Abstract
Background
The aim of the study was to evaluate exhaled nitric oxide (eNO) derived from different areas of airway in obstructive sleep apnea hypopnea syndrome (OSAHS) patients with NO exchange model and investigate the potential application and interpretation of eNO in clinical setting.
Methods
This study was divided into two parts. Firstly, we performed a case control study in 32 OSAHS patients and 27 non-OSAHS participants. Fractional eNO (FeNO) and eNO from the central airway (J’awNO) and from alveoli (CANO) were compared in OSAHS and control groups. Also, correlation of eNO to severity of OSAHS was analyzed. Secondly, a prospective study was conducted in 30 severe OSAHS patients who received a short-term nasal continuous positive airway pressure (nCPAP) treatment. We evaluated eNO, plasma ET-1 concentration, and echocardiography during the treatment process and explored the potential relationship among them.
Results
FeNO and J’awNO were higher in OSAHS and associated with disease severity, while CANO was relatively lower. After nCPAP treatment in severe OSAHS patients, FeNO and J’awNO decreased and CANO increased significantly. Substantial agreement was shown between the elevation of CANO and the decrease of plasma ET-1 concentration after nCPAP by Kappa analysis for consistency. Tei index, which is considered indicative of global right ventricular function, might be predicted by plasma ET-1 levels in severe OSAHS patients.
Conclusions
NO exchange model provides us with more information of eNO derived from different areas. eNO is not only confirmed to be an effective method for airway inflammation evaluation in the follow-up of OSAHS, CANO may also serve as a useful marker in monitoring endothelial function, resistance of pulmonary circulation, and right ventricular function for clinical implication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea hypopnea syndrome (OSAHS) is a syndrome characterized by intermittent nocturnal hypoxemia due to upper airway obstruction. It is estimated that about 9 and 4 % of middle-aged males and females suffer from this disease [1]. Repeated decreases in oxygen saturation and rapid reoxygenation increase the production of reactive oxygen species (ROS) and inflammatory cytokines which also contribute to worsening of OSAHS and development of its complications, especially cardiovascular diseases [2–4]. OSAHS has been confirmed as a critical risk factor of cardiovascular diseases which have high morbidity and mortality [5]. At present, treatment with nasal continuous positive airway pressure (nCPAP) is a well-accepted therapy which can reverse sleep disorder-related events quickly and effectively for OSAHS patients [6].
As a non-invasive method for lung inflammatory status evaluation, exhaled nitric oxide (eNO) has been applied in clinical settings with excellent feasibility and repeatability. It is believed that eNO increases in eosinophilic conditions such as asthma, atopy, and allergic rhinitis and previous findings suggested that fractional eNO (FeNO), which represents upper airway and bronchial inflammation, might help with diagnosis and treatment adjustment of asthma [7]. With the development and standardization of methods, procedure, and instruments for eNO measurement, eNO derived from central airway and alveoli could be evaluated separately by multiple flow rate measurements and nitric oxide exchange models [8, 9]. Moreover, apart from eosinophilic inflammation, endotoxins, exotoxins, as well as pre-inflammatory cytokines such as TNF-α and IL-1β have also been demonstrated to increase the release of NO synthesized by inducible nitric oxide synthase (iNOS) in different pathological conditions [10].
Evaluation of eNO derived from the central airway and alveoli with exchange models has not been well investigated in previous studies. Only two researches performed this assessment and the results were not consistent and the clinical implication of eNO in OSAHS has not been fully illustrated yet [11, 12]. Based on these above, we designed this study to explore whether eNO derived from different regions of the airway are meaningful indicators for OSAHS in clinical setting and to provide more insights on potential implications of eNO.
Methods
Participants and study design
OSAHS patients were recruited from Sleep Laboratory, Department of Pulmonary Medicine, Zhongshan Hospital, from September 2012 to January 2013, while non-OSAHS individuals were recruited from health examination center in Zhongshan Hospital as control. Firstly, a case-control study was conducted between non-OSAHS and OSAHS patients. In the second part, severe OSAHS patients who were indicated for CPAP treatment were recruited. A prospective study was performed to analyze the effect of the treatment on eNO.
