Abstract
Background
Selective upper airway stimulation of the hypoglossal nerve is a novel therapy option for obstructive sleep apnea. Different tongue motions were observed after surgery during active therapy.
Methods
We examined tongue motions in 14 patients (mean age 51 ± 10 years) who received an implantation of an upper airway stimulation system (Inspire Medical Systems) from September 2013 to February 2014 in three different implantation centers in Germany after surgery. Sleep recording was performed preoperatively: 2 months (M02) and 6 months (M06) after surgery.
Results
There were three different tongue motions observed after surgery at 1 month (M01), M02, and M06 after surgery: bilateral protrusion (BP), right protrusion (RP), and mixed activation (MA). At M01: 10 BP, 2 RP, and 2 MA; at M02: 12 BP, 0 RP, and 2 MA; and at M06: 12 BP, 0 RP, and 2 MA could be detected. The average apnea-hypopnea index (AHI) was reduced from 32.5 ± 14.2/h before surgery to 17.9 ± 23.3/h at M02 and 14.1 ± 19.8/h at M06. An increased reduction in AHI was found in BP and RP group (Baseline: 29.6 ± 12.6/h; M02: 12.06 ± 14.1/h; M06: 9.7 ± 12.6/h) compared to the MA group (Baseline 49.6 ± 13.8/h; M02: 49.7 ± 5.1/h; M06: 40.5 ± 4.1/h).
Conclusions
These findings suggest that the postoperative tongue motions in upper airway stimulation are associated with the therapy outcome. The stimulation electrode placement on the hypoglossal nerve for selective muscle recruitment may play a role in the mechanism of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is one of the most common forms of sleep-disordered breathing, [1] with a prevalence of 2–4 % among the middle-aged population [2]. Patients with OSA experience intermittent and repeated collapses in the upper airway (UA) during sleep, which leads to irregular breathing and typical daytime sleepiness [3].
OSA is associated with hypertension [4], congestive heart failure and ischemic heart disease [5], obesity [6], diabetes [7], and stroke [8]. The continuous positive airway pressure (CPAP) is the first-line treatment in patients with OSA. When OSA is effectively treated, daytime symptoms and cardiovascular morbidity significantly improve or may even completely disappear [9]. However, CPAP is limited by acceptance issues with 50–80 % of patients accepting its use but only 17–71 % of them adhering to the treatment in the long term [10]. Alternative treatments to CPAP are mandibular advancement device and various UA surgeries [11–13].
The reduced activity of the UA dilatator muscle during sleep contributes to the collapse of UA [14]. The treatment of the functional loss of the neuromuscular tone is an option to treat the cause of OSA. Therefore, during the 1990s and 2000s, various studies on the submental transcutaneous electrical stimulation were performed. Miki et al. attached bipolar electrodes to the submental skin of patients with OSA and showed that the nocturnal stimulation decreases the incidence of apnea episodes [15]. However, further studies could not confirm these results [16–18].
Some other research groups started to investigate fine-wire stimulation by using steel electrodes inserted into the submental muscles [19, 20]. These studies could show that a selective stimulation of the protrusor (genioglossus) muscle and/or retractor (styloglossus and hyoglossus) muscles reduced the UA obstructions. A further study showed that stimulation near the proximal hypoglossal nerve could cause obstructions because of the stimulation of retractor muscles only (e.g., styloglossus and hyoglossus) [21]. Following these studies, some other researchers examined the effect of hypoglossal nerve stimulation in patients with OSA using different types of stimulators [22–24]. These results demonstrate the effectiveness of such stimulation techniques in patients with OSA, although they showed various side effects in terms of patient’s safety. In 2012, in a feasibility study, van de Heyning et al. showed that the best responders to UA stimulation have a body mass index (BMI) of ≤32 kg/m2 and an apnea-hypopnea index (AHI) of ≤50/h, and no complete concentric palatal collapse was observed in drug-induced sleep endoscopy [25]. With these selected patient criteria, the AHI reduced from 38.9 ± 9.8/h to 10.0 ± 11.0/h.
