Abstract
Purpose
The objectives of this study were to examine the prevalence of obstructive sleep apnea (OSA) in primary school children, relationships between OSA and tonsillar hypertrophy, adenoid, and patency of the nasal cavity, and the effects of the tonsillar hypertrophy and adenoid on the nasal patency.
Methods
An examination of the palatine tonsils and anterior rhinoscopy, completion of a questionnaire survey, a measurement of nasal resistance, an examination for sleep apnea at home using portable polysomnography device, and radiography of pharyngeal tonsil were performed in 152 primary school children.
Results
The obstructive apnea hypopnea index (O-AHI) increased with enlargements in the palatine tonsils. The adenoidal nasopharyngeal ratio (A/N ratio) decreased with advances in grade. A significant difference was observed in the O-AHI between those with and without adenoid. The median value of the O-AHI increased with advances in grade. The nasal resistance was significantly higher in the group with adenoid compared as in the group without. It was also higher in the nasal disease group with OSA than in the group without. Full polysomnography(PSG) was recommended in 16 (10.5 %) of 152 who underwent the examination using the portable polysomnography device, and of the eight who underwent PSG, six (75 %) were confirmed to have OSA, while its prevalence in all subjects was estimated as 7.9 %.
Conclusions
Disturbances in nasal respiration as well as tonsillar hypertrophy and adenoid were found to be a risk factor of OSA in primary school children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary causes of obstructive sleep apnea (OSA) in children are tonsillar hypertrophy, adenoid, and nasal diseases. The palatine and pharyngeal tonsils reach their largest at the ages of 3–6 years old [1] and these narrows the airway. Papaioannou et al. [2] reported that in children without snoring, growing adenotonsillar tissue narrows the upper airway lumen to variable degrees only during the first 8 years of life. In contrast, in children with snoring, appreciable pharyngeal lymphoid tissue enlargement is present during the preschool years and persists beyond the eighth birthday. If a nasal respiration disorder exists, it is compensated for to an extent by conscious oral respiration during awakening; however, this conscious compensation ceases during sleep, and respiratory disturbance occurs as patients try to breathe unconsciously through the narrowed nasal cavity. Nasal obstruction causes exacerbates apnea because negative pressure in the airway during inspiration increases due to an increase in resistance to the passage of air through the nasal cavity, inducing stenosis of the soft and weak pharyngeal cavity. Konno et al. [3] induced nasal obstruction by blocking the nostrils with cotton balls soaked with vaseline during sleep in infants and demonstrated the occurrence of sleep apnea or hypopnea. OSA in children, unlike that in adults, is diagnosed when the obstructive apnea hypopnea index (O-AHI) is ≥1 [4]. OSA in children is often mitigated, and polysomnographic findings were normalized in the majority of children (79 %) by adenotonsillectomy [5]. The prevalence of OSA in children has been reported to be 0.7–10.3 % [6–8]; however, there has been no report on the prevalence of OSA according to the AASM international classification of sleep disorders [4]. In our questionnaire survey in 2008, OSA was noted in 7.4 % of 900 primary school children. However, the prevalence of OSA has been hardly reported using a testing instrument in Asia. The prevalence of allergic rhinitis is also increasing in Japanese children, and although the nocturnal disturbance of nasal respiration may be an exacerbating factor of OSA, no report has been conducted on the relationship between OSA and patency of the nasal cavity in all primary school grades combined. In this study, we investigated the prevalence of OSA in Asian primary school children, relationships between OSA and tonsillar hypertrophy, adenoid, and patency of the nasal cavity, and the effects of tonsillar hypertrophy and adenoid on nasal patency by portable polysomnography device, rhinomanometry, and radiography.