All of the patients had been referred to outpatient clinic with suspicious symptoms of OSAHS and were newly diagnosed according to results of polysomnography (PSG) which indicated that apnea-hypopnea index (AHI) was more than 5 or the lowest SpO2 was less than 90 %. Severe OSAHS was defined as AHI over 30. Non-OSAHS individuals were included based on overnight SpO2 monitoring (nadir SpO2 > 90 %). For all of the subjects in this research, we excluded those with atopy or other respiratory diseases, previous treatment for OSAHS, gastrointestinal, kidney, rheumatic, and neuromuscular diseases, or history of malignant tumor, acute illness, or medications within the past week. In addition, based on the international recommendations for the measurement of FeNO [13], patients with conditions that might affect FeNO levels were also excluded.
Medical records and Epworth Sleepiness Scale (ESS) were recorded; eNO and lung function test were performed for them as well. For severe OSAHS patients recruited in the second part of our study, they would receive a three-night nCPAP treatment after titration. In this process, exhaled NO measurement (before and after nCPAP every day), plasma collection, and echocardiography (only before and after the treatment) were performed.
The study was approved by the ethics committee of Zhongshan Hospital Affiliated to Fudan University. Informed consent was obtained from all individual participants included in the study.
Evaluation of daytime sleepiness
Chinese version of ESS was used to evaluate daytime sleepiness in all participants when they were included in this study. ESS consists of eight items, rated on a scale of 0–3, in which the total score is computed through adding up answers to each item [14]. The total score represents a measure of subjective daytime sleepiness (ranged 0–24, higher results indicating greater probability to fall asleep) recorded for each participant.
Polysomnography
Overnight PSG (Alice 4, Respironics, USA; Medcare MONET, TMS International B.V., USA) was performed in suspected OSAHS patients who had excessive daytime sleepiness and/or related comorbidity according to the guidelines of the American Academy of Sleep Medicine Standards of Practice Committee of the American Sleep Disorders Association [15]. Apnea was defined as complete cessation of airflow of at least 10 s in duration and hypopnea as >50 % amplitude reduction of airflow lasting 10 s, associated with 4 % oxyhemoglobin desaturation during sleep. All PSG tracing records were analyzed by a specialist in the sleep lab. The following results were collected: AHI, mean and nadir SpO2, and duration of sleeping with a SpO2 <90 % and its proportion in the whole sleeping period.
Overnight SpO2 monitoring
Overnight SpO2 monitoring (Apnealink, ResMed, USA) was performed in each participant in non-OSAHS group to exclude those who had hypoxemia (less than 90 %) and in those who accepted nCPAP treatment to evaluate or confirm the therapeutic effect.
nCPAP treatment
Severe OSAHS patients who met the criteria for nCPAP treatment would undergo titration for one night with automatic nCPAP device (S8 AutoSet, ResMed, USA) and SpO2 monitoring. The ninety-fifth percentile pressure on the download was used to determine the treatment pressure for each subject. After that, OSAHS patients received a three-night nCPAP treatment in the sleep lab in the hospital.
Exhaled NO measurement
eNO measurements were preformed between 06:00 and 07:00 a.m. after the participants woke up and 20:00 to 21:00 p.m. before they went to sleep. eNO was measured by an analyzer (Nano Coulomb Nitric Oxide Analyzer, Sunvou, China) which was calibrated every day before each testing period. eNO was measured at multiple flow rates of 50, 100, 120, 180, and 250 ml/s which were enabled by resistors supplied by Sunvou. For each flow rate, we took the mean value of two measurements using the standard on-line technique. Single exhaled fractional NO (FeNO) was measured at a flow rate of 50 ml/s. Then, a mathematical approach based on a two-compartment model published by Tsoukias et al. was used to estimate steady-state NO levels in alveoli (CANO, ppb) and flux of NO from the airway wall (J’awNO, nl/s) [9]. According to this model, the lung comprises two separate regions: a non-expansible airway compartment and an expansible alveolar compartment. Two parameters, CANO and J’awNO, define the exhaled NO contributions from each compartment. CANO and J’awNO can be estimated from the slope and intercept of the equation. This technique has proved to be able to provide more specific information about lung inflammation as contributions of the two compartments can be analyzed separately [8].