In a large multicenter prospective study, Strollo et al. confirmed that the AHI reduced by 68 % from 29.3 to 9.0 events per hour in patients with OSA by using selective hypoglossal nerve stimulation (Inspire Medical Systems) [26]. Yet, 34 % of the patients did not respond sufficiently to the treatment. We hypothesized that tongue motion during stimulation could have an impact on therapy outcome.
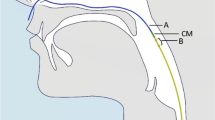
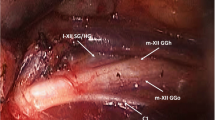
The main trunk of the hypoglossal nerve divides into lateral (l-XII) and medial (m-XII) branches [27]. The l-XII branches supply the hyoglossus and styloglossus muscles [28]. Both these muscles are retractors. The m-XII branches innervate the intrinsic muscles (vertical and transverse muscles) and the genioglossus muscle, which is the principal protrusor muscle of the tongue.
To assess whether the postoperative tongue motion is associated with the outcome regarding the treatment of sleep apnea measuring AHI, we recorded the postoperative tongue motions of all patients who received an implantation of UA stimulation system (Inspire Medical Systems, Maple Grove, USA) for their OSA in three different implantation centers.
Methods
Selection of patients
The study was performed in three different implantation centers in Germany (Luebeck, Mannheim, Munich). All consecutive patients from September 2013 till February 2014 who received an implantation of UAS system (Inspire Medical Systems) were included in this study [26]. The inclusion and exclusion criteria were:
-
Moderate to severe OSA (15/h < AHI < 65/h)
-
BMI < 35 kg/m2
-
Ruling out a complete concentric collapse at the soft palate with a drug-induced sedated endoscopy
-
Patients had to be non-compliant to CPAP and should have tried using the device
-
No severe diseases such as chronic obstructive pulmonary disease, New York Heart Association class III or IV congestive heart failure, neuromuscular diseases
The study was approved by the local ethics committees.
Time of investigation
Preoperatively, a polysomnography (PSG) in a sleep laboratory, medical and surgical consultations, and a drug-induced sedated endoscopy (DISE) to rule out a complete concentric collapse at the soft palate were performed [29]. Any other types of collapse during DISE were allowed (e.g., complete anterior-posterior collapse at the soft palate). A complete collapse at the tongue base was not mandatory.
An 18-channel in-laboratory polysomnography examination was performed at baseline and 2 (M02) and 6 months (M06) after surgery according to the American Academy of Sleep Medicine (AASM 2.0) guidelines from 2012 [30]. PSG included the following electrodes: LSO-A2, RIO-A1, EMG chin, F4-A1, C3-A2, C4-A1, O2-A1, EMG legs, electrocardiogram, flow with thermistors, flow with nasal cannula, thoracic and abdominal effort, position. In some patients, a home sleep study (HST) was performed 6 months after surgery. For all sleep studies, the same standard scoring criteria were used. [31] Hypopneas were scored based on 30 % reduction in airflow and 4 % oxygen desaturation. Apneas were scored based on 90 % reduction in airflow. After 2 months of surgery, PSG was performed to identify the therapeutic stimulation amplitude needed to abolish respiratory events during sleep. For the HST, 6 months after surgery, MiniScreen Plus (Heinen und Löwenstein, Bad Ems, Germany) or SOMNOCheck (Weinmann GmbH, Hamburg, Germany) was used.
All participants underwent surgical procedure using the Inspire Medical System for selective hypoglossal nerve stimulation [26]. Approximately 1 month after implantation (M01), all patients received their devices activated and were instructed regarding the use of a controller to initiate and terminate the therapy for night-time home use. Furthermore, the sensation threshold (the level of voltage at which the patient first feels the stimulation) and the functional threshold (the level of voltage at which bulk tongue motion is achieved) were recorded. The home amplitude (HomeAMP) is the level of voltage which was installed, when the device was first activated after 1 month (M01). The installed home amplitude (installed HomeAMP) is the level of voltage, which was titrated under a polysomnography in the sleep laboratory 2 months (M02) after surgery. At M01 no sleep recording was performed.
