Abstract
Rationale
Temporomandibular disorder (TMD) has been the most common contraindication for mandibular advancement device (MAD) as a treatment for obstructive sleep apnea syndrome (OSAS). Exercising the mandible is a recommended form of therapy for TMD.
Objectives
To assess the efficacy of mandibular exercises in the control of pain, changes of quality of life and to assess the impact of MAD compliance in OSAS patients with previously diagnosed TMD.
Methods
A blind, randomized, and controlled trial was used to evaluate 29 OSAS patients with TMDs were divided in two groups: the exercise support therapy (ST) and placebo therapy (PT), who were evaluated prior to and 120 days after MAD treatment. Treatment outcomes were measured using the Fletcher and Luckett sleep questionnaire, Epworth sleepiness scale, SF-36 inventory of quality of life, polysomnography, diary of MAD usage, and the research diagnostic criteria for TMD.
Measurements and main results
ST group showed significant improvement in their sleep quality and life quality when compared to the PT group (p < 0.05). Higher number of patients with persistent pain was observed in the PT group (p = 0.01). There was a reduction of pain intensity in the ST group, but not in the PT group (p < 0.05). Higher MAD compliance was observed in the ST group (p < 0.05).
Conclusions
Mandibular exercises enable patients with TMD to use MAD; exercises were found to be effective in reducing pain and increasing MAD compliance and produced a significant improvement in the quality of life and quality of sleep.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A mandibular advancement device (MAD) is an effective treatment for obstructive sleep apnea syndrome (OSAS) [1, 2]; in addition, it is a noninvasive modality that is reversible and easy to build. It is widely recommended as a treatment for snoring and for mild and moderate OSAS [3, 4]. Such devices are also recommended for mild and moderate OSAS patients who have not responded to continuous positive airway pressure therapy (CPAP), who are not appropriate candidates for CPAP, or whose previous attempts to use the CPAP failed [5]. It has been widely described that the use of MAD may have several short-term side effects, such as excessive salivation, dry mouth, tooth discomfort, pain in the soft tissue within the mouth, and pain or discomfort in the masticatory muscles and/or temporomandibular joints [6–8]. Pain originating either from the masticatory muscles or the temporomandibular joint (TMJ) is referred as a temporomandibular disorder (TMD); this pain may be one of the main reasons for poor compliance or abandonment MAD treatment [9–14]. TMD patients most frequently report pain or discomfort in the region of the face. TMD pain can be exacerbated by mandibular function, resulting in limited mouth opening. The condition may produce noise from the temporomandibular joint, headaches, and alterations of sleep quality [15–18]. For pain control and recovery of mandibular function, noninvasive and reversible modalities of treatment are the first choices of treatment [18, 19]. Yet another noteworthy issue is the reduced quality of life endured by with TMD pain [20, 21] which is also seen in OSAS patients. Mandibular exercises, known as support therapy (ST), are used as a treatment modality for TMD. ST is widely accepted by patients and is efficient in the management of muscular and joint dysfunction [22–28].
It is noteworthy that in studies of MAD therapy, different diagnostic criteria have been used to evaluate TMD pain [6–10] and various authors have contraindicated the MAD when any sign of TMD was present [10]. Others have reported noncompliance or suspension of the MAD usage because of the development of TMD related to MAD usage [9–14].
However, to our knowledge, there are no studies which did simultaneously assess therapies controlling TMD pain and MAD treatment. The purpose of this study was to assess the efficacy of support therapy (ST) in the reduction of pain, improvement in the quality of life, and compliance to MAD treatment in OSAS patients with TMD pain.