Endothelin-1 measurement
In OSAHS group, venous blood anticoagulated with EDTA-2K was collected twice (before and after nCPAP treatment). Plasma was preserved after centrifuge. Plasma endothelin-1 (ET-1) concentration was measured as previously described [16].
Lung function test
All of the participants underwent a lung function test (Jaeger Toennies, Jaeger, Germany) on the same day after the first eNO measurement by specialists in a lung function lab according to the recommendation of the American Thoracic Society guideline [17]. Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC, total lung capacity (TLC), and diffusing capacity of carbon monoxide (DLCO) were recorded.
Echocardiography
For 30 severe OSAHS patients who underwent nCPAP treatment, echocardiography examinations (iE33 xMATRIX Echocardiography System, Philips, USA) were performed by a professional doctor of echocardiography in the morning (06:00–07:00) twice (before and after their nCPAP treatment) blindly. Right ventricular function was analyzed and assessed as follows: Doppler tissue imaging of the velocity of tricuspid annular systolic motion, early diastolic motion and late diastolic motion (cm/s), right ventricular isovolumic relaxation time (ms), and Tei index [18] (also known as myocardial performance index which is defined as the sum of the isovolumic contraction and the isovolumic relaxation time divided by ejection time and thus incorporates elements of both systolic and diastolic phases in the assessment of global right ventricular function).
Statistical analysis
Quantitative variables were presented as mean ± standard error (SE), and comparison of independent groups was performed with independent samples T test or analysis of variance (ANOVA). Categorical variables were compared via chi-square and Fisher’s exact tests. Correlation between different variables was evaluated with Pearson or Spearman correlation test based on normal distribution or not. The kappa statistic was also used as a measure of consistency between variables. All analyses were conducted using SPSS 19.0 (SPSS Inc, Chicago, IL, USA). Statistical difference was taken as p < 0.05.
Results
Baseline characteristics of subjects
Finally, 32 OSAHS patients and 27 non-OSAHS subjects were enrolled in the study from an outpatient clinic of pulmonary disease and health examination center. The baseline characteristics of them are shown in Table 1. Briefly, age, gender, smoking status, alcohol history, and lung function were comparable in these two groups. Hypertension, diabetes mellitus, and hyperlipidemia were the main comorbidities in all participants among which hypertension was more commonly seen in OSAHS group. In addition, as one of the major risk factors of OSAHS, BMI was significantly higher in OSAHS individuals compared with non-OSAHS ones.
Exhaled NO measurements
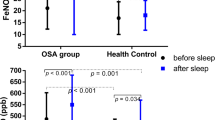
We measured eNO in all participants in this research when they were recruited. As shown in Fig. 1, FeNO and J’awNO which represents eNO derived from the central airway were dramatically higher in OSAHS group than those in non-OSAHS group (19.99 ± 1.23 vs. 9.45 ± 0.87 ppb, p < 0.0001; 837.1 ± 80.43 vs. 362.4 ± 30.84 pl/s, p < 0.0001), while CANO was relatively lower in OSAHS patients although no significance was found (3.25 ± 0.31 vs. 3.83 ± 0.34 ppb, p = 0.22). A subgroup analysis was performed based on their smoking status which is considered as a novel factor in eNO measurement, and the results were similarly demonstrated as above (Table 2).
Additionally, correlation between eNO and some critical indicators of sleep evaluation including PSG in OSAHS group was analyzed further on. Among them, J’awNO and FeNO were found to be positively correlated with AHI. For the durations of hypoxemia (minutes and percentage of slept with a SpO2 <90 %), the longer they were recorded, the higher level of J’awNO would be evaluated. However, NO concentration in alveoli did not seem to be affected by disease severity or duration of hypoxemia (Table 3).