The following three different tongue motions with functional threshold were recorded for the different time points after surgery (M02, M06): right protrusion (RP), bilateral protrusion (BP), and mixed activation (MA). BP shows a clear protrusion of the tongue over the lower teeth without deviation to one side. RP shows a protrusion of the tongue over the lower teeth with deviation to the left side. MA includes every other kind of tongue motion such as shortening or furling of the tongue (Figs. 1, 2, 3).
a (Rest): stimulation turned off: the tongue is in a resting position with the tip of the tongue not extending over the lower teeth. b (Activated): stimulation turned on: the tongue moves forward with deviation to the left side (right protusion) with the tip of the tongue extending over the lower teeth
The outcome measurements and definition of responders and non-responders were performed to the definition in the STAR trial and publication of Elshaug et al. in 2007: A response as measured by means of the AHI was defined as a reduction of at least 50 % from baseline in the AHI score and an AHI score on the 6-month sleep recording of less than 20 events per hour [32, 33].
Finally, we also recorded the stimulation amplitudes (in voltage) and using hours per night for every patient at M01, M02, and M06.
Statistical analysis
Descriptive statistic was calculated for demographic variables. AHI at the 2- and 6-month follow-up was compared with the baseline (preoperative) AHI. P values were calculated using a paired t test. The significance level for all statistical tests was set at a level of 5 %. Data are given as means ± standard deviation. The software program Prism 6.0f (GraphPad, Inc.; CA, USA) for Mac computer was used for all statistical analyses.
Results
A total of 14 patients (mean age 51 ± 10 years; 14 males and 0 female; BMI 28.7 ± 4.9 kg/m2) were included in this study, and their average AHI was 33 ± 14/h. The patients’ characteristics are depicted in Table 1.
Tongue motions
Two patients exhibited MA during the study period. One patient showed a soft retraction of the tongue at M01, M02, and M06, and the other patient showed a curling of the tongue and a soft retraction at all the three time points (Table 2).
The other 12 patients (86 %) showed a BP at M02 and M06. Two patients exhibited RP at M01 and BP at M02 and at M06.
The implantation of the UA device was successful in all patients without adverse events. During surgery, a nerve integrity monitoring (NIM) system (Medtronic, USA) was used to differentiate a tongue retraction from a tongue protrusion. All patients showed a clear protrusion of the tongue during implantation as assessed by the NIM system. Exactly at the end of the surgery, a protrusion of the tongue could be detected in each of the 14 patients.
Tongue motions and the outcome in terms of AHI
Overall, the baseline (pre-implant) AHI of all patients was reduced from 32.5 to 14.1/h at M06 (Fig. 4). In the MA group, AHI of the two patients was not reduced at M06 (from 49.6/h at baseline to 40.5/h at M06). In patients with BP, AHI was statistically significantly reduced at M06 (from 29.6 to 9.7/h) (Fig. 4).
Effect of tongue protrusion on AHI after implantation at several study time points (pre-implant, M02, and M06). All protrusions means: all patients included in this study regardless of their tongue motion. In groups “all” and “bilateral,” AHI could be significantly reduced. Only in the “mixed activation” group a decrease of AHI was not possible. *p < 0.05
For the two patients with the RP response, the pre AHI and post AHI (M02) was 54.0 to 13.7/h and 28.0 to 3.9/h. The change of the tongue motion was between the two visits of M01 and M02.
At the 6-months visit, the criteria for the clinical outcome of a reduction of at least 50 % in the AHI score from baseline and an AHI score of less than 20 events per hour were met by 92 % (11 of 12) of the patients in the BP group and 0 % (0 of 2) of the patients in the MA group. The overall responder rate across all groups (BP + MA) was 79 % (11 of 14).