Methods
Study population
Adult patients diagnosed with mild and moderate OSAS [29], with TMD diagnosed by the Research Diagnostic Criteria (RDC/TMD) [30] adapted and translated to Portuguese [31, 32] and referred for MAD treatment, were assessed in the sleep ambulatory unit of Universidade Federal de Sao Paulo (UNIFESP), Brazil. This experimental investigation had the approval of the Ethics in Research Board of the UNIFESP. The inclusion criteria was adopted for the following indication of MAD described in the literature [4]: age between 18 and 60 years, apnea–hypopnea index (AHI) over 5 and under 30, and body mass index (BMI) less than or equal to 30 kg/m2. Both genders were included. Patients, who presented fewer than 10 teeth per arch, active periodontal disease, a need of overall dental treatment, a mandible protrusion less than 5 mm, limited mouth opening, use of alcohol, drugs or hypnotic substances, and sleep disturbances other than OSAS with or without previous treatment for OSAS, were excluded. As cervical myofascial pain can refer pain to the face, patients with cervical myofascial pain were not included in this study.
Study design
This study was a double-blind, randomized, and controlled trial in which patients were distributed into ST and placebo therapy (PT) groups.
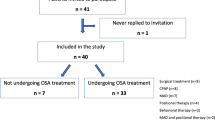
To ensure patients were blinded to the study, both therapies were explained to the patients as being effective therapies. The investigator who was blinded to the randomization has only applied all study instruments of evaluation such as the RDC, while a second investigator did the randomization and was responsible for explaining the exercises to the patients. Patients were evaluated at the start of the protocol and after 120 days, according to the experimental design described in Fig. 1. Sleep evaluation was assessed by polysomnography and by the Flecher and Luckett questionnaire [33]. Sleepiness was measured by the Epworth sleepiness scale (ESS) [34], and the quality of life was assessed by the quality of life inventory (SF-36) [35]. TMD was evaluated by the investigator (PAC) throughout the study, who was also responsible for advancing and adjusting the appliance. The presence and type (persistent or recurring) of TMD pain was assessed after the first week of MAD use, in the week when MAD advancement reached two-thirds of maximum mandible protrusion, and in the week when it reached maximum protrusion. Intensity of pain was assessed prior to and after treatment. Compliance to the treatment was registered 1 week after insertion and after final titration of the MAD by sleep log book. This book of sleep show that the use of MAD throughout the night, part of the night, or when the patient did not using the MAD. These data were also collected by the second investigator blind while the principal investigator (PAC). The MAD appliance used is the Brazilian repositioning device (BRD®), an adjustable device, made in a specialized laboratory, developed at UNIFESP (Fig. 2a and b). The appliance was inserted at 50% of the maximum mandible protrusion advancement; patients were instructed to return for reevaluation every month and to advance the appliance 0.5 mm/week. This advancement was monitored until the maximum mandible protrusion was achieved.
Outcome measures
Questionnaires
The Fletcher and Luckett [33] questionnaire consists of questions about sleep, snoring, nocturnal apnea, and daytime somnolence. This questionnaire classifies the answers as never (0), rarely (1), occasionally (2), and often (3). Daytime somnolence was evaluated by the Epworth sleepiness scale [34]. These questionnaires were use to quantify the sleep complaints of patients. To assess patient’s quality of life, we used the quality of life inventory (SF-36) [35] which refers to the subjective quality of life in day-by-day activities, work, pain, and other activities. All three questionnaires were used prior to MAD treatment and after 120 days of the treatment.
TMD diagnostic
We employed the “Research Diagnostic Criteria for Temporomandibular Disorders” (RDC/TMD) [30], a tool developed to quantify and qualify signs and symptoms of TMD, to classify TMD. This questionnaire, adapted to Portuguese, has been used previously [31, 32]. It evaluates the presence of pain in the facial region and in the TMJ in the previous 30 days, especially during mandibular function. All patients were asked to fill out the protocol to score their chronic TMD pain; clinical exams for classification and confirmation of TMD were carried out according to the clinical examination for the RDC/TMD. Three sites of pain in the masticatory muscles and/or in TMJ, in the clinic evaluation, coinciding with the same sites of pain complaint by the patient determined the clinical diagnosis [30]. The RDC/TMD was applied before treatment and in the week during which the patients completed 120 days of treatment.