Exhaled NO after nCPAP treatment
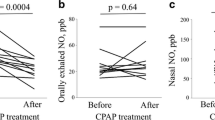
A total of 30 severe OSAHS patients indicated for nCPAP treatment were enrolled in this section. Their characteristics were shown in Table 4. After three-night treatment of nCPAP, both FeNO and J’awNO measured before and after each treatment markedly decreased, while CANO increased (Fig. 2). Notably, eNO levels in both central airways and alveoli in severe OSAHS patients were affected significantly only after one night of satisfactory treatment.
eNO changes in severe OSAHS patients during the process of nCPAP treatment. eNO exhaled nitric oxide, OSAHS obstructive sleep apnea hypopnea syndrome, FeNO fractional nitric oxide, nCPAP nasal continuous positive airway pressure. J’awNO represents NO derived from the central airway. CANO means NO concentration in the alveoli. a–c Variations of FeNO (29.97 ± 2.22 vs. 24.2 ± 2.03 vs. 19.22 ± 1.23 ppb), J’awNO (1353.03 ± 110.96 vs. 1004.48 ± 105.14 vs. 672.99 ± 55.77 pl/s), and CANO (2.90 ± 0.39 vs. 4.39 ± 0.48 vs. 5.91 ± 0.49 ppb) levels, respectively, which were measured before each time of nCPAP at night. d–f Variations of FeNO (33.71 ± 2.96 vs. 26.12 ± 2.12 vs. 21.51 ± 1.67 ppb), J’awNO (1571.60 ± 158.95 vs. 1102.11 ± 109.96 vs. 860.98 ± 86.56 pl/s), and CANO (2.17 ± 0.42 vs. 4.43 ± 0.61 vs. 4.39 ± 0.37 ppb) levels, respectively, which were measured after each time of nCPAP in the morning. Black circles eNO levels before nCPAP at night, black triangles eNO levels after nCPAP in the morning. *p < 0.05; **p < 0.01
Concentration of plasma ET-1 in OSAHS patients
Among severe OSAHS patients who received nCPAP, plasma was collected for ET-1 measurement in 20 of them before and after treatment with informed consents. We found that effective nCPAP treatment could decrease plasma ET-1 concentration significantly (0.79 ± 0.07 vs. 0.63 ± 0.08 pg/ml, p = 0.0098). Furthermore, although no correlation between ET-1 and eNO was shown statistically, it was noticed that the elevation of CANO after nCPAP usually suggested the decrease of plasma ET-1 concentration. Thus, Kappa analysis for consistency of CANO and ET-1 variation was performed and results showed that the Kappa value was 0.748 (p < 0.001) which suggested substantial agreement between the variation of CANO and ET-1 concentration after nCPAP.
Right cardiac function evaluation in OSAHS patients
Finally, 22 patients accepted right cardiac function evaluation and echocardiography both before and after nCPAP were performed in 13 of them with informed consents. Tei index decreased significantly after nCPAP treatment as shown in Fig. 3 and Table 5, while no significant changes were observed for other indices. Also, relationships between eNO, plasma ET-1 concentrations, and Tei index with which 18 sets of data were recorded in the same period were explored. Significant positive correlation between Tei index and ET-1 concentrations was demonstrated (r = 0.6394, p = 0.0043), while no correlation between eNO (FeNO, J’awNO, or CANO) and Tei index was found. Linear regression analysis also showed that Tei index considered indicative of global right ventricular function might be predicted by plasma ET-1 levels in severe OSAHS patients with the following equation: plasma ET-1 concentration (pg/ml) = 0.8779 (SE = 0.2639) × Tei index + 0.4138 (SE = 0.1140).