Material and methods
Study design
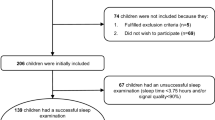
Health screening based on Japanese laws was conducted in 270 first (6–7-year-olds) to sixth graders (11–12-year-olds) in Ayagawa Town between May and June, 2012. In those who consented to the present survey, (1) a questionnaire survey including snoring, sleep apnea, nasal symptoms, and history of surgery was conducted, (2) nasal resistance was measured using a rhinomanometer, (3) sleep apnea was examined at home using a portable polysomnography device, and (4) radiography was performed at a hospital to check for the presence or absence of adenoid. The study received approval from the Hospital Ethics Committee. Of the 270 children, 152 (56.3 %) submitted informed consent and underwent the questionnaire survey, rhinomanometry, examination for sleep apnea, using a portable polysomnography device, and radiography of pharyngeal tonsil, respectively.
Anterior rhinoscopy and rhinomanometry
Anterior rhinoscopy was performed in 270 children in May as a part of health screening based on Japanese laws. We used the data of 152 who underwent all examinations among 270 children in this study. Rhinomanometry was performed in 152 of these children.
This period was chosen because it was when children were least affected by the common cold (December to March) and Japanese cedar or cypress pollinosis (February to April). The checkup was performed on a school day between 9:00 and 11:00. Anterior rhinoscopy was performed by a single operator in all subjects, and nasal resistance was measured in the bilateral nasal cavities once in all subjects by another single operator. The measurement was not repeated. The two examinations were separately performed at distant sites so that the operators were blinded from the other test results. The test results were finally combined based on the subject ID.
Nasal resistance was measured using rhinomanometry. The instrument (MPR-3100 Rhinomanometer, Nihonkoden, Japan) was connected to a personal computer. The rhinometry technique we used involved the nozzle active anterior method [9, 10]. Nozzle’s internal diameter was 8 mm. Left and right unilateral measurements were performed, and all nasal resistance measurements were calculated according to Ohm’s law automatically. The evaluation method adopted a resistance value of 100 Pa for resting respiration.
The normal group
We questioned schoolchildren and their parents about the presence or absence of nasal symptoms (sneezing, rhinorrhea, and nasal obstruction) for several days of the testing. An otolaryngologist checked for nasal diseases with an anterior rhinoscope. The nasal disease group was defined as subjects with rhinitis, rhinosinusitis, or septal deviation. The normal group was defined as those without nasal diseases.
Tonsil size
The size of the palatine tonsils was checked in all 270 children at school in May as a part of health screening based on Japanese laws. We used the data of 152 who underwent all examinations among 270 children in this study. Tonsillar hypertrophy was defined as grade +3 or +4 according to Brodsky’s classification [11].
Examination for sleep apnea
One hundred fifty-two children took home portable polysomnography device (Smart Watch PMP-300E®, Pacific Medico, Tokyo, Japan), and the examination was performed by their parents at night. The portable polysomnography device was returned by children to school the next day and collected by us. Data on body position, chest-wall movements, nasal pressure, snoring, SpO2, and pulse rate were obtained using this portable monitor device and analyzed by a polysomnographic technologist. All portable monitor device data were manually scored by a polysomnographic technologist according to the 2007 American Academy of Sleep Medicine (AASM) scoring rules [12]. The apnea hypopnea index (AHI) minus central apnea was defined as the obstructive apnea hypopnea index (O-AHI).
Radiography
One hundred fifty-two children underwent lateral radiography in the seated position at Sue Hospital. Patency of the nasopharynx was evaluated using the A/N ratio [13]. Nasopharygeal fibrercopy is superior to X-ray for the evaluation of the volume of adenoid. But, in consideration of a risk of a pain and bleeding, we chose X-ray in this study.
Polysomnography (PSG)
Of the 152 children who underwent an examination for sleep apnea using a portable polysomnography device, a close examination by full PSG (Alice5®, Respironics, PA, USA) was considered necessary in 16 in whom the AHI was ≥5 or 3 % ODI (oxygen desaturation index) was ≥5. We explained this to the 16 children and their parents, and 8 consented and underwent the examination. The following parameters were measured: four-channel electroencephalography (C3-A2,C4-A1, O1-A2, and O2-A1), electrooculography, electromyography, electrocardiography, oronasal airflow recordings by an oronasal thermistor, thoracic and abdominal motion by piezo sensors, oxygen saturation by pulse oximetry, and tracheal sounds by a microphone. All full-night PSG data were manually scored by a polysomnographic technologist according to the 2007 American Academy of Sleep Medicine (AASM) scoring rules [12].