Stimulation parameters
In every group, the installed home amplitude (HomeAMP) was increased from M01 to M02 (Fig. 5). There was no statistical difference between the incoming and installed home amplitude at M02 and M06. Regardless of the group, these results were the same. Only at M01, when the devices were first turned on, there was a statistical significance between the BP and MA groups.
The different amplitudes for night-time stimulations at different study time points (M01, M02, and M06). AMP stands for amplitude (measured in volts). HomeAMP installed M01 represents the amplitude for the patient, which was installed when the device was first activated 4 weeks after surgery. HomeAMP incoming m02 represents the amplitude, which the patient self-titrated over the last 4 weeks using his remote at home. HomeAMP installed M02 is the amplitude for the patient, which was titrated under a polysomnography in the sleep laboratory 8 weeks after surgery. HomeAMP incoming M02 represents the amplitude, which the patient titrated in his range over the last 6 months after surgery using his controller at home. HomeAMP installed M06 is the amplitude, which was titrated under polysomnography in the sleep laboratory 6 months after surgery. *p < 0.05
Usage per night
There was no statistical significance in the average usage of the device per night at M02 and M06 among the different groups (Fig. 6). Even in the MA group with an insufficient outcome in terms of AHI reduction, the average usage per night was 7.1 h.
Discussion
Different kinds of surgery are available for the treatment of OSA [34]. In most cases, the anatomy of the pharynx is altered using surgery. Removing or modifying the tissues at the pharynx is performed to reduce the UA obstruction during sleep [35]. The UAS system approach is different from previous surgical approaches. UAS therapy opens UA by selectively stimulating the hypoglossal nerve fibers, which innervate the muscles responsible for maintaining airflow patency. Previous studies showed that this selective stimulation decreases AHI and has a positive effect on UA [25, 32].
The STAR trial showed short- and long-term follow-up of patients with an UAS system [32], and it was the first surgical randomized therapy withdrawal study for the treatment of OSA [36]. The overall success rate was over 66 %, and AHI was reduced by 68 % on average. In the current study, we examined if the different tongue motions observed after surgery were associated with therapy outcome that might explain the mechanism of action. We have shown that patients with BP demonstrated a greater reduction of AHI than patients with an MA during 6 months of follow-up.
To understand what happens in during hypoglossal nerve stimulation, UA the mechanism and activation of the tongue muscles and the corresponding nerve fibers need to be considered. It is known that opening of the tongue base results from stimulation of the genioglossus muscle, but the opening of the soft palate cannot be explained by this kind of stimulation [37]. The anterior palatal pillars link the soft palate to the tongue base [38]. This mechanical linkage may explain the retropalatal effect during stimulation. The palatoglossus muscle forms the anterior pillar from the uvula and inserts into the sides of the tongue. It seems that this muscle shows an activation to negative pressure in patients with OSA [38], which could play a role in restoring airway patency. Different forward tongue motions could have different effects on the soft palate. Normally, when the tongue protrudes forward, then the anterior pillar and the palatoglossus muscle moves in an anterior direction thereby causing the increase in the retropalatal cross-sectional area. Safiruddin et al. showed that responders and non-responders to the UA stimulation therapy had similar degrees of retrolingual opening to stimulation, but responders had a greater increase in the retropalatal area. This might to be linked to the different postoperative tongue motions and the different outcome results of therapy in terms of AHI. For a better assessment, it would certainly be helpful to repeat a drug-induced sleep endoscopy with stimulation. Furthermore, the tongue movement can primarily be due to not only the cuff placement but also the very individual crossing of the nerve plexus in each individual case or during surgery-related (preparation) effects due to dissection. However, surgery-related effects seem to be unlikely as the MA was stable over the follow-up period. Mu and Sanders were able to show in a human cadaver research that the genioglossus muscle in the tongue is innervated by separate primary nerve branches [27]. This very individual branching of the nerve endings and the resulting complex innervation of the tongue can be an explanation for the different postoperative tongue motions. This needs to be discussed in further studies using larger clinical trials in patients with UA stimulation.
