Abstract
Introduction
Neonatal treatment with clomipramine (CLI) has been shown to have reliable behavioral and biological changes that mimic major symptomatic and biochemical changes found in depression. This paper further explores a common feature of depression, the comorbidity of seizure activity and depressive behaviors in this mode.
Methods
Rat pups were neonatally treated with 40 mg/kg/day of CLI from postnatal day 8 through 21. In adulthood, they were instrumented with electroencephalographic (EEG) and electromyographic (EMG) electrodes for 24 h of polysomnogram (PSG) recordings. PSG data were analyzed for: (1) sleep-wake cycle; (2) spectral power; and (3) epileptiform activity, including NREM-to-REM transition (NRT) bursts.
Results
Neonatal treatment with CLI reliably produces enhanced levels of REM (p < 0.01) and reduced sexual activity (p < 0.05). Theta power was enhanced during NREM sleep in the CLI group (p = 0.02). CLI-treated animals experienced increased frequency at the NRT (p < 0.01), as well as additional epileptiform activity of continuous (CTS; p < 0.05) and petite-continuous (P-CTS; p < 0.01) types, across the sleep–wake cycle. There is a strong temporal correlation with increased REM sleep duration, increased frequency of NRT bursts, and increased theta power during NREM sleep in CLI-treated animals.
Discussion
Neonatal CLI-treated animals experienced significantly more epileptiform activity as a whole, in addition to comorbid features of depression in adulthood. Neonatal exposure to CLI will not only produce depressive phenotype but may also enhance risk for epilepsy in some individuals. This warrants further investigation into currently acceptable medicinal use in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal exposure to the tricyclic antidepressant, clomipramine (CLI), has been investigated in many species for its ability to produce depressive pathophysiology during adulthood [1–4]. The model displays adult onset sleep disturbances, an increased percentage of REM sleep, and a decreased REM sleep onset latency [5] as seen in human depression [6–9]. Spectral power changes within the sleep–wake cycle, as indicated by the electroencephalogram (EEG), and power levels of specific EEG rhythms also vary with epileptic activity [10], treatment of depression in humans [11], and response to clomipramine [12]; however, none of these studies suspected or studied neonatal risks, but rather, reported findings in adults.
The Long-Evans strain of rat is more sensitive to neonatal treatment with clomipramine than strains of Sprague–Dawley and Wistar strains [13]. In the absence of neonatal CLI, the Long-Evans strain of rat exhibits freezing behavior that electoencephalographically is characterized by epileptiform bursting, resembling absence seizures [14, 15]. A recent study demonstrated that adult WAG/Rij rats treated with acute injections of clomipramine experienced increased levels of epileptiform spike and wave discharges within 1 to 4 h post-injection [16]. Given these observations and the clinical co-relationship of depression and epilepsy in sleep disturbances [17]; one might suspect that neonatal CLI treatment will alter adult brain excitability.
In the current study, we examined epileptiform bursting and spectral power levels across the sleep–wake cycle in adult Long-Evans rats after neonatal CLI treatment. We hypothesize that CLI-treated animals, who exhibit depressive traits, will also have increased epileptiform activity, namely bursting activity at the NRT point, as well as outside of the NRT point, relative to control animals [14]. In addition, spectral power changes, namely in theta frequency, would give insight into mechanisms putting individuals at risk for epileptiform activity.
Methods
Subjects
Male Long-Evans hooded newborn rats were acquired from Harlan, Inc. (Indianapolis, IN) at postnatal day 4 along with their mothers and were housed at the Louis Stokes VAMC. The light–dark cycle of the rat colony was 6:00 a.m. (light-on) to 6:00 p.m. (light-off). Rat pups were treated twice daily (at 7:30 a.m. and 5:30 p.m. ±15 min) with 20 mg/ kg of CLI (n = 8) or equivolume of saline (SAL, n = 8) from postnatal day 8 to postnatal day 21. Animals were weaned at day 21 and littermates were evenly distributed between groups. Animals were socially housed and other than routine husbandry were left undisturbed until surgery. All guidelines of the Institutional Animal Care and Use Committee were adhered to, and protocols were approved by the IACUC of Louis Stokes Cleveland Department of Veteran Affairs.
Surgery and polysomnographic recording
At 5 to 6 months of age, the animals were anesthetized for surgical electrode implantation with sodium pentobarbital (50 mg/kg with additional 25 mg/kg if needed). According to our previous publication, commercially available electrodes were used (Plastics One, Roanoke, VA). Stainless steel jewel screws were stereotaxically screwed into the skull. The location of the three electrodes were: E1: AP = 2.0, L = 1.5; E2: AP = −2.0, L = 3.0; E3: AP = −6.0, L = 3.0. EMG electrodes were sutured onto the nuchal muscles [18]. After surgery, rats were given 8 to 10 days to recover and were switched to a 9:00 a.m. (light-on) to 9:00 p.m. (light-off) (to match previous publications using this protocol) schedule throughout recovery and PSG recording. After the recovery period, animals were connected to the recording cable and housed in special acrylic glass cages where water and food were freely accessible. Animals were given 96 h of adaptation to the chamber prior to the 24-h baseline recording.