Changes and correlation of ET-1 and Tei index before and after nCPAP in severe OSAHS patients. ET-1 endothelin-1, nCPAP nasal continuous positive airway pressure, OSAHS obstructive sleep apnea hypopnea syndrome. a Plasma ET-1 concentration decreased after short-term nCPAP treatment. b Tei index which reflects global right ventricular function also decreased after nCPAP treatment. c ET-1 levels positively correlated with Tei index in severe OSAHS patients with significance. *p < 0.05; **p < 0.01
Discussion
In this study, we confirmed the changes of exhaled NO in OSAHS patients. According to 2CM model of NO exchange which was published before, eNO derived from the central airway and alveoli were analyzed separately in addition to FeNO measured at a constant flow rate of 50 ml/s with single-breath technique which has been widely used in clinical practice. Results showed that FeNO and J’awNO were significantly higher in OSAHS patients and were correlated with duration and severity of nocturnal hypoxemia; as for NO levels in alveoli, CANO slightly decreased in OSAHS instead. On the other hand, effective nCPAP treatment could reduce high levels of FeNO and J’awNO and increase CANO rapidly in severe OSAHS patients. Furthermore, we attempted to illustrate underlying reasons and interpretation of eNO changes in OSAHS and found a potential relationship between alveoli NO concentration, plasma ET-1 levels, and right ventricular function which provided a new perspective to eNO and its application.
eNO was firstly noticed in OSAHS by Olopade et al. who demonstrated high levels of nasal NO in moderate and severe patients [19]. Afterwards, oral NO was also found to increase in OSAHS in relation to upper airway obstructive episodes and severity of hypoxemia [20]. Previous studies also proved that a large number of inflammatory cells including neutrophils and lymphocytes were found in induced sputum, mucosa, and muscular layer of the upper airway in OSAHS patients, which implicated higher levels of local inflammatory reaction and oxidative stress caused by nocturnal intermittent hypoxemia [21–23]. Our findings of higher levels of FeNO and J’awNO in OSAHS patients are consistent with the results of a Spanish study which concluded that eNO (FeNO and J’awNO) was an easy way to monitor local upper airway inflammation and predict therapeutic response [11]. As a key risk factor of OSAHS, obesity is believed to be associated with systemic inflammation which may also affect local airway. Some researches demonstrated that FeNO was positively correlated with BMI in obese patients without OSAHS [24, 25]. However, only FeNO levels in OSAHS group had a weak correlation with BMI in our study and J’awNO was not associated with BMI in either group. This phenomenon suggested that FeNO might be more suitable for non-obese individuals who did not suffer from increased systemic inflammation, while for OSAHS patients with higher BMI, high levels of FeNO might be a result of hypoxemia combined with obesity. Thus, J’awNO, which was also independent of BMI in OSAHS group, might be superior to FeNO in terms of representing local central airway inflammation and oxidative stress in obese patients, although the underlying mechanism still needs further investigation.
Main indicators of disease severity in OSAHS, including AHI and length and percentage of sleeping with SpO2 < 90 % recorded in PSG, were found to be correlated with FeNO and J’awNO in this study. It proved that increased central airway inflammation could be a consequence of intermittent hypoxemia. On the other hand, these correlations also reminded us that local inflammation might contribute to disease worsening or development in OSAHS, as Culla et al. have shown before [20].
For severe OSAHS patients, nCPAP could alleviate central airway inflammation effectively. Both FeNO and J’awNO decreased after 3-day qualified treatment. We also noticed that only one night of satisfactory nCPAP could largely relieve central airway inflammation levels as FeNO and J’awNO changed after that. Similar conclusion has also been drawn in a long-term study of Fortuna et al. [11]. Thus, we conclude that eNO could work as a simple and effective method for airway inflammation monitoring in the treatment and follow-up of severe OSAHS patients.
