Abstract
Purpose
The relationship between obstructive sleep apnea (OSA) syndrome and metabolic syndrome is far from conclusion for obesity as a confounding factor. The aim of the present study was to investigate the association between OSA and some components of metabolic abnormality in nonobese patients.
Methods
We consecutively recruited nonobese subjects who underwent polysomnography and analyzed some components of metabolic abnormality in subjects with and without OSA. Multiple linear regression was used to evaluate the independent risk factor of some components of metabolic abnormality.
Results
A total of 154 subjects were enrolled and were divided to control group (45 subjects) and OSA group (113 subjects). Body mass index was no different between groups. Systolic blood pressure, triglycerides, and insulin concentration were significantly higher among OSA group compared with control group (p = 0.000, 0.043, and 0.006, respectively), and the prevalences of dyslipidemia, hypertension, and at least two of the metabolic abnormalities were significantly greater in OSA group (p = 0.003, 0.031, and 0.000, respectively). After adjusting for confounding factors, lowest O2 saturation was the major contributing factor for elevated systolic blood pressure (p = 0.001), and independent associations were found between apnea–hypopnea index and the following parameters of metabolic abnormality: triglycerides and homeostasis model assessment of insulin resistance (all p = 0.000).
Conclusions
Our finding was consistent with previous studies that OSA was independently associated with dyslipidemia, hypertension, and at least two of metabolic abnormalities in nonobese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) syndrome affects 2–4% of men and 1–2% of women in the general population [1]. It is a common disorder characterized by recurrent episodes of partial or complete obstruction of the upper airway during sleep, resulting in intermittent hypoxia. The symptoms of OSA include snoring and airflow cessation during sleep, excessive daytime sleepiness, morning headaches, fatigue, etc. OSA has been proven to be associated with many disease systems, including hypertension, stroke, cardiovascular disease (CVD), etc. [2–4]; however, much of the causal role and mechanisms are still unraveled.
The prevalence of metabolic syndrome (MS) is 22.8% of men and 22.6% of women in general population [5]). MS [6], known as insulin-resistant syndrome, is recognized as a constellation of central obesity, glucose intolerance, dyslipidemia, and hypertension, predisposing patients to the development of CVD and diabetes.
MS is caused by the accumulation of visceral fat and an increase in insulin resistance. OSA is associated with the accumulation of visceral fat, too, and OSA may be related to other metabolic abnormalities that comprise the diagnostic criteria of MS, such as impaired fasting glucose, hypertension, dyslipidemia. Even so, the evidence for the contribution of OSA to the development of MS is far from conclusion. To the author’s knowledge, many investigators tried to elucidate the relationship between OSA and MS, and the results in these studies were controversial. Several studies [7–9] have shown a direct relationship between OSA and MS, independently of obesity, while other studies have demonstrated that adiposity, as a confounding factor, contributed to MS among OSA patients [10, 11]. The subjects among many previous studies were adipose [10, 12], and few studies have focused on lean weight patients [8, 13].
The aim of our study was to find whether OSA was associated with some components of MS in nonobese patients independently of confounding factors.
Materials and methods
Subjects
We consecutively recruited nonobese subjects who presented to our sleep laboratory between January 1, 2008 and January 31, 2011, with a history of snoring duration from 5 to 20 years and witnessed episodes of apnea during sleep or excessive daytime sleepiness duration from 1 to 10 years. Nonobese was defined as body mass index (BMI) <25 kg/m2 and waist circumference according to the recommended criteria for Asians [14], at <90 cm in men and <80 cm in women. Participants were questioned about their sleep symptoms, medical history, current smoking, alcohol consumption, and medications by special physicians who were trained before this study began. According to their previous medical histories, which were from their written or self-reported medical record, physical examination, electrocardiography, and lung function test, subjects who had any acute or apparent chronic inflammatory disorders, chronic obstructive pulmonary disease, and coronary heart disease were excluded. Subjects with other sleep disorders, such as upper airway resistance syndrome, central sleep apnea, periodic limbs movements, or narcolepsy, were also excluded. Written informed consents were obtained from all subjects before the study, and our study had been approved by the local institutional review board.