Analysis of PSG data
Sleep–wake scoring
Sleep–wake states were scored in 30 s epoch as REM sleep, NREM sleep, active wake (AW), and quiet wake (QW) according to previous publications from this lab [18]. Epochs were visually identified as a particular state when that state occupied 50% or greater of the 30-s period (one epoch). All epochs were identified as one of the above states, and all were included in PSG analyses.
Spectral power analysis
Separate analyses were done for the light-on and light-off phases. Results are displayed either as a percent of power of that state relative to the total power across states or as an averaged absolute power. Due to variability in reported sigma ranges (ex. [19, 20]), we report a combined alpha-sigma range. The Somnologica program was used to calculate the amount of power for alpha-sigma (8.0–13.9 Hz), beta (14.0–29.9 Hz), delta (0.5–3.9 Hz), and theta (4.0–7.9 Hz) rhythms. Power analyses were calculated on frontal cortical EEG activity in 30-s epochs via Somnologica® Fast Fourier transformation algorithms. State specific power bands for each of the above rhythms were analyzed, and significant differences between groups were reported. Also, targeted power analyses were calculated for independent NREM and REM epochs at the transition point.
NRT bursts and epileptiform activity scoring
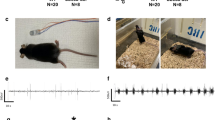
Special events in the EEG were observed and denoted as bursts or epileptiform activity. The events were categorized based on waveform characteristics, such as: duration, correlational EMG activity, and state of onset preference. Six snapshots of three event types shown in two durations (a longer snapshot in minutes and a close-up of a portion of the longer in seconds) are displayed as: NRT bursts, CTS activity, and P-CTS activity. NRT bursts were a priori defined as having an onset (first burst) that occurred within the 60-s epoch of the transition from NREM-to-REM sleep (Fig. 1a), which was chosen based upon known changes [21]. Continuous events were operationally defined as having a duration lasting 4 s or longer (Fig. 1b). Continuous events were more heterogeneous in nature than the other two types having no predictable duration of time, co-occurring EMG activity or lack thereof (approximately half of the time), and moderate-to-low state preference (later discussed in further detail). Petite-continuous events were scored in the same manner as CTS with the differences being a shorter duration lasting 3 s or less and increased state preferences (Fig. 1c).
a Two representations of each type of quantified hypersynchronous activity. Frontal and parietal EEGs show three sample spindles (denoted by arrows) occurring at the NREM-to-REM transition point (left side) and a closer view (right side) of the second and third events (1A). b Spindle activity is noticeable in the frontal and parietal EEGs, but not in the EMGs. b An example of a long CTS event (denoted by arrow) occurring at the transition from active wake to quiet wake (left side) and a closer view (right side). Hypersynchronous activity is noticeable in all three traces. c Four examples of P-CTS activities (denoted by arrows) occurring in quiet wake (left side) and a closer view of the first event (right side). Epileptiform activity is noticeable in all three traces
Events were scored as part of a series where an event was considered within series if it occurred within 15 s of the preceding event. The total number of events were summed and averaged and the number range spanned from1 to 64 events within one series. Series of events could span across multiple epochs and across state changes. In addition to denoting the number of events in a series, the state of onset of the first event of the series was also recorded. State changes were broken down according to the state of onset for the total number of events in the series. Finally, the amount of time spent in each sleep–wake cycle was also taken into account to reflect the relative number of epileptiform events that occurred across the state of onset. The relative number of events given the amount of time spent in that state is the most accurate representation of state preferences for the onset of epileptic activity but does not take into account the possible state changes that may have occurred within a long series of events; however, the longer series applied only to CTS events and were less frequent. The vast majority of series occurred within one to two epochs.
Behavioral testing
Sexual activity
Twenty days after the last day of sleep recording, sexual activity testing began, and female Long-Evans hooded animals of 4 to 5 months of age were used as partners (n = 9). In order to control for the female estrous cycle, at 3 months of age, females were ovariectomized. Prior to testing, females were twice subcutaneously injected with 5 μg/0.05 ml of the sex hormone β-estradiol at 48 and 24 h, as well as one injection with 0.5 mg/0.1 ml of progesterone at 4 h prior to testing. All males were sexually naïve prior to their first testing session and were tested in three separate sessions with 1 to 5 days in between each session. Males were placed in the testing chamber for 5 min of adaptation prior to females being introduced, and testing sessions were 30 min each. Partner pairing, testing order, and intersession interval were counterbalanced across groups. Sexual activity testing was conducted in the dark phase within 1 to 5 h post-light offset, allowing for 1 h of adaptation to light offset. Sexual mounting, latency to mount, and the number of ejaculations were scored live and video-taped for verification purposes. The CLI-treated rats showed no ejaculatory behavior and therefore the number of ejaculations is not presented.