BP and RP are probably common with a distal cuff placement at the hypoglossal nerve with almost activation of the horizontal and oblique genioglossus muscle fibers. In most of the cases, the soft palate can open unhindered. This could be due to activation of the palatoglossus muscle. The activation does not occur because of contraction of the muscle but the tongue motion brings the palatoglossal muscle also forward. MA shows any other kind of motion or protrusion of the tongue, such as a retrusion or shortening of the tongue. This could likely happen because of placing the stimulation cuff too proximally. Nevertheless, it may lead to a fully open tongue base but with different variations on the soft palate (obstruction or opening). The reason for these phenomena is the fact that the retractor/depressor muscles (e.g., styloglossus and hyoglossus muscles) are considerably activated. One explanation may be that the placement of the cuff includes the nerve fibers, which belong to the I-XII branches or the cudd is too proximal—including only medial branches but the generated electric field activates retractor nerves/muscles as well. Table 3 gives an overview of the different tongue motions and the cuff placement, how the different tongue motions could be explained.
Recently, Safiruddin et al. showed that unilateral stimulation of the hypoglossal nerve increases airway area at multiple levels [39]. The degree of UA opening corresponded to higher amplitudes of stimulation. Based on the results of the present study that different tongue motions have different effects on the results, the cuff placement seems to be very important. Different muscle groups can be stimulated by different cuff placements with the result that different nerve fibers are enclosed. Two other studies showed an improvement of the retropalatal cross-sectional area during direct genioglossus muscle stimulation under anesthesia [40, 41]. These anatomical changes are very important in the surgical treatment of OSA. In most patients with a higher AHI, the obstructions are found on different levels such as the tongue base and the retropalatal region. Most importantly, opening the airway in an anterior-posterior dimension during hypoglossal nerve stimulation is relevant particularly concerning the role of airway shape for OSA therapies [39].
Another important point is the higher BMI of the two patients with MA. Looking at other surgical procedures for OSA, it is known that the clinical outcome is worse with a higher BMI. Whether this is also true for the UAS remains to be seen in growing trials. Age seems not be a problem because the MA patients were even younger.
The study has few limitations including the low patient number (only two patients with an MA) and the retrospective design. However, considering the fact that this therapy is available only in few hospitals in Germany because of the reimbursement issue, it is difficult to collect a large number of patients. A further prospective study would be needed to verify the data using higher patient numbers. Perhaps, a future study could produce a marker for successful outcome during surgery if we could manage in a better way to detect the tongue motion type during the implantation.
In conclusion, selective stimulation of the hypoglossal nerve is an effective surgical treatment therapy for OSA. Different tongue motions can be detected postoperatively and during surgery which might result in different outcomes. To obtain the best surgical outcome, the placement of the cuff is very important to receive a multilevel opening effect on UA during stimulation. The cuff needs to be placed distally including the m-XII fibers of the hypoglossal nerve supplying the genioglossus muscle (oblique and horizontal) and the transverse and horizontal intrinsic muscles.