Anthropometric and biochemical measurements
Body weight and height were measured in bare feet and light clothing in the morning. BMI was defined as weight (kilograms)/height2 (meters). Waist circumference was measured at a level midway between the lower costal margin and the iliac crest. Hip circumference was measured at the maximum circumference of buttocks. Waist circumference/hip circumference ratio was calculated. Blood pressure was measured on the right arm with the subjects in a sitting position and after a 5-min rest, using a mercury sphygmomanometer: The average of two measurements, 1 min apart, was considered. Fasting blood was taken in the morning for the measurement of serum glucose, insulin, and lipid profile comprising total cholesterol, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C) and triglycerides. All blood samples were analyzed using the H-7600 autoanalyzer (Hitachi). Fasting insulin sensitivity was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR) [15] in all patients. The value of HOMA-IR was calculated by the following equation: fasting insulin (micro-units per milliliter) × fasting glucose (millimoles per liter)/22.5.
Polysomnography
Polysomnography (P Series Sleep System; Compumedics; Melbourne, Australia) was performed overnight between 2200 and 0600 hours. Polysomnography consisted of continuous polygraphic recording from surface leads for electroencephalography, electrooculography, electromyography, thermistors for nasal and oral airflow, thoracic and abdominal impedance belts for respiratory effort, pulse oximetry for oxyhemoglobin level (SaO2), and tracheal microphone for snoring and sensor to assess changing of the position during sleep. Polysomnography records were staged manually according to the criteria of American Academy of Sleep Medicine published in 2007 [16]. Apnea was defined as the absence of airflow for at least 10 s and indicated by the thermistors and nasal cannula. Hypopnea was defined as a reduction in airflow by at least 30%, lasting for at least 10 s, and associated with a 4% or more decrease in oxyhemoglobin saturation or arousal, as indicated by the electroencephalogram. The apnea–hypopnea index (AHI) was calculated as the number of episodes of apnea and hypopnea per hour of sleep. The oxygen desaturation index (ODI) was defined as the total number of episodes of oxyhemoglobin desaturation ≥4% from the immediate baseline. The percentage of total sleep time spent with SaO2 <90% (TST) and lowest O2 saturation (LaSO2) were also recorded. OSA group was defined as AHI ≥5/h, while control group was defined as AHI <5/h.
Definition of metabolic abnormalities
The definition of metabolic abnormalities was based on the clinical diagnostic criteria of the Third Report of the National Cholesterol Education Program (NCEP) [17]. Parameters of metabolic abnormality were as following: (1) hypertension as defined by a recorded systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg according to the average of two consecutive measurements; (2) glucose intolerance as defined by a fasting blood glucose concentration ≥6.1 mmol/L; and (3) dyslipidemia as defined by a HDL-C in men <1.03 mmol/L or in women <1.29 mmol/L or a serum total triglycerides concentration ≥1.7 mmol/L. Patients who were already receiving therapy for hypertension, dyslipidemia, or diabetes were considered to have these diseases. Since our study had strict enrolment criterion, subjects who had BMI ≥25 [14] and waist circumference at ≥90 cm in men and ≥80 cm in women were excluded and MS as an entity could not be diagnosed when using NCEP criterion.
Data analysis
Statistical analysis was performed using the SPSS statistical software version 13.0 for Windows (SPSS, Inc, Chicago, IL, USA). All data were tested for normal distribution prior to analysis. Normally distributed data were expressed as mean ± SD and were compared by Student’s t test between the OSA and the control group, while skewed data (including AHI, ODI, TST, LaSO2, triglycerides, insulin, and HOMA-IR) were expressed as median (interquartile range) and were compared using Mann–Whitney U nonparametric test between groups. Categorical variables were expressed as number (percentage) and were compared by the chi-square test or Fisher’s exact test. All skewed data were log-transformed before correlation, and regression analysis was performed. Spearman’s rank correlation was used to evaluate the interrelation between clinical, polysomnographic, and metabolic parameters, and multiple linear regression analysis was performed to determine the independent predictors of some parameters of metabolic abnormality. A p < 0.05 was considered to be statistically significant.
