Abstract
Background
Cardiovascular diseases are frequent in patients with obstructive sleep apnea (OSAS). There is evidence that the day–night pattern of myocardial infarction and sudden cardiac death observed in the general population is altered in patients with OSAS. This study investigates potential abnormalities in the circadian profiles of platelet activity in OSAS.
Methods
We studied 37 patients with OSAS [7 of whom were also studied after 3 months on continuous positive airway pressure (CPAP) treatment] and 11 controls. In each subject, we obtained six different blood samples during 24-h period (2200, 0200, 0600, 1000, 1400, and 1800 hours). Platelet activity was determined by flow cytometry immediately after sampling.
Results
We found that nocturnal platelet activity was significantly increased in patients with OSAS (p = 0.043) and that effective treatment with CPAP decreased platelet activity in these patients but differences just failed to reach statistical significance (p = 0.063).
Conclusions
OSAS is associated with increased platelet activity during the night, and that this appears to be improved by chronic use of CPAP. These results may contribute to explain the high prevalence of cardiovascular events during sleep in OSAS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The obstructive sleep apnea syndrome (OSAS) is a frequent disease characterized by the occurrence of numerous episodes of absence of respiratory flow (apnea) during sleep. Each episode of apnea is followed by a marked decrease in arterial oxygen saturation that is rapidly normalized when ventilation resumes [1]. Treatment with continuous positive airway pressure (CPAP) prevents the occurrence of apneas and the hypoxia-reoxygenation episodes that follow them [2].
There is evidence that patients with OSAS have an increased risk for cardiovascular diseases including premature death from vascular events [3–6]. The mechanisms underlying this association are unclear but candidate mechanisms include increased activity of the sympathetic nervous system, endothelial dysfunction, hypercoagulability, oxidative stress, systemic inflammation, and metabolic dysregulation [7–14]. It is well-documented that, in the general population cardiovascular (CV) events occur preferentially in the first few hours after awakening [15–17]. This has been explained on the basis of the circadian variations of heart rate, blood pressure, platelet aggregation, and fibrinolytic activity [18–23]. In contrast, patients with OSAS show a marked variation of the day–night pattern of myocardial infarction and sudden cardiac death observed in the general population [24, 25] such that CV events occur preferentially in the middle of the night, while patients are sleeping. Whether the coagulation-fibrinolytic profiles are also altered in patients with OSAS has not been explored before. We hypothesized that the repetition of apneas and oxygen desaturation events that characterize OSAS modify the normal coagulation-fibrinolytic circadian rhythm, rendering patients with OSAS more susceptible to suffer a CV event during sleep. To test this hypothesis, we compared the 24-h circadian profiles of platelet activity in patients with OSAS and control subjects without OSAS. A few patients could also be studied 3 months after being effectively treated with CPAP to investigate the effects of the normalization of respiration during sleep on the circadian profile of platelet activity.
Methods
Subjects and ethics
We studied 37 male patients with OSAS and 11 healthy controls. Participants were recruited and studied at sleep unit of our institution. They had all referred to the sleep laboratory for snoring or suspected OSAS. Each participant was interviewed and was informed in detail of the purpose of this study. The diagnosis of OSAS was established by full polysomnography (E-Series Compumedics, Abbotsford, Australia) and included recording of oronasal flow, thoracoabdominal movements, electrocardiography, submental and pretibial electromyography, electrooculography, electroencephalography, and transcutaneous measurement of arterial oxygen saturation. Apnea was defined by the absence of airflow for more than 10 s. Hypopnea was defined as any airflow reduction that last more than 10 s and resulted in arousal or oxygen desaturation. We considered desaturation as a decrease in SaO2 greater than 4%. The apnea-hypopnea index (AHI) was defined as the sum of the number of apneas plus hypopneas per hour of sleep. Patients and controls were selected based on the diagnosis of OSAS and their hematological profile. The case or control status was defined by the AHI threshold of ten or greater. The number of circulating erythrocytes, leucocytes, and platelets were similar between groups. Participants were considered obese when their body mass index (BMI) was higher than 30 Kg m2. Arterial hypertension was diagnosed if systolic blood pressure was ≥140 mmHg and/or diastolic pressure was ≥90 mmHg or the individual was on specific treatment.
Seven patients were studied twice: at diagnosis and after effective treatment with CPAP (REM Star; Respironics®, Murrysville, PA, USA) during 3 months. Compliance with treatment was checked by the timer built up in the CPAP device, and it was higher than 4 h/night in all patients. Fourteen patients who did not use the device for a minimum of 4 h/night were excluded from the follow-up analysis. On the other hand, 16 patients refused to be reevaluated.