Locomotor activity
The males were tested in the locomotor activity test using automated MotorMonitor equipment (Kinder Scientific, Inc., Poway, CA). This device has a rectangular testing frame, which has a set of sensors in all directions for detecting broken beams. Each rat was placed in a standard rat tub within the testing frame for a 10-min testing session for a total of three testing days. Rats were tested in the dark phase at 1 to 3 h following light offset. Total activity was automatically recorded, and data was analyzed using MotorMonitor® software (Kinder Scientific). Activity was distinguished in movement metrics that have been previously reported [1, 22].
Statistical analysis
Descriptive statistics, including group means and standard error of the means, were reported for all data. A repeated measure analysis of variance (RM-ANOVA) was used to test for significant differences between treatment groups (SAL vs. CLI) for sleep–wake cycles across time, as well as sexual activity across testing sessions. T tests were used to test for differences in raw data of NRT bursts (planned), CTS activity, P-CTS activity, and the locomotor activity test. A univariate ANOVA test was used to test for treatment differences of events by state onset per unit of time for NRT burst, CTS activity, P-CTS activity, and spectral analysis measures. Statistical significance for overall analyses is described at a 95% confidence level. Group comparisons were made using the Bonferonni post hoc test to control for multiple comparisons.
Results
PSG data
Sleep–wake cycle
Table 1 summarizes the average amount of REM, NREM, active wake, and quiet wake for each state in 6-h blocks of time. Similar to previous studies, we found significant differences between CLI- and SAL-treated animals at multiple time points for REM sleep [df (1, 8), F = 16.63, p < 0.01]. CLI-treated animals (n = 5) averaged 36 ± 3 min and SAL-treated animals had 26 ± 2 min of REM sleep. There were no significant differences between groups during NREM sleep [df (1, 8) F = 1.18, p > 0.05]. CLI-treated animals averaged 167 ± 11 min of NREM sleep and SAL-treated animals averaged 179 ± 12 min.
There were no differences for total wake time between groups as CLI-treated animals had 156 min and SAL-treated animals had 155 min (p > 0.05). However, after separating quiet and active wake, modest trends in activity levels emerged. Although CLI-treated animals had more time in QW 42 ± 9 min relative to the SAL-treated animals 28 ± 6 min, this was not statistically significant [df (7, 56) F = 0.95, p > 0.05]. Even the largest point of difference, from 9 a.m. to 3 p.m., did not reach significance (t = −1.88, p > 0.02). CLI-treated animals had 45 ± 9 min and SAL-treated animals had 26 ± 5 min.
Similarly, there was no significant difference between CLI- and SAL-treated animals in active wake [df (1, 8), F = 0.04, p > 0.05], where CLI-treated animals averaged 114 ± 12 min relative to SAL-treated animals who averaged 127 ± 15 min. Interestingly, the greatest point of divergence during active wake was also from 9 a.m. to 3 p.m., where CLI-treated animals had 36 ± 12 min and SAL-treated animals had 63 ± 12 min (difference was in the opposite direction from QW). These results suggest that while there are no differences in the amount of total wake time, CLI-treated animals tended to be slightly less active than their control counterparts during their subjective peak sleeping time of day (Table 1).
Power analyses
There were no differences in the percent of power between groups for any rhythm during quiet or active wake, during light-on or light-off phases (data not shown). During the light-on NREM sleep phase, power analysis revealed a modest difference between CLI- and SAL-treated animals for the alpha-sigma rhythm [df (1, 9), F = 5.60, p = 0.05]; however, after controlling for multiple comparisons, this was not significant. There was a statistically significant difference between groups for the theta rhythm [df (1, 9), F = 8.42, p = 0.02], but there were no differences between groups for beta or delta rhythms (Fig. 2a). CLI-treated animals had moderately less alpha-sigma power and significantly more theta power than SAL-treated animals. Interestingly, during REM sleep, we saw a switch in direction, in that CLI-treated animals had less theta power than SAL-treated animals, but this did not reach statistical significance [df (1, 9), F = 1.73, p > 0.05] (Fig. 2b). There were no other differences during REM sleep.