Polysomnography
Polysomnography, used for diagnosis and treatment follow-up, consisted of the computerized system Sonolab Meditron (version 2003.a). Surface electrodes were used to record electroencephalographs (EEG; C3-A2, C4-A1, O2-A1, O1-A2); submentonian and tibial electromyograph recordings (EMG); bilateral electrooculograms (EOG); and electrocardiographs (ECG-modified derivation V1). Breathing was monitored with a nasal cannula, in which air flow is gauged by pressure transduction, and oral flow was monitored with a thermal sensor. Thoracic and abdominal movements were measured with noncalibrated pletismography. Measurements of oxygen saturation were collected from a wrist oxymetry device (Nellcor). The position used for the recordings, lying down, allowed the placement of a sensor over the sternum bone to define body position, and an attached tracheal microphone allowed recording of snoring. Staging was made according to the directives set forth by Rechtschaffen and Kales [36], and respiratory events were scored following the criteria adopted by the American Academy of Sleep Medicine (AASM) board [37]. Awakenings were scored by the criteria of the American Sleep Disorders Association (ASDA) [38].
Support therapy
Patients were blind to their assigned type of therapy. ST consisted of coordinated exercises to stretch the mandibular muscles, and it was adopted to control pain as well as to restore movement otherwise hampered by TMD pain. For coordinated movements, patients were instructed to perform sequences of exercises by controlled mouth opening. This was achieved by maintaining the tongue in contact with the palate, followed by a sequence of lateral left–right movement of the mandible against light-hand resistance. This type of movement was used with the intent to exercise the lateral pterygoid muscles and the TMJ (Fig. 3a, b, and c). For stretching, we used the movement of opening the mouth against light resistance of the hand, followed by a maximum opening of the mouth assisted by the fingers, with the intent of stretching the temporal and masseter muscles (Fig. 2d and e). Patients were instructed to exercise twice a day, in three sets of five repetitions of each movement, prior to and after use of the MAD. These mandibular exercises, adopted as ST, have proven to be efficient and effective in the treatment of TMD [19]. The patients were highly reinforced to do the exercises on a regular basis, but we could not be sure if patients had followed our recommendations.
ST exercises used for coordination and stretching, with the goal to restore the movements function and control pain. For coordination, movements of opening and closing the mouth with tongue limitation (a), and lateral movements against mild hand resistance were directed to the lateral pterygoide muscles and temporomandibular joints (b and c); For stretching, the movement of opening the mouth against hand resistance (d), and the forced assisted movement of widely opening the mouth, forced by fingers (e), gave stretching to temporal and masseter muscles
Placebo therapy
PT consisted of two different types of assisted cervical movement. At first, the patient was instructed to rotate the head left and right (Fig. 4a and b) and then to tilt the head to the each side, recovering the vertical position against the resistance of the hand placed contralaterally (Fig. 4c and d). This type of exercise was chosen because patients had no cervical myofascial pain (muscular cervicalgia), a condition that could point to pain in the face and mimic TMD pain [39]. Patients in the PT group were instructed to perform three sets of five repetitions for each movement, twice a day, prior and subsequent to use of the MAD; this is the same frequency used for the ST group. Although these exercises are indicated for myofascial cervical pain, like we said in the description of study population, patients with cervical myofascial pain were not included in this study.
Treatment outcome
The efficacy of MAD therapy was measured by the Fletcher and Luckett questionnaire, ESS, and polysomnography (PSG). The efficacy of ST in reducing pain intensity was determined by comparing the intensity of pain, in both ST and PT groups at baseline settings and after 120 days of treatment, by the RDC/TMD. We also assessed the occurrence of TMD pain, which was deemed persistent when there was pain at the morning MAD removal and during mastication, preventing the subject from using the MAD that day. When pain or discomfort was reported only during removal of the MAD, without compromising its use or mastication, it was considered recurrent pain.
Changes in quality of life in both groups were determined by comparing the baseline and end-of-protocol results of the SF-36 test. In order to determine what influence ST had in MAD compliance, log books of MAD use of ST adhesion were maintained by the patients.
Statistical analyses
Results are presented means and standard errors. For quantitative comparisons between groups, the Student’s t test for independent samples, or the intra-group Student’s t test for dependent variables, was used. The Chi-squared test was used for the categorical data. For statistical calculations, the software Statistic® 6.0 was used, and the value of p = 0.05 was considered as significant.