No participant suffered from any chronic disease (chronic obstructive pulmonary disease, liver cirrhosis, thyroid dysfunction, rheumatoid arthritis, chronic renal failure, and/or psychiatric disorders), or was taking any type of medication. The study was approved by the ethics committee of our institution, and all participants signed their consent after being fully informed of its goal and characteristics.
Protocol
Participants arrived at the sleep unit of our institution at 9 pm, after fasting for at least 6 h. A heparinized venous catheter (Introcan Safety ®, Braun, Melsungen, Germany) was inserted into an antecubital vein to allow serial blood sampling through the night without disturbing sleep. From this catheter, six different samples (20 ml each) were obtained during the next 24 h (2200, 0200, 0600, 1000, 1400, and 1800 hours). Blood was collected into tubes containing EDTA (10 ml) and into tubes containing sodium citrate (10 ml). The sample obtained at 1000 hours was followed by an additional one (10 ml) collected into tubes without anticoagulant for general biochemical assessment. Blood samples were immediately processed to determine platelet activity (citrate anticoagulated blood) and blood cell count (EDTA tubes) or centrifuged during 15 min at 2,500 revolutions per min (Jouan S.A, model CR4 22, Saint- Herblain, France). Serum and plasma were frozen at −80°C until analysis.
During the study, participants remained in the hospital. During the daytime, they were allowed to rest or to just perform low-activity tasks and they ate a standardized three meal diet.
Platelet flow cytometry
Blood platelets are easily activated in vitro [26]. To avoid this effect, measurements of platelet activity were performed within 30 min of sampling, without stirring, at room temperature using a flow cytometer (Epics XL-MCL Flow Cytometer, Beckman-Coulter, FL, USA) that measured the fraction of positive platelets and the arbitrary fluorescence intensity units (as an index of surface antigen expression in the total platelet population) according to standard protocols [26]. Platelet activity was determined with respect to α-granule degranulation (surface expression of CD62p antigen or P-selectin). A fluorescein isothiocyanate-conjugated antibody to GPIIIa (CD61; Beckman Coulter, Marseille, France) was used as an activation-independent marker of platelets. A phycoerythrin (PE)-conjugated anti-CD62 antibody (CD62; Beckman Coulter, Marseille, France) was used to assess α-granule degranulation. Platelets were identified on the basis of size and association with CD61 antibody. The control ligand (IgG-PE conjugate, Beckman Coulter, Marseille, France) was used to detect any nonspecific associations. The percentage of platelets expressing P-selectin was defined as the fraction exhibiting specific binding minus that exhibiting nonspecific binding of the 20,000 platelets sorted. The lowest percentage of activated platelets that could be detected was 0.1% [26, 27].
Hematological and biochemical analysis
Blood cell count was done on fresh samples using automatic electronic cell counter (XE 2100, Sysmex Corp, Japan). Measurements of glucose, cholesterol, triglycerides, uric acid, creatinine, and liver enzymes [alanine transaminase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transpeptidase (GGT)] were performed using a standard automated enzymatic methods on a Hitachi 917 biochemical analyzer (Roche Diagnostics, Indianapolis, USA). HDL cholesterol was measured by a homogeneous, enzymatic colorimetric method using a commercial reagent set (Roche Diagnostics). LDL cholesterol was calculated using the Friedewald equation [28].
Statistical analysis
Results are presented as median or mean ± standard error of the mean. Mann–Whitney U test was performed to assess the statistical significance of differences between OSAS patients and controls during the night. The method of summary measures was also applied following the comparison of the areas under the curves (AUC) of platelet activity vs time between patients and controls.
The study was powered not to miss a difference of 0.15% in platelet activity, (assuming a within subject SD of 0.10%) at a significance level of 5% and with a power of 90%, which required ten subjects in each group.
The effects of CPAP therapy were analyzed using Wilcoxon signed-rank test.
Correlations between variables were explored using the Spearman rank test. All statistical analyses were performed using SPSS version 15.0.
Statistical significance was defined as p < 0.05.
Results
Table 1 shows the main clinical characteristics and biochemical parameters investigated in both groups. By definition, patients with OSAS showed abnormal sleep parameters, whereas these variables were normal in controls. According to the AHI, the population studied suffered from severe OSAS (Table 1).
BMI, systolic and diastolic pressure, glucose, triglycerides cholesterol, creatinine, AST, ALT, and GGT levels were similar in patients and controls, although the latter were slightly younger (Table 1). Yet, age differences were minor in absolute terms and probably are of marginal biological significance. Patients with OSAS showed higher waist circumference than controls but the difference was not significant (p = 0.07). The number of circulating erythrocytes, leucocytes, and platelets was similar between groups (Table 1).