A comparison of power levels between CLI- and SAL-treated animals. a No difference between groups for alpha-sigma, beta, and delta rhythms during NREM sleep for the light-on phase, but there was a significant difference in theta power. b No difference between groups for any rhythm during REM sleep for the light-on phase. c No difference between groups for any rhythm during NREM sleep for the light-off phase. d No difference between groups for alpha-sigma, beta, and delta rhythms during REM sleep for the light-off phase, but CLI-treated animals had significantly less theta power
During the light-off phase, although theta rhythms for CLI-treated animals were still higher, they were no longer significantly different from SAL-treated animals during NREM sleep [df (1, 9), F = 0.633, p > 0.05] (Fig. 2c). The data during REM sleep (Fig. 2d) consistently show that CLI-treated animals had less theta power than their control counterparts, and furthermore during the light-off period, this difference approached significance [df (1, 9), F = 6.91, p = 0.03], but when controlling for multiple comparisons was only moderate.
Targeted spectral power analyses were done at the NRT point. NREM and REM epochs were separated from all other epochs outside of the NRT, including other NREM and REM epochs. Each NREM (Fig. 3a) and REM epoch (Fig. 3b) pair were separated and averaged independently to see if one state could account for the differences noted above. For the light-on phase, there were no statistically significant differences for the averaged NREM epochs [df (1, 9), F = 3.01, p > 0.05], which indicates that the NREM epoch of the NRT does not account for the increase in theta power found across all NREM epochs.
Independent NRT power level comparisons for the NREM and REM epochs. a There were no differences between groups for any rhythm during the NREM epochs for NRTs. b There was no difference between groups for alpha-sigma and delta rhythms, but it shows that CLI-treated animals had moderately more beta power and moderately less theta power during the REM epoch of the NRT (p < 0.05, but not significant when controlling for multiple comparisons)
For the separated NRT REM epoch, the pattern of results is trending in the same direction as the overall pattern did with slight increases in beta and decreases in theta levels; however, once again, these trends were not significant, beta [df (1, 9), F = 4.49, p > 0.05] and theta rhythms [df (1, 9), F = 3.88, p > 0.05]. These results suggest that while the REM epoch of the NRT may account for a portion of the difference in overall power level trends, the difference is not entirely accounted for by the NRT; or, is maximally only a modest difference.
The same NRT analyses were also done for the light-off phase. There was neither main effect of group for NREM [df (1, 7), F = 2.087, p > 0.05] nor for REM [df (1, 7), F = 0.198, p > 0.05]. Also, there was no significant interaction for group by range for the NREM epochs [df (7, 39), F = 0.117, p > 0.05] nor for the REM epochs [df (7, 39), F = 1.957, p > 0.05]. No significant differences in power levels were seen between groups during the light-off phase at either state.
Epileptiform activity: NRT bursts
The averaged total number of NRT bursts in CLI-treated animals was 255 ± 68, and in SAL-treated animals it was 104 ± 22, which were significantly different from each other (t = 4.804, p < 0.05) (Fig. 4a). The EMG trace showed 0% accompanying muscle activity. NRT bursts typically occurred in a series of 2 to 4 bursts and the average number in a series for CLI-treated animals was 4.1 ± 0.4, and for SAL-treated animals, it was 2.7 ± 0.1. Table 1 shows the average number of events that occurred across the sleep–wake cycle for the state of onset. There was an overall effect for treatment by state of onset [df (1, 39), F = 3.116, p = 0.01]. Also, there was a main effect of group [df (1, 32), F = 5.934, p < 0.05], as well as a main effect of state [df (3, 32), F = 3.289, p < 0.05]. A Bonferroni post hoc analysis showed that SAL- and CLI-treated animals significantly differed in NREM sleep (p < 0.05).
A comparison of CLI-treated and SAL-treated animals for NRT burst events. a The average number of NRT burst events collapsed across states. CLI-treated animals experienced significantly more events than SAL-treated animals. b The number of NRT burst events per unit time for drug-treated and SAL-treated animals. There were no differences between groups for REM sleep, but CLI-treated animals experienced significantly more events during NREM sleep
In addition to the raw average number of events reported above, it is important to consider the amount of opportunity to experience an event by taking into account the relative amount of time spent within that state [total number of events for each state of onset ÷ (time in state ÷ total time across all states)]. When looking at the data more closely, we see that given the amount of time per state relative to the total time, more events occurred per unit of time during REM sleep (716 ± 169, SAL) and (670 ± 124, CLI) than in NREM sleep (104 ± 21, SAL) and (403 ± 119, CLI). An ANOVA revealed an overall effect of NRT events per unit time [df (3, 19) F = 4.987, p < 0.05]. There was no main effect of group [df (1, 19), F = 0.002, p > 0.05], but there was a main effect of state [df (1, 16), F = 4.219, p = 0.05]. Group comparisons revealed that CLI-treated animals had significantly more events 403 ± 119 in NREM sleep relative to SAL-treated rats (104 ± 21, p < 0.05), there was no difference between groups during REM sleep (716 ± 169 versus 670 ± 124, p > 0.05) (Fig. 4b). The raw data and relative NRT analysis combined suggest that the additional bursts the CLI-treated animals experienced relative to control animals occurred in the NREM epoch immediately preceding REM sleep.