Statistical analysis
The Student’s t test was used for intergroup comparisons between the two patient groups. Probability values of p < 0.05 were regarded as significant. IBM SPSS Statistic version 20.0 (SPSS Inc. Chicago, IL, USA) was used for all statistical analyses.
Results
The questionnaire survey, measurement of nasal resistance, examination for sleep apnea using portable polysomnography device, and radiography were performed in 152 (56.3 %) subjects.
When grades +3 and +4 by Brodsky’s classification were defined as tonsillar hypertrophy, it was observed in 5 (29.4 %) of the 17 first graders, whereas this percentage decreased with advances in grade to 19.4 % (6/31) of sixth graders (Table 1).
The obstructive apnea hypopnea index (O-AHI) was 0.9–1.4 and 2.0–2.5 times/h for grade +2 or milder and +3 or severer tonsillar hypertrophy, respectively. It was significantly higher in children with +4 than +2 tonsillar hypertrophy (p = 0.007) (Table 2).
The A/N ratio was 0.61 ± 0.16 in first graders, decreased with advances in grade, and was 0.45 ± 0.17 in the sixth graders (Table 3).
A significant difference was observed in the O-AHI between the two groups divided according to the A/N ratio at a cutoff value of 0.55 (p = 0.048) (Table 4).
According to the results of PSG (O-AHI ≥1: ICSD II criteria) (Table 5), the cutoff value of the O-AHI for OSA using a portable polysomnography device (Smart Watch PMP-300E®) was between 3 and 4 (Table 6).
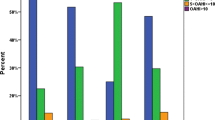
The median value of the O-AHI increased slightly with advances in grade, except for it being lower in second graders than in first graders (Fig. 1).
No significant difference was noted in 87 children in the normal group when nasal resistance was compared between those with and without tonsillar hypertrophy (0.42 ± 0.21 vs. 0.39 ± 0.20 Pa/cm3/s).
Nasal resistance was significantly higher in children with adenoid (A/N ratio ≥0.55) than in those without in the normal group (A/N ratio <0.55) (0.46 ± 0.24 vs. 0.32 ± 0.10 Pa/cm3/s).
When the relationship between OSA and nasal resistance was evaluated in 152 children, no difference was noted in nasal resistance between those with (O-AHI ≥ 4, 0.40 ± 0.26 Pa/cm3/s) and without (O-AHI < 4, 0.40 ± 0.20 Pa/cm3/s) OSA in the normal group, while it was higher in those with (O-AHI ≥ 4, 0.70 ± 0.57 Pa/cm3/s) than without (O-AHI ≥ 4, 0.59 ± 0.30 Pa/cm3/s) OSA in the nasal disease group (Table 7).
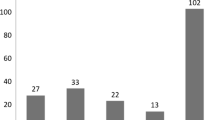
Of the 152 children who underwent the examination for sleep apnea using a portable polysomnography device, a more detailed evaluation was considered necessary in 16, and eight of them consented to full PSG. A diagnosis of sleep apnea syndrome was made in 6 (75 %) of the eight children who underwent full PSG. Treatment was considered necessary in five of the eight children, and three and two were treated surgically and conservatively, respectively (Table 5).
Discussion
This study is the first report that determined OSA and tonsillar hypertrophy, adenoid, association with the nasal patency with anterior rhinoscopy, rhinomanometry, X-rays, and portable polysomnography device. Also, we determined the effect that adenoid (Nasal resistance was significantly higher with adenoid (A/N ratio ≥0.55) (0.46 ± 0.24 Pa/cm3/s) than in those without (A/N ratio <0.55) (0.32 ± 0.10 Pa/cm3/s)) gave in nasal resistance.