Results
A total of 45 (52% of 87 patients) with mild to moderate cases of OSAS and referred for MAD therapy presented signs or symptoms of TMD pain. Two patients were excluded by the inclusion criteria, and 11 never returned for the initial assessment. Thus, 32 patients were selected for randomization; 29 completed the protocol.
Comparisons were made between the 14 patients who either did not finish (n = 3) or did not accept to participate in the study (n = 11) and the 29 patients who completed the protocol. There was no statistical significance between these groups with regard to age (44.5 ± 10.7 × 49.7 ± 9.8 years), BMI (25.2 ± 3.8× 25.9 ± 4.1 kg/m2) or AHI (13.7 5.25 × 16.5 4.1;).
At baseline, none of the groups presented any statistically significant differences, except that the PT group was on average younger (Table 1).
The Fletcher and Luckett Sleep questionnaire showed significant changes, related to treatment, only in the ST group (Table 2). Comparison of the SF-36 before and after MAD titration showed a significant improvement in a higher number of quality of life domains in ST group, as compared to PT group (in 5 of the 8 domains in the ST group, and in three of the eight domains for the PT group). Differences were significant in domains related to pain (pain, general health) and psychoemotional aspects (limitation by emotional and mental health; Table 2). We found no difference in polysomnographic parameters between groups at baseline (Table 1); ST and PT groups both showed a significant reduction in their AHIs with the MAD. There was no difference between groups in terms of AHI improvement, but only the ST group presented a significant alteration in the minimum oxygen saturation parameter (Table 2). There was no significant difference in the type of complaints of TMD pain in the first week (8 of 15 and 8 of 14 patients in the ST and PT groups, respectively). At two-thirds of the maximum MAD advancement, there was a greater number of patients with persistent pain in the PT group (14 patients) compared to the ST group (three patients). This condition remained for the maximum advancement setting, when a higher number of complaints concerning persistent pain was observed in PT group (ten patients) compared to ST group (four patients; Fig. 5).
By the criteria of the RDC/TMD, the intensity of pain decreased between the baseline and the final condition of the treatment in the ST group, whereas in the PT group, pain was slightly increased, but without significance (Fig. 6).
Compliance to the MAD was similar between groups during the first week. After advancement of the MAD, greater compliance was observed in the ST group (Fig. 7).
Discussion
This is the first study to assess an ST for TMD pain control in patients who undergo MAD therapy and who have been diagnosed with TMD by RDC/TMD prior to MAD treatment. Patients in the ST group reported less pain, adhered better to MAD therapy, and experienced significant improvements in their quality of life and sleep as compared to patients in the placebo group (PT).
The MAD therapy in the present study resulted in a significant improvement in AHI and a decrease in the number of micro-arousals, regardless of the therapy adopted (ST or PT). The improvements achieved with the use of the MAD confirm the well-established literature demonstrating the efficacy of MAD therapy [5, 11, 14].
The quality of life and quality of sleep in OSAS patients are compromised, and according to some studies, this condition can be improved by MAD treatment [40, 41]. In the present investigation, the sleep complaints assessed by the Fletcher and Luckett questionnaire [33] were reduced only in the ST group. We also found a significant improvement in, and in a higher number of, quality of life domains in the ST group compared with the PT group. Such differences were represented by domains related to pain (pain, general health) and psychoemotional aspects (limitation by emotional and mental health). Although some improvement was expected in both groups after the same treatment was offered to the ST and PT groups, MAD treatment, we hypothesize that persistent pain could be related to the worsening of the quality of sleep. We observed that, independent of group (PT or ST), the excessive daytime sleepiness measured with Epworth sleepiness scale remained high after the treatment with the MAD. Although a significant change in sleepiness after MAD usage has been shown [6, 11, 12, 42], there are other studies that, in agreement with ours, did not find a significant change [1, 43]. The ESS is a subjective measurement tool, and it does not always correlate well with objective measures of excessive sleepiness, such as the multiple latency sleep test or the maintenance of vigil test [44]. Because this study did not apply objective measures of sleepiness, we hesitate to conclude that the use of the MAD did not reduce patients’ sleepiness. Another possibility of EDS persistency could be a chronic pain condition with long period of sleep fragmentation leading to alteration in the awake–sleep brain system.