Epileptiform activity: CTS and P-CTS
On average, CTS events (see Fig. 1b) had correlated and patterned EMG activity 43% (SAL) and 46% (CLI) of the time and P-CTS (see Fig. 1c) had it 17% (SAL) and 23% (CLI) of the time (i.e., EMG onset and offset occurred simultaneously or within 1 s after the EEG burst). The average total number of CTS events (collapsed across all sleep–wake states) in CLI-treated animals was 578 ± 125 and in SAL-treated animals was 299 ± 162, which were significantly different (t = 4.710 p < 0.05; Fig. 5a). The average total number of P-CTS events in CLI-treated animals was 133 ± 61 and 27 ± 14 for SAL-treated animals, which were also significantly different (t = 8.604, p < 0.01; Fig. 5b).
A comparison of the average number of CTS and P-CTS events for CLI- and SAL-treated animals. a The average number of CTS events collapsed across all states. CLI-treated animals experienced significantly more events. b The average number of P-CTS events collapsed across all states. CLI-treated animals experienced moderately more events. c The number of CTS events per unit time. All animals experienced the most number of relative CTS events in quiet wake, followed by REM, NREM, and the fewest in active wake. There were no differences between groups for quiet wake, REM sleep, or active wake; however, drug-treated animals experienced significantly more events during NREM sleep. d There was no main effect of state for P-CTS events; however, there was a main effect of group. Once more, CLI-treated animals had significantly more events during NREM sleep
When considering the amount of time within each state, the results show that relatively more CTS events were experienced during quiet wake than any other state (982 ± 159, SAL) and (1142 ± 107, CLI). The next highest was during REM sleep (476 ± 73, SAL) and (762 ± 36, CLI), then during NREM there were (177 ± 17, SAL) and (582 ± 23, CLI) with the fewest relative events occurring during active wake at (285 ± 43, SAL) and (296 ± 34, CLI). An ANOVA revealed an overall significant difference in CTS events per unit time [df (7, 39), F = 3.116, p = 0.01]. Between-group comparisons revealed a main effect of group [df (1, 32), F = 5.934, p < 0.05], as well as a main effect of state [df (3, 32), F = 3.289, p < 0.05]. A pairwise comparison controlling for multiple comparisons found that CLI-treated animals experienced significantly more CTS events per unit time in NREM than SAL-treated animals (p < 0.01) (Fig. 5c).
For P-CTS events, we saw a similar pattern of results with (72 ± 17, SAL) and (352 ± 203, CLI) relative events occurring in quiet wake, and occurring in REM were (30 ± 17, SAL) and (130 ± 51, CLI) events, during NREM there were (15 ± 7, SAL) and (125 ± 44, CLI) and during active wake (31 ± 9, SAL) and (64 ± 35, CLI) events. There was an overall main effect [df (7, 39), F = 2.521, p < 0.05]. Additionally, we see a main effect of group [df (1, 32), F = 9.166, p < 0.01]; however, we see no main effect of state [df (3, 32), F = 1.466, p > 0.05]. Pairwise group comparisons showed that CLI-treated animals experienced significantly different per unit of time rates relative to SAL-treated animals in NREM sleep (Fig. 5d).
Behavioral testing of depression
Sexual behavior
The sexual behavior test replicated previous findings of a deficit in sexual function for CLI-treated animals [23]. CLI-treated animals (n = 7) were impaired in the number of mounts 0.2 ± 0.2 relative to SAL-treated animals (n = 8) which had 15 ± 7, as well as latency to mount (seconds) 1,699 ± 145 and 1,072 ± 176, respectively. There was a significant interaction of testing session by group where CLI-treated animals were severely impaired in mounting activity and the magnitude of the deficit increased across testing session relative to SAL-treated animals [df (1, 13), F = 5.36, p < 0.05]. Although we saw the same trend in latency to mount, the difference was not significant [df (1, 13), F = 4.141, p = 0.06].
Locomotor activity
There were no differences in total activity levels with 4,286 ± 433 movements for CLI-treated animals versus 4,149 ± 428 for SAL-treated animals (t = 0.38, p > 0.05). Similarly, 14 of the 15 measures showed no difference between groups; however, CLI-treated animals showed significantly more horizontal movements (t = 5.84, p = 0.03) with 73 ± 15 than SAL-treated animals, which showed 54 ± 16 movements; however, due to multiple measurements in the current test, it is not clear that one significant measure represents a robust finding and in agreement with previous publication [1], this effect is not always consistently found in the CLI model, nor is psychomotor agitation consistently reported as a reliable measure of anxiety [24] and therefore, this result is considered cautiously.