The AHI determined by PSG has been reported to show seasonal variations due to the effects of viruses and pollens [14, 15]. In our present study, all examinations were carried out between May and June, during which the effects of upper respiratory tract infections and pollen allergies were the lowest, to minimize variations in the results of each examination.
A diagnosis of OSA was made when the AHI was ≥1 [4]. The major causes of OSA in children are tonsillar hypertrophy and adenoid and nasal diseases.
Brodsky’s classification [11] is used in the 2011 guidelines of the American Academy of Otolaryngology-Head and Neck Surgery Foundation concerning the palatine tonsils [16]. In this study, we defined hypertrophy of the palatine tonsils as grades +3 and +4 of Brodsky’s classification. Tonsillar hypertrophy was observed in 5 (29.4 %) of the 17 first graders (6–7-year-olds), whereas its prevalence decreased with advances in grade. It was noted in 8 (14.5 %) of the 55 fifth graders (10–11-year-olds) and sixth graders (11–12-year-olds) (Table 1). The size of the palatine tonsils has been shown to reach its largest at the ages of 3–6 years old [1], 7–8 years old without snoring [2], and then gradually decreases after school age. In contrast, in children with snoring, appreciable pharyngeal lymphoid tissue enlargement is present during the preschool years and persists beyond the eighth birthday [2]. In our present study, the prevalence of tonsillar hypertrophy in fifth graders and sixth graders (8/55) (14.5 %) was about half that in first graders (29.4 %), which suggests that about half of children with tonsillar hypertrophy in the first grade still have the condition after 4–5 years.
The O-AHI was 0.9–1.4 and 2.0–2.5 times/h in children with grade +2 or milder and +3 or more severe tonsillar hypertrophy, respectively, and was significantly higher for grade +4 than grade +2 (Table 2). Therefore, we consider grade +3 or more severe tonsillar hypertrophy to be an indication for tonsillectomy.
In the 87 children in the normal group (no nasal disease), no significant difference in nasal resistance was observed between those with (0.42 ± 0.21 Pa/cm3/s) and without (0.39 ± 0.20 Pa/cm3/s) tonsillar hypertrophy. In our prior study [17] of 892 children, the presence of tonsillar hypertrophy did not influence nasal resistance (0.40 ± 0.18 and 0.43 ± 0.52 Pa/cm3/s with and without tonsillar hypertrophy, respectively). In another study [18] of 494 children with no nasal disease, nasal resistance differed significantly between those with (0.41 ± 0.17 Pa/cm3/s) and without (0.35 ± 0.15 Pa/cm3/s) tonsillar hypertrophy. Nasal resistance has been reported to decrease significantly in adults after tonsillectomy [19]. Since nasal resistance is calculated from the pressure difference between the anterior and posterior parts of the nostril by the nozzle active anterior method, the value is theoretically not affected by the palatine tonsils located in the oropharynx. However, nasal resistance may be affected if the superior pole of the palatine tonsils pushes the soft palate up to a large extent, and further evaluations are necessary to elucidate the relationship between nasal resistance and tonsillar hypertrophy in more detail in children.
The A/N ratio was 0.61 ± 0.16 in first graders, decreased with advances in grade, and was 0.45 ± 0.17 in sixth graders (Table 3), which is consistent with the report by Fujioka M et al. [13]. They used an A/N ratio of 0.5 as a cutoff value between the presence and absence of adenoidal hypertrophy. Joshua B et al. [20] evaluated the size of the pharyngeal tonsils by the percentage of the upper pharynx it occupied using nasopharyngeal fiberscopy and considered that symptomatic nasal obstruction may be caused when 51–75 % of the upper pharynx is occupied by adenoidal tissue. In our present study, the O-AHI differed significantly between above and below the A/N ratio of 0.55 (Table 4); therefore, we propose an A/N ratio of 0.55 as a criterion for adenoidal hypertrophy in Japanese children.
In 87 children with no nasal disease, nasal resistance was significantly higher in those with (0.46 ± 0.24 Pa/cm3/s) than without (0.32 ± 0.10 Pa/cm3/s) adenoid (cutoff value, A/N ratio = 0.55) (p = 0.001). Therefore, adenoid is considered to be a factor that affects nasal resistance.