CANO, the steady state of concentration of eNO in alveoli, was slightly lower in OSAHS group compared with non-OSAHS participants. After a short-term nCPAP treatment, CANO increased significantly. These results are similar to Foresi and Fortuna’s findings, but no relationships between CANO and comorbidities, BMI, or disease severity were demonstrated in our research [11, 12]. As discussed above, type II pneumocytes and macrophages in alveoli also distribute iNOS which contributes to large amounts of NO generation. When OSAHS patients suffer from hypoxemia during sleep, NO could be synthesized as it was in the central airway. However in alveoli, NO can diffuse across air-blood barrier rapidly for its lipid-soluble feature. Reactive oxygen species produced by excessive oxidative stress in untreated OSAHS has been confirmed to inhibit phosphorylation of endothelial nitric oxide synthase (eNOS) and reduce NO levels [26, 27]. Beyond that, increased levels of inflammatory cytokines, adhesion molecules, and microparticles in OSAHS have been proved to be responsible for endothelial dysfunction [28, 29]. Thus, we speculate that dysfunction of endothelial cells and eNOS caused by inflammation and oxidative stress makes it difficult to catalyze the generation of NO in pulmonary microcirculation, while NO produced in physiologic or pathological status in alveoli serve as a compensatory mechanism contributing to the regulation of pulmonary circulation and CANO decreased to a level lower than normal as a result. This process occurred rapidly and constantly for the reason that NO could bind to hemoglobin and oxidize it quickly. While after nCPAP treatment, endothelial function was restored with improvement of hypoxemia and so was endothelial NO level in pulmonary microcirculation. Therefore, diffusion of NO from alveoli to capillary decreased and NO concentration in alveoli elevated. On the other hand, as a substrate of NO synthesis, the lack of oxygen in OSAHS might also be a cause of lower CANO. Effective nCPAP treatment made local oxygen adequate enough so that NO production in alveoli could also increase to a certain extent.
Then, we further explored whether there was any relationship between CANO and pulmonary circulation and found that reduction of plasma ET-1 levels after nCPAP usually predicted the increasing of CANO although no proportional association was demonstrated. Through assessment of right cardiac function by echocardiography, Tei index, which represents global right ventricular function as mentioned above, was shown to be correlated with ET-1 concentration significantly. These findings indicated that decreased resistance of pulmonary circulation leads to the improvement of right ventricular function. So, our study proved for the first time that CANO could also be used in monitoring endothelial function and resistance of pulmonary circulation and right ventricular function apart from local levels of inflammation and oxidative stress in OSAHS patients. We also considered that evaluation of eNO especially NO derived from alveoli might help with the prevention of long-term cardiovascular complications as evidences have shown that inflammation, oxidative stress, endothelial injuries, and dysfunction are the main mechanisms for hypertension, atherosclerosis, and pulmonary hypertension in OSAHS patients [4, 30].
Our study also has limitations. Restricted by time and patients’ willingness, the number of participants was relatively small, especially those who accepted ET-1 and echocardiography evaluation. We just investigated clinical value and significance of eNO in severe OSAHS patients and focused on effects of short-term nCPAP treatment in this research, so that we could not tell outcomes of long-term follow-up or in patients with mild and moderate OSAHS as well. We also think that the existed models still cannot reflect the real status of NO production and exchange in the lung. Larger number of patients that cover various disease severities and longer duration of follow-up based on better responsive models are expected to confirm our findings in future researches.
In conclusion, this pilot study showed that FeNO and J’awNO which represent eNO derived from the central airway were higher in OSAHS and associated with disease severity. While eNO from alveoli, evaluated as CANO, was relatively lower. After nCPAP treatment in severe OSAHS individuals, FeNO and J’awNO decreased and CANO increased significantly. Combined with assessment of ET-1 and right cardiac function, eNO was confirmed to be an effective method for airway inflammation evaluation in the follow-up of OSAHS and we demonstrated for the first time that CANO may serve as a useful marker in monitoring endothelial function and resistance of pulmonary circulation and right ventricular function for clinical implication, so that cardiovascular complications of OSAHS could be prevented at an early stage. A larger and long-term study is still needed to confirm these findings in the future.