Discussion
Novel findings of this study were that rats that were neonatally exposed to CLI show increased amounts of NRT bursts that correlated with modest changes in power levels and epigenetic effects on epileptiform activity (the CLI-treated animals experienced significantly more events than SAL-treated) comorbid with behavior and physiology seen in depressive phenotype. This study replicates previous studies which have shown a depressive phenotype in animals neonatally treated with CLI; namely, enhanced amounts of REM sleep and depressive phenotypic behavior [1, 5]. Additionally, we saw several effects of neonatal drug treatment on spectral power levels in the EEG.
Power analyses
The most robust effect between groups found from spectral power analysis was the increase in theta activity in CLI-treated animals during NREM sleep. Theta rhythm is typically associated with intention to move or voluntary activity and has been seen during REM sleep [25]. Kesic and colleagues found significantly augmented sigma-theta coupling as a result of MAO lesions and suggest that decreased monoaminergic signaling disrupted NREM sleep consolidation and altered theta generation [20]. Our finding implies that CLI-treated animals are experiencing a less restful sleep during NREM sleep. Neonatal treatment with CLI may mimic some of the effects seen with MAO lesions.
Additionally, although the decreases in REM theta power for CLI animals were modest, the more interesting part of this finding is not only the switch in direction, but that it was exaggerated in CLI rats. This pattern of results implies that NREM theta power is influencing subsequent REM theta power to a greater degree in CLI animals because one would expect no effect or an effect in the same direction and of a similar magnitude if it were not.
NRT bursts
CLI-treated animals experienced more NRT bursts than SAL-treated animals, which resulted from longer REM sleep periods and more opportunity, because there was not a disproportionately higher frequency of NRT bursts during REM sleep. Even though the burst occurrence is not disproportionately higher in REM sleep, it may be that the higher average burst occurrence is directly related to enhancing the amount of time spent in REM sleep. One study suggested that NREM sleep can promote seizure activity and that REM sleep can suppress it and further that events in NREM sleep may propagate subsequent events in REM and facilitate seizure induction [26]. Given that epileptiform activity is known to respond in a dichotomous manner from NREM versus REM sleep, and we report an exaggerated switch in direction in our theta spectral power, our results suggest a correlation of theta spectral power, the additional bursting activity at the NRT point, and an increased propensity for REM sleep seen in CLI animals.
Epileptiform activity
Another major finding is that the frequency of CTS and P-CTS events was disproportionately higher for CLI-treated animals with the greatest proportion of events for both groups of animals having occurred during quiet wake. CLI-treated animals experienced a disproportionately higher frequency of events during REM sleep, which suggest an additional CLI-related abnormal phenotype. All animals experienced a disproportionately low frequency of events during AW, which suggest that movement or perhaps more likely, a cognitive state associated with movement (i.e., attention or arousal level), provided a “protective” mechanism against event occurrence. One study reported a direct link between immobility and the onset of absence seizures in Long-Evans hooded rats [15] and neonatal treatment with CLI [27]. In the current study, all animals had accompanying EMG activity 44% of the time for CTS events and 20% of the time for P-CTS events, which suggests that animals may experience enhanced amounts of epileptiform activity from maintaining a vulnerable cognitive state for a longer amount of time. Cognitive states which are more synchronous (i.e., NREM and QW) may be more vulnerable to fluctuation because the system is more labile [28]. The reticular neurons of the thalamus have been shown to be sensitive to modulation of the reticular activating system (RAS) and reductions are theorized to lead to instability in the thalamo-cortical spindle generating system that could result in hypersynchronous activity (see [29] for review).
How neonatal treatment induces adult epileptiform activity remains to be explored. Evidence indicated that seizure-like activity may be a part of depressive pathology or a side effect of antidepressant treatment. In humans, not only CLI, but other antidepressants also induce seizure in a small percentage of treated patients (for review, see [30]). Depressive symptoms have been reported in about 50% of patients with epilepsy [17, 30]. If CLI is selectively overdriving serotonergic and noradrenergic neurons during development, that could lead to system-wide alterations that are important for maintaining homeostasis. Previous studies have shown that CLI-treated rats are deficient in 5-HT neurons, including decreased brain levels of 5-HT [31] and decreased 5-HT neuronal firing rate [32], which implicates 5-HT in the regulation of the cognitive, behavioral, and physiological deficits found in this model. In adult rats, it has been reported that serotonergic and noradrenergic blockade reduce NRT frequency and that cholinergic blockade increases NRT frequency [33]. We have recently demonstrated that the CLI rats have elevated brain level of orexins [34], which are regulated by 5-HT as well [35]. These findings may implicate that the interaction between reduced 5-HT and elevated brain orexin plays a role in the pathology of the occurrence of epileptic activity. However, further study is needed.