Since the cutoff value of the O-AHI for OSA by PSG is ≥1 according to the ICSD II criteria [4], the cutoff value of the O-AHI for OSA by PSG using portable polysomnography device was between 3 and 4 (Table 5). Therefore, using portable polysomnography device, an O-AHI of ≥4 is judged to indicate OSA (Table 6).
The median O-AHI was lower in second graders than in first graders, but thereafter increased with advances in grade (Fig. 1). The median O-AHI increased in spite of progressive decreases in the size of the palatine and pharyngeal tonsils with advances in grade, which may have been because of disturbances in nasal respiration due to allergic rhinitis, the prevalence and severity of which increase with advances in grade in Japan.
When the relationship between OSA and nasal resistance was evaluated in 152 children, nasal resistance was not significantly different between those with (O-AHI ≥ 4, 0.40 ± 0.26 Pa/cm3/s) and without (O-AHI < 4, 0.40 ± 0.20 Pa/cm3/s) OSA in the normal group, but was higher in those with (O-AHI ≥ 4, 0.70 ± 0.57 Pa/cm3/s) than without (O-AHI < 4, 0.59 ± 0.30 Pa/cm3/s) OSA in the nasal diseases group (Table 7). Therefore, a disturbance in nasal respiration is considered to be an exacerbating factor of OSA. Rizzi et al. [21] reported that rhinomanometry, which is an objective test of patency of the nasal cavity, was useful for screening children for sleep apnea and that nasal resistance was correlated with the severity of OSA. However, according to our evaluation, such a correlation was not observed. The reason for this discrepancy may be that the O-AHI is determined by various factors including nasal diseases, adenoid and tonsillar hypertrophy, and obesity, while nasal resistance is an index of patency from the nasal cavity to the upper pharynx.
Based on the results of the examination for sleep apnea using portable polysomnography device, a more detailed evaluation was considered necessary in 16 of the 152 children, and eight of them consented to an evaluation by full PSG, which established the diagnosis of OSA in six (75 %). If OSA is assumed to have been present in 75 % (12) of the 16 children, its prevalence is estimated to be 7.9 % (12/152). The prevalence of OSA was reported to be 0.7 % in 4–5-year-olds by Ali et al. [6] using pulse oximetry and video recorders, 2.9 % in 0.5–6-year-olds by Gislason et al. [7] using PSG by the criterion of AHI > 3, and 1.6 % for AHI > 10 and 10.3 % for AHI > 5 in 2–18 -year-olds by Rendline et al. [8] using home PSG. In the present study, we estimated the prevalence of OSA in Asian primary school children to be 7.9 % using portable polysomnography device and full PSG by the criterion of the O-AHI ≥ 1 according to the AASM rules for scoring respiratory events [4]. Discussions with standardized testing instruments and diagnostic criteria are desired for the future.
In conclusion, we estimated the prevalence of OSA in Asian primary school children using portable polysomnography device and full PSG. Disturbances in nasal respiration as well as tonsillar hypertrophy and adenoid were found to be a risk factor of OSA in primary school children. Nasal obstruction and the high nasal resistance may predict the presence of OSA and adenoid.