References
Steier J, Martin A, Harris J, Jarrold I, Pugh D, Williams A (2014) Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision across the UK. Thorax 69(4):390–392
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328(17):1230–1235
Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP (2010) Pathophysiology of sleep apnea. Physiol Rev 90(1):47–112
Narkiewicz K, Somers VK (1999) Obstructive sleep apnea as a cause of neurogenic hypertension. Curr Hypertens Rep 1(3):268–273
Martinez D, Klein C, Rahmeier L, da Silva RP, Fiori CZ, Cassol CM, Goncalves SC, Bos AJ (2012) Sleep apnea is a stronger predictor for coronary heart disease than traditional risk factors. Sleep Breath; 16(3):695–701
Levy P, Bonsignore MR, Eckel J (2009) Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J; 34(1):243–260
Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL (2002) Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165(5):677–682
Palomaki H, Partinen M, Juvela S, Kaste M (1989) Snoring as a risk factor for sleep-related brain infarction. Stroke J Cereb Circul 20(10):1311–1315
Barbe F, Duran-Cantolla J, Capote F, de la Pena M, Chiner E, Masa JF, Gonzalez M, Marin JM, Garcia-Rio F, de Atauri JD, Teran J, Mayos M, Monasterio C, del Campo F, Gomez S, de la Torre MS, Martinez M, Montserrat JM, Spanish S, Breathing G (2010) Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med 181(7):718–726
Catcheside P. G. (2010) Predictors of continuous positive airway pressure adherence. F1000 medicine reports; 2
Vanderveken OM, Devolder A, Marklund M, Boudewyns AN, Braem MJ, Okkerse W, Verbraecken JA, Franklin KA, De Backer WA, Van de Heyning PH (2008) Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 178(2):197–202
Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB, Katz SG, Harwick JD (2010) Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 33(10):1396–1407
Stuck BA, Dreher A, Heiser C, Herzog M, Kuhnel T, Maurer JT, Pistner H, Sitter H, Steffen A, Verse T (2015) Diagnosis and treatment of snoring in adults-S2k Guideline of the German Society of Otorhinolaryngology, Head and Neck Surgery. Sleep Breath; 19(1):135–148
Heiser C, Zimmermann I, Sommer JU, Hormann K, Herr RM, Stuck BA (2013) Pharyngeal chemosensitivity in patients with obstructive sleep apnea and healthy subjects. Chem Senses 38(7):595–603
Miki H, Hida W, Chonan T, Kikuchi Y, Takishima T (1989) Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. Am Rev Respir Dis; 140(5):1285–1289
Edmonds LC, Daniels BK, Stanson AW, Sheedy 3rd PF, Shepard Jr. JW (1992) The effects of transcutaneous electrical stimulation during wakefulness and sleep in patients with obstructive sleep apnea. Am Rev Respir Dis; 146(4):1030–1036
Decker MJ, Haaga J, Arnold JL, Atzberger D, Strohl KP (1993) Functional electrical stimulation and respiration during sleep. J Appl Physiol (1985); 75(3):1053–1061
Guilleminault C, Powell N, Bowman B, Stoohs R (1995) The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest 107(1):67–73
Eisele DW, Smith PL, Alam DS, Schwartz AR (1997) Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 123(1):57–61
Oliven A, Schnall RP, Pillar G, Gavriely N, Odeh M (2001) Sublingual electrical stimulation of the tongue during wakefulness and sleep. Respir Physiol 127(2–3):217–226
Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith PL (1996) Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol (1985); 81(2):643–652
1996) Practice parameters for the treatment of obstructive sleep apnea in adults: the efficacy of surgical modifications of the upper airway. Report of the American Sleep Disorders Association. Sleep 19(2):152–155
Schwartz AR, Barnes M, Hillman D, Malhotra A, Kezirian E, Smith PL, Hoegh T, Parrish D, Eastwood PR (2012) Acute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnea. Am J Respir Crit Care Med 185(4):420–426
Eastwood PR, Barnes M, Walsh JH, Maddison KJ, Hee G, Schwartz AR, Smith PL, Malhotra A, McEvoy RD, Wheatley JR, O'Donoghue FJ, Rochford PD, Churchward T, Campbell MC, Palme CE, Robinson S, Goding GS, Eckert DJ, Jordan AS, Catcheside PG, Tyler L, Antic NA, Worsnop CJ, Kezirian EJ, Hillman DR (2011) Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 34(11):1479–1486