Results
A total of 154 subjects were included in our study. One hundred thirteen subjects whose AHI ≥5/h were as OSA group, and the remainders of 45 participants were grouped as control group. Subjects with and without OSA did not significantly differ in sex, age, current smoking, alcohol consumption, medications, BMI, and waist circumference (all p > 0.05). AHI, ODI, TST, and LaSO2 were markedly greater in OSA group (all p = 0.000) (Table 1).
Systolic blood pressure (SBP), triglycerides were significantly higher in subjects with OSA (p = 0.000 and 0.043, respectively). Insulin concentration and HOMA-IR were higher in subjects with OSA (p = 0.006 and 0.002, respectively), but fasting glucose was similar to controls (p = 0.084). There were no significant difference in diastolic blood pressure, total cholesterol, HDL-C, and LDL-C between two groups (Table 1).
The percentages of dyslipidemia and hypertension in OSA group were significantly higher compared with control group (60.2% vs. 33.3%, p = 0.003; 46.0% vs. 26.7%, p = 0.031, respectively). There were 38.9% of patients in OSA group, compared with only 8.9% in control group, who had at least two of the metabolic abnormalities (p = 0.000) (Table 1).
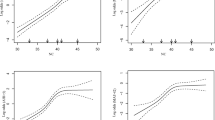
Table 2 shows that the polysomnographic parameters, including log AHI, log ODI, log TST, and log LaSO2, were all significantly correlated with SBP, log triglycerides and log HOMA-IR (all p < 0.01). Multiple linear regression showed that when using SBP as a dependent variable, log LaSO2 was the major contributing factor for elevated SBP (β = −0.316, adjusted r 2 = 0.341, p = 0.001; Fig. 1), When using log triglycerides as dependent variable, log AHI was the independent predictor of log triglycerides (β = 0.323, adjusted r 2 = 0.098, p = 0.000; Fig. 2) and when using log HOMA-IR as dependent variable, log AHI was independently associated with log HOMA-IR (β = 0.317, adjusted r 2 = 0.130, p = 0.000; Fig. 3).
Correlation between log LaSO2 and SBP (r = −0.484, p = 0.000). We entered gender, age, current smoking, alcohol consumption, antihypertensive agents, BMI, log AHI, log ODI, log TST, log LaSO2, total cholesterol, log triglycerides, HDL-C, LDL-C, fasting glucose, and log insulin as independent variables. Stepwise linear regression showed that age, log triglycerides, log TST, and log LaSO2 were included in the final model. Log laSO2 was the independent predictor of SBP (β = −0.316, adjusted r 2 = 0.341, p = 0.001). LaSO 2 Lowest O2 saturation, SBP systolic blood pressure, BMI body mass index, AHI apnea–hypopnea index, ODI oxygen desaturation index, TST the percentage of total sleep time spent with SaO2 <90%, HDL-C high density lipoprotein-cholesterol, LDL-C low density lipoprotein-cholesterol
Correlation between log AHI and Log TG (r = 0.360, p = 0.000). We entered gender, age, current smoking, alcohol consumption, antilipemic agents, BMI, log AHI, log ODI, log TST, log LaSO2, SBP, DBP, total cholesterol, HDL-C, LDL-C, fasting glucose, and log insulin as independent variables. Stepwise linear regression showed that only log AHI was included in the final model, and it was the independent predictor of log TG (β = 0.323, adjusted r 2 = 0.098, p = 0.000). TG Triglycerides, AHI apnea–hypopnea index, BMI body mass index, ODI oxygen desaturation index, TST the percentage of total sleep time spent with SaO2 <90%, LaSO 2 lowest O2 saturation, SBP systolic blood pressure, DBP diastolic blood pressure, HDL-C high density lipoprotein-cholesterol, LDL-C low density lipoprotein-cholesterol
