI obtained by RP could underestimate the real value, though the differences between AHI and ODI have shown similar behavior in both diagnostic methods.
Obese individuals may have fall of SO2 without association with the presence of apneas or hypopneas with the usual methods of measurement in RP or PSG (nasal pressure, bands, and thermistors). This can cause a reduced specificity of pulse oximetry (increased false positives) when used as a tool to diagnose OSA [27, 28]. The severity of oxygen desaturation during sleep disorders has been correlated with the degree of obesity expressed as body mass index (BMI). These findings have been previously described by Ling, who evaluated more than 11,000 patients with PSG [11]. According to our findings, we have observed similar phenomenon, although these differences seem to be magnified with the use of RP.
The correlation between AHI and ODI was larger in patients with normal weight and decreased proportionately to the increase in BMI, both in men and in women by both methods. These findings highlight the important role of oximetry in obese patients.
References
Yaggi HK, Strohl KP (2010) Adult obstructive sleep apnea/hypopnea syndrome: definitions, risk factors, and pathogenesis. Clin Chest Med 31(2):179–186
Swinburn BA, Sacks G, Hall KD, et al. (2011) The global obesity pandemic: shaped by global drivers and local environments. Lancet 378(9793):804–814
Xu HJ, Lan XF, Li QY, et al. (2015) Factors affecting blood pressure profile in pre and postmenopausal women with obstructive sleep apnea hypopnea syndrome. Sleep and Breathing 19(1):169–174
Report of a WHO Consultation (WHO Technical Report Series 894) Obesity: preventing and managing the global epidemic. Online supplement. http://www.who.int/gho/ncd/risk_factors/bmi_text/en/index.html
Elgart J, Pfirter G, Gonzalez L, et al. (2010) Obesity in Argentina: epidemiology, morbimortality and economic impact. Rev Argent Salud Pública 1:6–12
Arnardottir ES, Mackiewick M, Gislason T, Teff KL, Pack A (2009) Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 32:447–470
Bonsignore MR, McNicholas WT, Montserrat JM, Eckel J (2012) Adipose tissue in obesity and obstructive sleep apnoea. Eur Respir J 39:746–767
Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T (2005) Progression and regression of sleep-disordered breathing with changes in weight: the sleep heart health study. Arch Intern Med 165:2408–2413
Schwartz A, Patil S, Laffan A, Polotsky V, Schneider H, Smith P (2008) Obesity and obstructive sleep apnea. Pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc 5:185–192
Serafini F, MacDowell A, Rosemurgy A, Strait T, Murr M (2001) Clinical predictors of sleep apnea in patients undergoing bariatric surgery. Obes Surg 11:28–31
Ling I, James A, Hillman D (2012) Interrelationships between body mass, oxygen desaturation, and apnea-hypopnea indices in a sleep clinic population. Sleep 35:89–96
Bixler EO, Vgontzas AN, Lin HM, et al. (2001) Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163(3):608–613
Block AJ, Wynne JW, Boysen PG (1980) Sleep-disordered breathing and nocturnal oxygen desaturation in postmenopausal women. Am J Med 69:75–79
Chiner E, Arriero JM, Signes-Costa J, Marco J, Fuentes I (1999) Validation of the Spanish version of the Epworth Sleepiness Scale in patients with a sleep apnea syndrome. Arch Bronconeumol 35:422–427
Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester
Farre R, Rigau J, Montserrat JM, Buscemi L, Ballester E, Navajas D (2003) Static and dynamic upper airway obstruction in sleep apnea: role of the breathing gas properties. Am J Resp Crit Care Med 168:659–663
O’Connor C, Thornley KS, Hanly PJ (2000) Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 161:1465–1472
Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ (1997) Prevalence of sleep-disordered breathing in ages 40-64 yr: a population-based survey. Sleep 20:65–76
Sil A, Barr G (2012) Assessment of predictive ability of Epworth scoring in screening of patients with sleep apnoea. J Laringol Otol 126(4):372–379
Araghi MH, Jagielski A, Neira I, et al. (2013) The complex associations among sleep quality, anxiety-depression, and quality of life in patients with extreme obesity. Sleep 36:1859–1865
Cambursano VH (2015) SAHOS, una vez más, favorecidas por la naturaleza. RAMR 3:161–163
Peppard PE, Ward NR, Morrell MJ (2009) The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med 180(8):788–793
Berry RB, Budhiraja R, Gottlieb DJ, American Academy of Sleep Medicine, et al. (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8:597–519
Redline S, Kapur VK, Sanders MH, et al. (2000) Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment. Am J Respir Crit Care Med 161:369–674
Fredheim JM, Røislien J, Hjelmesth J (2014) Validation of a portable monitor for the diagnosis of obstructive sleep apnea in morbidly obese patients. J Clin Sleep Med 10:751–757
Rapoport DM (2015) Point: is the AHI the best way to quantify the severity of sleep disordered breathing?—Yes. Chest. doi:10.1378/chest.15-1319
Chiner E, Signes-Costa J, Arriero JM, Marco J, Fuentes I, Sergado A (1999) Nocturnal oximetry for the diagnosis of the sleep apnoea hypopnoea syndrome: a method to reduce the number of polysomnographies? Thorax 54:968–971
Nigro CA, Aimaretti S, Gonzalez S, Rhodius E (2009) Validation of the WristOx 3100 oximeter for the diagnosis of sleep apnea/hypopnea syndrome. Sleep Breath 13(2):127–136
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Hospital Británico de Buenos Aires and Hospital Alemán committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ernst, G., Bosio, M., Salvado, A. et al. Difference between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI): proportional increase associated with degree of obesity. Sleep Breath 20, 1175–1183 (2016). https://doi.org/10.1007/s11325-016-1330-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-016-1330-3