Considering that the SAL-treated animals experienced many events as well, it is possible that stressor-induced effects from repeated handling, the injections or the surgery itself may have produced some damage that resulted in lower thresholds for the onset of epileptic activity; however, the neonatal period is stress hyporesponsive (SHRP), which is critically maintained by maternal interaction and less by other factors [36, 37]. Furthermore, handling itself benefits the development of brain and behavior as indicated by reducing brain levels of stress-related hormone, corticotrophin-releasing factor (CRF) mRNA levels [38]. It is also possible that the events in the SAL-treated rat are partly or wholly because Long-Evans are genetically susceptible [15, 39]. Nevertheless, it is clear that CLI-treated animals are experiencing significantly more events than controls. By whatever mechanism, the results shed light on the known comorbidity between depressive symptoms and reduced availability of serotonin in patients with epilepsy [40] and the high prevalence of depressive symptoms in all epilepsy patients [41].
Implications of the model
While neonatal exposure to CLI results in a reliable phenotype with features similar to those seen in depressed humans [1, 6, 42], the findings here document characteristics of epileptic activity. Given the significant increase of epileptic activity in the Long-Evans rat with neonatal CLI treatment, and that depression with melancholy is a comorbidity of epilepsy, the pathology in this model is relevant to both disorders. We speculate that neonates of depressed mothers who took antidepressants during postnatal nursing may also be at a higher risk for developing subtle neurocognitive deficits and epilepsy as well. Use of antidepressants during postnatal feeding is currently accepted as safe, but conclusions are limited to anatomic teratology and of incidence of pulmonary hypertension [43]. The current results suggest harmful effects induced by neonatal exposure to CLI could include epilepsy. Future epidemiologic studies should elaborate on hypothetical theta-driven epileptic activity in adults as a response to perinatal exposure to CLI.
References
Vogel G, Neill D, Hagler M, Kors D (1990) A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev 14:85–91
Dunleavy DL, Brezinova V, Oswald I, Maclean AW, Tinker M (1972) Changes during weeks in effects of tricyclic drugs on the human sleeping brain. Br J Psychiatry 120:663–672
Schimmell MS, Katz EZ, Shaag Y, Pastuszak A, Koren G (1991) Toxic neonatal effects following maternal clomipramine therapy. J Toxicol Clin Toxicol 29:479–484
Vijayakumar M, Meti BL (1999) Alterations in the levels of monoamines in discrete brain regions of clomipramine-induced animal model of endogenous depression. Neurochem Res 24:345–349
Mirmiran M, van de Poll NE, Corner MA, van Oyen HG, Bour HL (1981) Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res 204:129–146
Vogel WG (1999) REM sleep deprivation and behavioral changes. In: Mallick BN, Inoue S (eds) Rapid eye movement sleep. Narosa Publishing House, New Delhi, pp 355–366
Grunhaus L, Shipley JE, Eiser A, Pande AC, Tandon R, Remen A, Greden JF (1994) Shortened REM latency PostECT is associated with rapid recurrence of depressive symptomatology. Biol Psychiatry 36:214–222
Riemann D, Kammerer J, Low H, Schmidt MH (1995) Sleep in adolescents with primary major depression and schizophrenia: a pilot study. J Child Psychol Psychiatry 36:313–326
Berger M, van Calker D, Riemann D (2003) Sleep and manipulations of the sleep-wake rhythm in depression. Acta Psychiatr Scand Suppl 418:83–91
Douw L, van Dellen E, de Groot M, Heimans JJ, Klein M, Stam CJ, Reijneveld JC (2010) Epilepsy is related to theta band brain connectivity and network topology in brain tumor patients. BMC Neurosci 11:103
Buysse DJ, Hall M, Begley A, Cherry CR, Houck PR, Land S, Ombao H, Kupfer DJ, Frank E (2001) Sleep and treatment response in depression: new findings using power spectral analysis. Psychiatry Res 103:51–67
Dijk DJ, Strijkstra A, Daan S, Beersma DG, Van den Hoofdakker RH (1991) Effect of clomipramine on sleep and EEG power spectra in the diurnal rodent Eutamias sibiricus. Psychopharmacology (Berl) 103:375–379
Neill D, Vogel G, Hagler M, Kors D, Hennessey A (1990) Diminished sexual activity in a new animal model of endogenous depression. Neurosci Biobehav Rev 14:73–76
Siegel A, Grady CL, Mirsky AF (1982) Prediction of spike-wave bursts in absence epilepsy by EEG power-spectrum signals. Epilepsia 23:47–60
Shaw FZ (2004) Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J Neurophysiol 91:63–77
Kovacs Z, Czurko A, Kekesi KA, Juhasz G (2010) Clomipramine increases the incidence and duration of spike-wave discharges in freely moving WAG/Rij rats. Epilepsy Res 90:167–170