References
Yaggi HK, Strohl KP (2010) Adult obstructive sleep apnea/hypopnea syndrome: definitions, risk factors, and pathogenesis. Clin Chest Med 31:179–186
Ryan S, McNicholas WT (2008) Intermittent hypoxia and activation of inflammatory molecular pathways in OSAS. Arch Physiol Biochem 114:261–266
Selmi C, Montano N, Furlan R, Keen CL, Gershwin ME (2007) Inflammation and oxidative stress in obstructive sleep apnea syndrome. Exp Biol Med (Maywood) 232:1409–1413
Ryan S, Taylor CT, McNicholas WT (2009) Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgrad Med J 85:693–698
Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053
Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ (2006) Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev CD001106
From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. Available from: http://www.ginasthma.org/. Accessed on 2014 Dec 30
George SC, Hogman M, Permutt S, Silkoff PE (2004) Modeling pulmonary nitric oxide exchange. J Appl Physiol (1985) 96:831–839
Tsoukias NM, George SC (1998) A two-compartment model of pulmonary nitric oxide exchange dynamics. J Appl Physiol (1985) 85:653–666
Morris SJ, Billiar TR (1994) New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol 266:E829–E839
Fortuna AM, Miralda R, Calaf N, Gonzalez M, Casan P, Mayos M (2011) Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir Med 105:630–636
Foresi A, Leone C, Olivieri D, Cremona G (2007) Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest 132:860–867
American Thoracic Society, European Respiratory Society (2005) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 171:912–930
Johns M (1998) Rethinking the assessment of sleepiness. Sleep Med Rev 2:3–15
Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee (1997) Practice parameters for the indications for polysomnography and related procedures. Sleep 20:406–422
Ishizawa K, Yoshizumi M, Tsuchiya K, Houchi H, Minakuchi K, Izawa Y, Kanematsu Y, Kagami S, Hirose M, Tamaki T (2004) Dual effects of endothelin-1 (1-31): induction of mesangial cell migration and facilitation of monocyte recruitment through monocyte chemoattractant protein-1 production by mesangial cells. Hypertens Res 27:433–440
American Thoracic Society (1995) Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 152:1107–1136
Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB (1995) New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function—a study in normals and dilated cardiomyopathy. J Cardiol 26:357–366
Olopade CO, Christon JA, Zakkar M, Hua C, Swedler WI, Scheff PA, Rubinstein I (1997) Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest 111:1500–1504
Culla B, Guida G, Brussino L, Tribolo A, Cicolin A, Sciascia S, Badiu I, Mietta S, Bucca C (2010) Increased oral nitric oxide in obstructive sleep apnoea. Respir Med 104:316–320
Salerno FG, Carpagnano E, Guido P, Bonsignore MR, Roberti A, Aliani M, Vignola AM, Spanevello A (2004) Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med 98:25–28
Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ (2004) Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med 170:541–546
Inancli HM, Enoz M (2010) Obstructive sleep apnea syndrome and upper airway inflammation. Recent Patents Inflamm Allergy Drug Discov 4:54–57
Carpagnano GE, Spanevello A, Sabato R, Depalo A, Turchiarelli V, Foschino BM (2008) Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl Res 151:45–50
Depalo A, Carpagnano GE, Spanevello A, Sabato R, Cagnazzo MG, Gramiccioni C, Foschino-Barbaro MP (2008) Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med 263:70–78
Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, Kimura H (2005) Oxidative stress in obstructive sleep apnea. Chest 127:1674–1679
Tanaka T, Nakamura H, Yodoi J, Bloom ET (2005) Redox regulation of the signaling pathways leading to eNOS phosphorylation. Free Radic Biol Med 38:1231–1242
Lui MM, Lam DC, Ip MS (2013) Significance of endothelial dysfunction in sleep-related breathing disorder. Respirology 18:39–46
Feng J, Zhang D, Chen B (2012) Endothelial mechanisms of endothelial dysfunction in patients with obstructive sleep apnea. Sleep Breath 16:283–294
Kohler M, Stradling JR (2010) Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 7:677–685
Conflict of interest
The authors declare that they have no competing interests.
Funding
This research was supported by the Shanghai Leading Academic Discipline Project (B115), National Natural Science Foundation of China (81400043, 81100048, 81300055), Key Medical Grant from Shanghai Science and Technology Committee (13430720500), and Grant from Ministry of Education of China (20130071110044).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yuanlin Song, Shanqun Li and Chunxue Bai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, J., Li, Z., Liu, Z. et al. Exhaled nitric oxide from the central airway and alveoli in OSAHS patients: the potential correlations and clinical implications. Sleep Breath 20, 145–154 (2016). https://doi.org/10.1007/s11325-015-1198-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-015-1198-7