Van de Heyning PH, Badr MS, Baskin JZ, Cramer Bornemann MA, De Backer WA, Dotan Y, Hohenhorst W, Knaack L, Lin HS, Maurer JT, Netzer A, Odland RM, Oliven A, Strohl KP, Vanderveken OM, Verbraecken J, Woodson BT (2012) Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope 122(7):1626–1633
Maurer JT, Van de Heyning PV, Lin HS, Baskin J, Anders C, Hohenhorst W, Woodson BT (2012) Operative technique of upper airway stimulation: an implantable treatment of obstructive sleep apnea. Oper Tech Otolaryngol Head Neck Surg; 2012(23):227–233
Mu L, Sanders I (2010) Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat 23(7):777–791
Sanders I, Mu L (2013) A three-dimensional atlas of human tongue muscles. Anat Rec 296(7):1102–1114
Vanderveken OM, Maurer JT, Hohenhorst W, Hamans E, Lin HS, Vroegop AV, Anders C, de Vries N, Van de Heyning PH (2013) Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 9(5):433–438
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep M. (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med 8(5):597–619
Iber C, Ancoli-Israel S, Chessonn A, Quan SF (2007) In the AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Journal (Issue)
Strollo Jr. PJ, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP, Group ST (2014) Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 370(2):139–149
Elshaug AG, Moss JR, Southcott AM, Hiller JE (2007) Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep 30(4):461–467
Kezirian EJ, Goldberg AN (2006) Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg 132(2):206–213
Randerath WJ, Verbraecken J, Andreas S, Bettega G, Boudewyns A, Hamans E, Jalbert F, Paoli JR, Sanner B, Smith I, Stuck BA, Lacassagne L, Marklund M, Maurer JT, Pepin JL, Valipour A, Verse T, Fietze I, European Respiratory Society task force on non C. t. i. s. a. (2011) Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J 37(5):1000–1028
Woodson BT, Gillespie MB, Soose RJ, Maurer JT, de Vries N, Steward DL, Baskin JZ, Padhya TA, Lin HS, Mickelson S, Badr SM, Strohl KP, Strollo Jr. PJ, Investigators ST, Investigators ST (2014) Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg 151(5):880–887
Mortimore IL, Douglas NJ (1997) Palatal muscle EMG response to negative pressure in awake sleep apneic and control subjects. Am J Respir Crit Care Med 156(3 Pt 1):867–873
Van de Graaff WB, Gottfried SB, Mitra J, van Lunteren E, Cherniack NS, Strohl KP (1984) Respiratory function of hyoid muscles and hyoid arch. J Appl Physiol Respir Environ Exerc Physiol 57(1):197–204
Safiruddin F, Vanderveken OM, de Vries N, Maurer JT, Lee K, Ni Q, Strohl KP (2015) Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J; 45(1):129–138
Dotan Y, Golibroda T, Oliven R, Netzer A, Gaitini L, Toubi A, Oliven A (2011) Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur Respir J; 38(2):338–347
Oliven A, Tov N, Geitini L, Steinfeld U, Oliven R, Schwartz AR, Odeh M (2007) Effect of genioglossus contraction on pharyngeal lumen and airflow in sleep apnoea patients. Eur Respir J; 30(4):748–758
Conflict of interest
Heiser, C. is the study investigator and consultant of Inspire Medical Systems and received personal fees from Neuwirth, Heinen & Loewenstein, Sutter Medizintechnik outside the submitted work. Maurer, JT. is the study investigator and consultant of Inspire Medical Systems and received personal fees from GlaxoSmithKline, Weinmann, Olympus, ResMed, Neuwirth, Medtronic, and Heinen & Loewenstein and ReVent outside the submitted work. Steffen, A. is the study investigator of Inspire Medical Systems and received honorarium for invited talks from Resmed outside the submitted work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heiser, C., Maurer, J.T. & Steffen, A. Functional outcome of tongue motions with selective hypoglossal nerve stimulation in patients with obstructive sleep apnea. Sleep Breath 20, 553–560 (2016). https://doi.org/10.1007/s11325-015-1237-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-015-1237-4