Correlation between log AHI and Log HOMA-IR (r = 0.378, p = 0.000). We entered gender, age, current smoking, alcohol consumption, antidiabetic agents, BMI, log AHI, log ODI, log TST, log LaSO2, SBP, DBP, total cholesterol, log triglycerides, HDL-C, and LDL-C as independent variables. Stepwise linear regression showed that log AHI and gender were included in the final model. Log AHI was independently associated with log HOMA-IR (β = 0.317, adjusted r 2 = 0.130, p = 0.000). HOMA-IR Homeostasis model assessment-insulin resistance, AHI apnea–hypopnea index, BMI body mass index, ODI oxygen desaturation index, TST the percentage of total sleep time spent with SaO2 <90%, LaSO 2 lowest O2 saturation, SBP systolic blood pressure, DBP diastolic blood pressure, HDL-C high density lipoprotein-cholesterol, LDL-C low density lipoprotein-cholesterol
Discussion
Our study involved both sexes of nonobese subjects with and without OSA matched for age and BMI. The major findings of the present study were that the occurrences of hypertension, dyslipidemia, and at least two of the metabolic abnormalities were significantly higher in patients with OSA. Multiple linear regression elucidated that the polysomnographic parameter, AHI, was an independent predictor of increased triglycerides and insulin resistance. Meanwhile, LaSO2, also known as an important polysomnographic parameter, was negatively correlated to SBP.
OSA may promote the onset of MS through several mechanisms: intermittent hypoxemia [18], enhanced sympathetic activity [19], fragmentation of sleep, dysregulation of the hypothalamic–pituitary–adrenal axis [20], impaired vascular endothelial function [21], and abnormal production of inflammatory cytokines and adipokines. Many studies [7, 9, 22] demonstrated that the prevalence of MS among OSA patients was 56–87%. A study based on community in Hong Kong of China identified that subjects with OSA had five times increased risk to develop MS [9]. A Japanese study [13] found that at least two components of MS were identified in 39.6% of the lean male patients with OSA. Another study [8] elucidated 19% patients in the nonobese male OSA compared with only 4% in the non-OSA having least two of the following: hypertension, hyperglycemia, and dyslipidemia. The two studies have suggested that OSA itself may predispose to the development of various types of metabolic dysfunction and thus MS, without the presence of excess visceral adiposity. The present study was consistent with above mentioned studies, and the overall prevalence of at least two of metabolic abnormalities in nonobese OSA patients was 38.9%. However, an India report demonstrated that it was obesity rather than OSA that contributed to lipid abnormalities, insulin resistance, serum leptin, and adiponectin level [10]. It is obvious that prospective studies with longer follow-up and rigorous characterization of subjects would be needed to address the direction of causality between OSA and MS.
Our study confirmed the association of OSA with hypertension. The prevalence of hypertension in OSA subjects was 46.0%, higher than control group. After adjustment for confounding factors, the polysomnographic parameter, LaSO2, was an independent predictor of SBP. Our study was consistent with many epidemiologic and clinical studies. The Sleep Heart Health Study elucidated that the mean systolic and diastolic blood pressure, as well as the prevalence of hypertension, increased obviously with an increase in AHI [23]. In a study that covered 709 subjects after a 4-year follow-up period, it was found that OSA patient with AHI ≥15/h would have 2.03 times to predispose to hypertension [24]. Kono et al. [8] studied nonobese Japanese men, and they reported that the prevalence of hypertension was 45.2% in patients with OSA. Both previous data and our study revealed that nonobese OSA patients might be prone to develop hypertension. Several potential mechanisms may explain the association of OSA with hypertension: recurrent hypoxia/reoxygenation, arousal, sympathetic overactivation, etc. [25]. Both positive [3, 26] and negative [27] results were reported about the effect of continuous positive airway pressure (CPAP) on hypertension among OSA patients. Overall, these findings suggested that CPAP treatment of OSA could lower blood pressure, but its effect was slight.