Grozinger M, Kogel P, Roschke J (2002) Effects of REM sleep awakenings and related wakening paradigms on the ultradian sleep cycle and the symptoms in depression. J Psychiatr Res 36:299–308
Feng P, Vurbic D, Wu Z, Strohl KP (2007) Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res 1154C:163–172
Fogel SM, Nader R, Cote KA, Smith CT (2007) Sleep spindles and learning potential. Behav Neurosci 121:1–10
Kesic S, Kalauzi A, Radulovacki M, Carley DW, Saponjic J (2010) Coupling changes in cortical and pontine sigma and theta frequency oscillations following monoaminergic lesions in rat. Sleep Breath (1):35-47
Benington JH, Kodali SK, Heller HC (1994) Scoring transitions to REM sleep in rats based on the EEG phenomena of pre-REM sleep: an improved analysis of sleep structure. Sleep 17:28–36
Hartley P, Neill D, Hagler M, Kors D, Vogel G (1990) Procedure- and age-dependent hyperactivity in a new animal model of endogenous depression. Neurosci Biobehav Rev 14:69–72
Feng P, Guan Z, Yang X, Fang J (2003) Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine. Brain Res 991:195–205
Day RK (1999) Psychomotor agitation: poorly defined and badly measured. J Affect Disord 55:89–98
Rowe K, Moreno R, Lau TR, Wallooppillai U, Nearing BD, Kocsis B, Quattrochi J, Hobson JA, Verrier RL (1999) Heart rate surges during REM sleep are associated with theta rhythm and PGO activity in cats. Am J Physiol 277:R843–849
Shouse MN, Farber PR, Staba RJ (2000) Physiological basis: how NREM sleep components can promote and REM sleep components can suppress seizure discharge propagation. Clin Neurophysiol 111(Suppl 2):S9–S18
Hansen HH, Sanchez C, Meier E (1997) Neonatal administration of the selective serotonin reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression? J Pharmacol Exp Ther 283:1333–1341
Beenhakker MP, Huguenard JR (2009) Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron 62:612–632
Sinha SR (2011) Basic mechanisms of sleep and epilepsy. J Clin Neurophysiol 28:103–110
Pisani F, Spina E, Oteri G (1999) Antidepressant drugs and seizure susceptibility: from in vitro data to clinical practice. Epilepsia 40(Suppl 10):S48–56
Feenstra MG, van Galen H, Te Riele PJ, Botterblom MH, Mirmiran M (1996) Decreased hypothalamic serotonin levels in adult rats treated neonatally with clomipramine. Pharmacol Biochem Behav 55:647–652
Kinney GG, Vogel GW, Feng P (1997) Decreased dorsal raphe nucleus neuronal activity in adult chloral hydrate anesthetized rats following neonatal clomipramine treatment: implications for endogenous depression. Brain Res 756:68–75
Benington JH, Heller HC (1995) Monoaminergic and cholinergic modulation of REM-sleep timing in rats. Brain Res 681:141–146
Feng P, Vurbic D, Wu Z, Hu Y, Strohl KP (2008) Changes in brain orexin levels in a rat model of depression induced by neonatal administration of clomipramine. J Psychopharmacol 22:784–791
Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T (2004) Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci 24:7159–7166
Levine S (2001) Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol Behav 73:255–260
Levine S (2002) Regulation of the hypothalamic-pituitary-adrenal axis in the neonatal rat: the role of maternal behavior. Neurotox Res 4:557–564
Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18:195–200
Xu B, McIntyre DC, Fahnestock M, Racine RJ (2004) Strain differences affect the induction of status epilepticus and seizure-induced morphological changes. Eur J Neurosci 20:403–418
Lothe A, Didelot A, Hammers A, Costes N, Saoud M, Gilliam F, Ryvlin P (2008) Comorbidity between temporal lobe epilepsy and depression: a [18F] MPPF PET study. Brain 131:2765–2782
Kanner AM (2009) Depression and epilepsy: a review of multiple facets of their close relation. Neurol Clin 27:865–880
Feng P (2004) Neonates. In: Kushida CA (ed) Sleep deprivation. Marcel Dekker, Inc., New York, pp 121–150
Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL (1996) Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 335:1010–1015
Acknowledgements
This study was supported by the VA Merit award, NIH training grant T32-5THL007913-10, and Louis Stokes Cleveland VA Medical Service. The authors would like to thank Dr. van Lunteren for lending his equipment to us to measure locomotor activity and lab members who gave valuable feedback or contributed to this project: Afaf Akladious, Jiejin Zhang, Phil Smith, and Yufen Hu.
Conflicts of interest
None of the authors has any conflict of interest to disclose.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McDowell, A.L., Strohl, K.P. & Feng, P. Sleep-related epilepsy in a Long-Evans hooded rat model of depression. Sleep Breath 16, 1181–1191 (2012). https://doi.org/10.1007/s11325-011-0630-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-011-0630-x