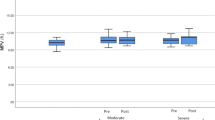
On average, global mean platelet activity was higher in patients with OSAS than controls (0.85 ± 0.1% vs 0.41 ± 0.03%, p = 0.037). Figures 1 and 2 show the median values of platelet activity at different intervals. A high variability in measures of platelet activity was detected between patients with OSAS (Fig. 1). As shown in Fig. 2, platelet activity at three different intervals during the study tended to be always higher in patients with OSAS than in controls but differences reached statistical significance during the night-time portion of the study (1.05 ± 0.19% vs 0.37 ± 0.05%, p = 0.043). Moreover, we detected a significant difference between the evening AUCs of OSAS patients and controls (0.82%, p = 0.042). In terms of frequency distribution, platelet activity was increased in 70% of patients as compared to 20% of controls. Yet, a different circadian pattern was observed in each group. Whereas healthy controls did not show evidence of circadian variation of platelet activity, a significant peak was observed in patients with OSAS during the night which progressively returned to normality during the day. In seven patients, we repeated the study after being under effective treatment with CPAP during 3 months. We found that this decreased platelet activity respect to that measured before therapy, but differences just failed to reach the statistical level of significance (night-time mean: 0.97 ± 0.2% vs 0.65 ± 0.1%, p = 0.063). Not significant differences in this time period were shown regarding BMI, systolic and diastolic pressure, glucose, triglycerides cholesterol, creatinine, AST, ALT, and GGT levels. We did not find any significant correlation between platelet activity at different time points and the age, AHI, mean or minimal arterial oxygen saturation at night or the arousal index.
Discussion
This study shows that, compared with healthy subjects, patients with OSAS present a profound alteration in the circadian rhythm of platelet activity, which reaches a peak in the late night hours. Furthermore, chronic treatment with CPAP reduced the platelet activity during the night. We speculate that this may contribute to the risk of acute CV events reported in patients with OSAS during sleep.
Many aspects of human physiology show circadian oscillations, including heart rate and blood pressure regulation. Other physiological factors affecting the vascular system, such as platelet activation and fibrinolysis are also regulated by the circadian clock, either directly or indirectly [22, 23, 29–34]. It is not surprising, therefore, that disrupted circadian rhythms had been implicated in the genesis of cardiovascular disease [35–38]. For instance, platelet activation has been related to onset of acute CV events. In the general population, acute CV events occur frequently in the early morning hours [15, 21], when there is a marked rise in neural and hormonal sympathetic activity [39, 40], increased platelet activity, and hypercoagulability [19, 20]. This suggests an interaction between arousal and acute thrombosis [19, 36]. By contrast, in patients with OSAS, the timing of myocardial infarction and sudden death shifts from the morning hours to the night, while the patient is actually sleeping [24, 25]. Experimental and clinical studies suggest that intermittent hypoxemia and increased sympathetic activation may be important mechanisms for a procoagulant state in OSAS [14, 41, 42]. Platelet activation and aggregation may be another contributing factor, as shown by previous studies that demonstrated that both are increased in patients with OSAS [43–45], particularly at night [46]. Our findings confirm these previous observations and extend them by showing that the 24-h circadian rhythm of platelet activation is disrupted in OSAS (Fig. 1), thus supporting the hypothesis that OSAS may lead to an elevated risk of nocturnal cardiovascular events by increasing nocturnal platelet activity. A different circadian pattern was observed in each group. Whereas healthy controls did not show evidence of circadian variation of platelet activity, a significant peak was observed in patients with OSAS during the night which progressively returned to normality during the day.
Differences occurred in the detection of peak platelet activation in general population between studies [19, 22, 47]. A consistent finding in all studies was that platelet is lowest when subjects were resting. In our controls, the platelet activity was minimal during the sleep hours but the differences were not significant between hours.