Dyslipidemia among OSA patients has been reported in many studies [7, 9, 28, 29]. However, adiposity, as a confounding factor, played an important role in the relationship between OSA and dyslipidemia. The prevalence of dyslipidemia among OSA patients was higher in our study matched for age, BMI, and waist circumference, and an positive correlation between AHI and triglycerides were observed. It was consistent with some previous investigations [7, 9]. In the Sleep Heart Health Study of over 6,000 men and women, there was a negative relationship between AHI and HDL-C and a positive correlation between AHI and triglycerides [29]. Some studies [7, 28] have pointed out that the severity of OSA was independently associated with low HDL. A Japanese study focused on lean men found that HDL-C and triglycerides did not differ between the OSA and the control group, but the incidence of dyslipidemia in OSA group was markedly greater [8]. Animal experiment research has supported that chronic intermittent hypoxia was the key factor in the pathogenesis of dyslipidemia in OSA [30]. There are limited interventional data on OSA and dyslipidemia showing controversial results. Borgel et al. [28] analyzed the effect of CPAP treatment on serum lipid profiles among 470 OSA patients, and they found that after 6 months CPAP treatment, the HDL-C level increased significantly. However, a randomized study [2] demonstrated that no changes in lipid profiles after a 6-week CPAP treatment in patients with OSA. Corroborative evidence is needed to delineate the causal relation between OSA and lipid profiles and the effect of CPAP on dyslipidemia in OSA patients.
Insulin resistance is a key factor in the diagnosis of MS [6]. There is accumulating evidence to support that OSA is associated with insulin resistance, independent of confounding factors [9, 31–34]. Some studies [8, 32] focused on nonobese subject with OSA, and they identified an association between sleep-disordered breathing/OSA and insulin resistance. Epidemiologic data suggested that subjects with mild or moderate to severe OSA have increased risks for fasting glucose intolence after adjustment for confounding factors [33]. The Wisconsin Cohort Study [34] found that 15% of individuals with and AHI of 15 or greater had twofold relative risk to be prone to diabetes after adjustment for age, sex, and BMI. Punjabi and colleagues [12] showed that a worsening of insulin resistance with increasing AHI, again independent of obesity. However, Sharma et al. [10] showed that it was adiposity playing an important role in the development of insulin resistance in the OSA population. Interventional data on OSA and insulin resistance showed conflicting results. One study [35] showed that CPAP treatment could make a reduction of insulin resistance in nonobese OSA patients. In our study, insulin level was significantly higher in subjects with OSA, and AHI was independently correlated with HOMA-IR. It may induce that nocturnal hypoxic episodes can deteriorate insulin resistance. The following animal studies supported this theory: Polotsky and co-workers [36] studied obese mice, and they delineated that leptin-deficient obese mice exposed to intermittent hypoxia for 12 weeks developed to an increase in fasting insulin concentration, impairing glucose tolerance and deterioration of insulin resistance. Another investigation found that after exposing lean mice to intermittent hypoxia, the whole-body insulin sensitivity decreased independent of autonomic nervous system [18]. The potential additive and interactive effects of intermittent hypoxia and sleep fragmentation on glucose metabolism still remain to be fully elucidated. Further well-designed longitudinal and interventional studies are clearly needed to clarify the direction of causal relationship between OSA and insulin resistance.
There were several limitations in the present study. First, since our study was a cross-sectional study, the causal relationship could not be drawn directly between OSA and parameters of metabolic abnormality. Second, our strict selection criteria allowed us to recruit only a small number of patients, and could not separately analyze by sex since female subjects were few in two groups (7 vs. 22).