References
Jeans WD, Fernando DC, Maw AR, Leighton BC (1981) A longitudinal study of the growth of the nasopharynx and its contents in normal children. Br J Radiol 54:117–121
Papaioannou G, Kambas I, Tsaoussoglou M, Panaghiotopoulou-Gartagani P, Chrousos G, Kaditis AG (2013) Age-dependent changes in the size of adenotonsillar tissue in childhood: implications for sleep-disordered breathing. J Pediatr 162:269–274
Konno A, Togawa K, Hoshino T (1980) The effect of nasal obstruction in infancy and early childhood upon ventilation. Laryngoscope 90:699–707
American Academy of Sleep Medicine (2005) International classification of sleep disorders: diagnostic and coding manual, 2nd edn. American Academy of Sleep Medicine, Westchester
Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, Mitchell RB, Amin R, Katz ES, Arens R, Paruthi S, Muzumdar H, Gozal D, Thomas NH, Ware J, Beebe D, Snyder K, Elden L, Sprecher RC, Willging P, Jones D, Bent JP, Hoban T, Chervin RD, Ellenberg SS, Redline S, Childhood Adenotonsillectomy Trial (CHAT) (2013) A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 368:2366–2376
Ali NJ, Pitson DJ, Stradling JR (1993) Snoring, sleep disturbance, and behaviour in 4–5 year olds. Arch Dis Child 68:360–366
Gislason T, Benediktsdóttir B (1995) Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest 107:963–966
Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G (1999) Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med 159:1527–1532
Unno T, Naitoh Y, Sakamoto N, Horikawa H (1986) Nasal resistance measured by anterior rhinomanometry. Rhinology 24:49–55
Georgitis JW (1985) The applicability of rhinomanometry in nonatopic children: comparison of three techniques. J Allergy Clin Immunol 75:614–620
Brodsky L (1989) Modern assessment of tonsils and adenoids. Pediatr Clin N Am 36:1551–1569
Iber C, Ancoli-Israel SA, Chesson A, Quan SF, for the American Academy of Sleep Medicine (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester
Fujioka M, Young LW, Girdany BR (1979) Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol 133:401–404
Nakayama M, Koike S, Kuriyama S, Suzuki M, Nakamura Y, Yamamoto K, Murakami S, Gozal D (2013) Seasonal variation in a clinical referral pediatric cohort at risk for obstructive sleep apnea. Int J Pediatr Otorhinolaryngol 77:266–269
Gozal D, Shata A, Nakayama M, Spruyt K (2011) Seasonal variability of sleep-disordered breathing in children. Pediatr Pulmonol 46:581–586
Baugh RF, Archer SM, Mitchell RB, Rosenfeld RM, Amin R, Burns JJ, Darrow DH, Giordano T, Litman RS, Li KK, Mannix ME, Schwartz RH, Setzen G, Wald ER, Wall E, Sandberg G, Patel MM (2011) American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg 144:S1–S30
Kobayashi R, Miyazaki S, Karaki M, Kobayashi E, Karaki R, Akiyama K, Matsubara A, Mori N (2011) Measurement of nasal resistance by rhinomanometry in 892 Japanese elementary school children. Auris Nasus Larynx 38:73–76
Kobayashi R, Miyazaki S, Karaki M, Hara H, Kikuchi A, Kitamura T, Mori N (2012) Nasal resistance in Japanese elementary school children: determination of normal value. Acta Otolaryngol 132:197–202
Nakata S, Miyazaki S, Ohki M, Morinaga M, Noda A, Sugiura T, Sugiura M, Teranishi M, Katayama N, Nakashima T (2007) Reduced nasal resistance after simple tonsillectomy in patients with obstructive sleep apnea. Am J Rhinol 21:192–195
Joshua B, Bahar G, Sulkes J, Shpitzer T, Raveh E (2006) Adenoidectomy: long-term follow-up. Otolaryngol Head Neck Surg 135:576–580
Rizzi M, Onorato J, Andreoli A, Colombo S, Pecis M, Marchisio P, Morelli M, Principi N, Esposito S, Sergi M (2002) Nasal resistances are useful in identifying children with severe obstructive sleep apnea before polysomnography. Int J Pediatr Otorhinolaryngol 65:7–13
Acknowledgments
We thank Ms. Naomi Kubo, Ms. Miho Shirafuji, and Mr. Takayuki Shinota for their assistance with the rhinomanometry measurements and statistical analysis. We are also grateful to Ms. Ikuko Morita, who analyzed the portable monitor device data.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, R., Miyazaki, S., Karaki, M. et al. Obstructive sleep apnea in Asian primary school children. Sleep Breath 18, 483–489 (2014). https://doi.org/10.1007/s11325-013-0909-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-013-0909-1