The precise mechanisms by which this occurs are unclear, since we did not find any significant relationship between platelet activity at different time points and the AHI, mean or minimal arterial oxygen saturation at night or the arousal index. The relatively small sample size of our study and/or the narrow range of disease severity of the patients studied here (all of whom had severe OSAS) may have limited our ability to find such relationships since it has been suggested that the relationship between sleep variables and procoagulant changes lies along a continuum of OSAS severity. On the other hand, despite the high variability detected in the measures of platelet activity between patients with OSAS, the increase during the night was detected in 70% of patients included in this study. We can exclude, however, several potential confounding factors that may influence platelet activity, such as obesity, diabetes, hypertension, or hyperlipidemia, because their distribution was similar in patients with OSAS and controls (Table 1). In our study, controls were slightly younger than OSAS but no correlation was evident for age and platelet activity at different time points. Furthermore, in keeping with these observations, Minoguchi et al. did not detect a correlation between age and platelet activity in patients with OSAS [45]. Finally, it was observed that chronic treatment with CPAP reduces the platelet activity during the night, suggesting that CPAP treatment may confer protection for cardiovascular events. A limitation of this observation is that the patients were selected because of excellent CPAP adherence and the trend toward decrease in platelet activity could be influenced to another additional factor such as healthier lifestyle or other therapies.
Although the exact mechanisms of platelet activation in OSAS are not understood, one factor that is important in these patients is the augmented sympathetic activity as a result of hypoxemia and repetitive arousals from sleep.
CPAP therapy has beneficial effects in abolishing both hypoxia and arousals related to obstructive respiratory events and sympathetic activation [48]. In any case, further studies are needed to unravel the mechanisms underlying the disruption of the circadian rhythm of platelet identified here. Likewise, it is possible that patients with OSAS might benefit from platelet anti-aggregant therapy. Future interventional studies are necessary to determine the impact of all these findings on the cardiovascular risk of OSAS patients.
In summary, our study has identified a disturbed circadian variation of platelet activity in patients with OSAS, with a clear nocturnal peak. We propose that this may contribute to the increased risk of acute CV events during the sleeping hours detected in patients with OSAS.
References
Stradling JR, Davies RJ (2004) Sleep 1: obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax 59:73–78
Sullivan CE, Berthon-Jones M, Issa FG, Eves L (1981) Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1:862–865
Pack AI, Gislason T (2009) Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis 51:434–451
Marin JM, Carrizo SJ, Vicente E, Agusti AGN (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnea. Effects of treatment with CPAP. Lancet 365 :1046–1053
Bradley TD, Floras JS (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373:82–93
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T (2008) Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118:1080–1111
Gozal D, Kheirandish-Gozal L (2008) Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 177:369–375
Somers VK, Dyken ME, Clary MP, Abboud FM (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96:1904
Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K (1995) Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J 8:222–229
Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK (1999) Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 100:2332–2335
Barcelo A, Miralles C, Barbe F, Vila M, Pons S, Agusti AGN (2000) Abnormal lipid peroxidation in patients with sleep apnea. Eur Respir J 16:644–647
Lavie L (2003) Obstructive sleep apnoea syndrome: an oxidative stress disorder. Sleep Med Rev 7:35–51
Ryan S, Taylor CT, McNicholas WT (2006) Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 174:824–830
von Känel R, Dimsdale JE (2003) Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest 124:1956–1967
Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T (1985) Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 313:1315–1322
Goldberg RJ, Brady P, Muller JE, Chen ZY, de Groot M, Zonneveld P, Dalen JE (1990) Time of onset of symptoms of acute myocardial infarction. Am J Cardiol 66:140–144
Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA (1997) Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol 79:1512–1516
Huikuri HV, Niemela MJ, Ojala S, Rantala A, Ikaheimo MJ, Airaksinen KE (1994) Circadian rhythms of frequency domain measures of heart rate variability in healthy subjects and patients with coronary artery disease. Effects of arousal and upright posture. Circulation 90:121–126
Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE (1987) Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med 316:1514–1518
Andreotti F, Davies GJ, Hackett DR, Khan MI, de Bart AC, Aber VR, Maseri A, Kluft C (1988) Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol 62:635–637