In conclusion, our study demonstrated that the prevalence of hypertension, dyslipidemia, and insulin resistance, as components of MS, were higher among nonobese adults with OSA. OSA is an independent risk factor to predispose some components of metabolic syndrome independently of obesity. Prompt recognition and effective treatment of MS in patient with OSA may reduce latent cardiovascular morbidity and mortality.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM (2007) Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J 29:720–727
Faccenda JF, Mackay TW, Boon NA, Douglas NJ (2001) Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea–hypopnea syndrome. Am J Respir Crit Care Med 163:344–348
Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM (2010) Obstructive sleep apnea–hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 182:269–277
Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB (2003) The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 163:427–436
Eckel RH, Alberti KG, Grundy SM, Zimmet PZ (2010) The metabolic syndrome. Lancet 375:181–183
Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP (2004) Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J 25:735–741
Kono M, Tatsumi K, Saibara T, Nakamura A, Tanabe N, Takiguchi Y, Kuriyama T (2007) Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest 131:1387–1392
Lam JC, Lam B, Lam CL, Fong D, Wang JK, Tse HF, Lam KS, Ip MS (2006) Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med 100:980–987
Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P (2007) Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med 8:12–17
Barcelo A, Barbe F, Llompart E, Mayoralas LR, Ladaria A, Bosch M, Agusti AG (2004) Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med 117:118–121
Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL (2002) Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165:677–682
Akahoshi T, Uematsu A, Akashiba T, Nagaoka K, Kiyofuji K, Kawahara S, Hattori T, Kaneita Y, Yoshizawa T, Takahashi N, Uchiyama M, Hashimoto S (2010) Obstructive sleep apnoea is associated with risk factors comprising the metabolic syndrome. Respirology 15:1122–1126
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Schulz H (2007) Phasic or transient? Comment on the terminology of the AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 3:752
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497
Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, Polotsky VY, O’Donnell CP (2007) Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175:851–857
Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE (2001) Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest 120:887–893
Carneiro G, Togeiro SM, Hayashi LF, Ribeiro-Filho FF, Ribeiro AB, Tufik S, Zanella MT (2008) Effect of continuous positive airway pressure therapy on hypothalamic–pituitary–adrenal axis function and 24-h blood pressure profile in obese men with obstructive sleep apnea syndrome. Am J Physiol Endocrinol Metab 295:E380–E384
Shiina K, Tomiyama H, Takata Y, Usui Y, Asano K, Hirayama Y, Nakamura T, Yamashina A (2006) Concurrent presence of metabolic syndrome in obstructive sleep apnea syndrome exacerbates the cardiovascular risk: a sleep clinic cohort study. Hypertens Res 29:433–441
Parish JM, Adam T, Facchiano L (2007) Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med 3:467–472
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283:1829–1836
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384
McNicholas WT, Bonsigore MR (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29:156–178
Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, Davies RJ (2002) Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 359:204–210
Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, Merino-Sanchez M, Gonzalez-Benitez MA, Beltran-Robles M, Almeida-Gonzalez C (2006) Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 129:1459–1467
Borgel J, Sanner BM, Bittlinsky A, Keskin F, Bartels NK, Buechner N, Huesing A, Rump LC, Mugge A (2006) Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J 27:121–127
Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, Quan SF (2001) Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol 154:50–59
Li J, Savransky V, Nanayakkara A, Smith PL, O’Donnell CP, Polotsky VY (2007) Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol 102:557–563
Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, Budhiraja R, Singer M, Redline S (2008) Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care 31:1001–1006
Shin C, Kim J, Lee S, Shim J, In K, Kang K, Yoo S, Cho N, Kimm K, Joo S (2005) Association of habitual snoring with glucose and insulin metabolism in nonobese Korean adult men. Am J Respir Crit Care Med 171:287–291
Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE (2004) Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 160:521–530
Reichmuth KJ, Austin D, Skatrud JB, Young T (2005) Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 172:1590–1595
Harsch IA, Schahin SP, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, Ficker JH (2004) Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 169:156–162
Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O’Donnell CP (2003) Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552:253–264
Acknowledgments
This word was supported by grant C07100008 for natural science foundation from Fujian province of China. The authors acknowledge the help by Bin Yang for blood analysis. Juan Wu and Xiao-Lan Lai are thanked for their help in statistics.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Zhang and X.-B. Zhang contributed equally to this work.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11325-011-0563-4.
Rights and permissions
About this article
Cite this article
Lin, QC., Zhang, XB., Chen, GP. et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep Breath 16, 571–578 (2012). https://doi.org/10.1007/s11325-011-0544-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-011-0544-7