Muller JE (1999) Circadian variation in cardiovascular events. Am J Hypertens 12:35S–42S
Jovicic A, Mandic S (1991) Circadian variations of platelet aggregability and fibrinolytic activity in healthy subjects. Thromb Res 62:65–74
Jafri SM, VanRollins M, Ozawa T, Mammen EF, Goldberg AD, Goldstein S (1992) Circadian variation in platelet function in healthy volunteers. Am J Cardiol 69:951–954
Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, Lopez-Jimenez F, Somers VK (2008) Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol 52:343–346
Gami AS, Howard DE, Olson EJ, Somers VK (2005) Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 352:1206–1214
Schmitz G, Rothe G, Ruf A, Barlage S, Tschope D, Clemetson KJ, Goodall AH, Michelson AD, Nurden AT, Shankey TV (1998) European working group on clinical cell analysis: consensus protocol for the flow cytometric characterisation of platelet function. Thromb Haemost 79:885–896
Harrison P (2000) Progress in the assessment of platelet function. Br J Haematol 111:733–744
Burtis C, Ashwood E, Bruns D (2005) Tietz textbook of clinical chemistry and molecular diagnostics, 4th edn. Saunders Ed
Bode-Boger SM, Boger RH, Kielstein JT, Loffler M, Schaffer J, Frolich JC (2000) Role of endogenous nitric oxide in circadian blood pressure regulation in healthy humans and in patients with hypertension or atherosclerosis. J Investig Med 48:125–132
Pinotti M, Bertolucci C, Portaluppi F, Colognesi I, Frigato E, Foa A, Bernardi F (2005) Daily and circadian rhythms of tissue factor pathway inhibitor and factor VII activity. Arterioscler Thromb Vasc Biol 25:646–649
Undar L, Ertugrul C, Altunbas H, Akca S (1999) Circadian variations in natural coagulation inhibitors protein C, protein S and antithrombin in healthy men: a possible association with interleukin-6. Thromb Haemost 81:571–575
Madden LA, Vince RV, Sandstrom ME, Taylor L, McNaughton L, Laden G (2008) Microparticle-associated vascular adhesion molecule-1 and tissue factor follow a circadian rhythm in healthy human subjects. Thromb Haemost 99:909–915
Kapiotis S, Jilma B, Quehenberger P, Ruzicka K, Handler S, Speiser W (1997) Morning hypercoagulability and hypofibrinolysis. Diurnal variations in circulating activated factor VII, prothrombin fragment F1+2, and plasmin-plasmin inhibitor complex. Circulation 96:19–21
Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V (2004) Early morning attenuation of endothelial function in healthy humans. Circulation 109:2507–2510
Keaney JF Jr, Weaver DR (2009) Vascular rhythms and adaptation: do your arteries know what time it is? Circulation 119:1463–1466
Decousus H, Boissier C, Perpoint B, Page Y, Mismetti P, Laporte S, Tardy B, Queneau P (1991) Circadian dynamics of coagulation and chronopathology of cardiovascular and cerebrovascular events. Future therapeutic implications for the treatment of these disorders? Ann NY Acad Sci 618:159–165
Maemura K, Takeda N, Nagai R (2007) Circadian rhythms in the CNS and peripheral clock disorders: role of the biological clock in cardiovascular diseases. J Pharmacol Sci 103:134–138
Walters J, Skene D, Hampton SM, Ferns GA (2003) Biological rhythms, endothelial health and cardiovascular disease. Med Sci Monit 9:RA1–RA8
Furlan R, Barbic F, Piazza S, Tinelli M, Seghizzi P, Malliani A (2000) Modifications of cardiac autonomic profile associated with a shift schedule of work. Circulation 102:1912–1916
Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG (1998) Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension 32:417–423
Olson LJ, Olson EJ, Somers VK (2004) Obstructive sleep apnea and platelet activation: another potential link between sleep-disordered breathing and cardiovascular disease. Chest 126:339–341
Dean RT, Wilcox I (1993) Possible atherogenic effects of hypoxia during obstructive sleep apnea. Sleep 16:S15–S22
Rangemark C, Hedner JA, Carlson JA, Gleerup G, Winther K (1995) Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep 18:188–194
Hui DS, Ko FW, Fok JP, Chan MC, Li TS, Tomlinson B, Cheng G (2004) The effects of nasal continuous positive airway pressure on platelet activation in obstructive sleep apnea syndrome. Chest 125:1768–1775
Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Oda N, Tanaka A, Yamamoto M, Ohta S, O’Donnell CP, Adachi M (2007) Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med 175:612–617
Sanner BM, Konermann M, Tepel M, Groetz J, Mummenhoff C, Zidek W (2000) Platelet function in patients with obstructive sleep apnoea syndrome. Eur Respir J 16:648–652
Brezinski DA, Tofler GH, Muller JE, Pohjola-Sintonen S, Willich SN, Schafer AI, Czeisler CA, Williams GH (1988) Morning increase in platelet aggregability. Association with assumption of the upright posture. Circulation 78:35–40
Narkiewicz K, Somers VK (2003) Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 177:385–390
Acknowledgments
We thank Margalida Bosch and Mónica Iglesias for their assistance in the coordination of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study is supported in part by SEPAR and Fondo de Investigaciones Sanitarias 07/906.
Rights and permissions
About this article
Cite this article
Barceló, A., Piérola, J., de la Peña, M. et al. Impaired circadian variation of platelet activity in patients with sleep apnea. Sleep Breath 16, 355–360 (2012). https://doi.org/10.1007/s11325-011-0501-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-011-0501-5