The contraindications of MAD treatment in TMD patients probably originated due to side effects like pain in the TMJ and masticatory muscles cause by MAD use [9, 10, 45]. Such exclusions, however, have been made in the absence of reproducible, systematic diagnostic criteria for TMD. As a result of the present study, we believe that the contraindication of MAD for patients with TMD is not based on proven results, and patients with TMD are still eligible for MAD therapy.
In our previous investigation [46], the prevalence of TMD was high (52%) in the OSAS population referred for MAD therapy. Of the individuals who showed TMD¸ 90% had myofascial masticatory muscles pain, and in 65% of those, joint pain was present. In 75% of the patients, the impact of TMD pain was of low intensity and low disability. Petit and colleagues [10] reported an active TMJ disorder was present in only 2% of patients.
The prevalence of side effects related to TMJ or masseter muscle pain was very different between studies [13, 40, 47]. As neither the degree of involvement nor the specificity of the TMD was determined, mild and severe cases were almost certainly lumped and assessed as one entity. The percentages of such side effects vary and can even be justified by the absence of a validated methodology with previous assessment of TMD [6–8]. Still, the authors did not use standardized classification criteria for the diagnosis of TMD prior to the treatment; therefore, it is possible that some of those 11 patients had TMD prior to the treatment. In 2001, Fritsch and collaborators [7] assessed side effects of the MAD in 22 individuals; mandibular pain was found to occur in nine patients (41%), and either stiffness or pain in the masseter was found in eight patients (36%). Prior systematic TMD evaluation was not assessed, similar to many other studies of MAD side effects [11–14]. This lack of diagnostic criteria for assessment, in our opinion, is responsible for such different findings. In the present investigation, the use of the RDC/TMD as a diagnostic instrument for TMD enabled us to assess the presence of TMD in OSAS patients prior to MAD treatment. Likewise, until the present report, there were no studies using a systematic protocol to evaluate TMD.
Yet another relevant issue is that none of the studies that assessed TMD as a side effect of MAD addressed alternative methods to minimize those effects and thus enabled continuation of the treatment [6–8]. Mandibular exercises, whether associated to other noninvasive treatment modalities (counseling and occlusion plates, for example) or not, have been used with satisfactory results in the control of pain caused by TMD. In those modalities, the observation period of patients submitted to exercises varied according to the protocol, but in general, good results were produced after 3 months [20–25]. In randomized controlled studies, mandibular exercises and counseling were compared to isolated counseling for the treatment of myofascial pain. The studies showed better results with the mandibular exercises; therefore, they suggest exercise therapy as a first choice intervention in muscular TMD [20, 21]. Also, passive mandibular exercises, used for the treatment of joint disc displacement with [22] and without reduction [23], showed a significant reduction of pain after 6 months. Mandibular exercises also improve significantly joint sounds, compared to a control group [25]. Ueda and collaborators [48] used mandibular exercises combined with a MAD and found that this type of therapy did improve occlusal contact area and bite force. The authors related those findings to tooth movement and did not evaluate systematically the presence or absence of TMD. The current study is, to our knowledge, the first to use mandibular exercises with MAD therapy for patients with TMD and OSAS. Similarly to previous studies, we also found that mandibular exercises produced a significant improvement in TMD. Differently than those studies, our patients used the MAD therapy at night and the ST during the day.
The amount of mandibular protrusion prescribed as necessary for the treatment of OSAS varies between half and maximum mandibular advancement. The results collected herein show that when the MAD is positioned at two-thirds of the maximum mandibular advancement, the ST group presented less pain when compared to the PT group (three vs. nine patients, respectively), and the same distribution of pain prevalence was seen at maximum mandibular protrusion (four vs. ten, respectively). According to the RDC/TMD criteria, pain intensity decreased from pre-treatment to final mandibular protrusion only in the ST group. Contrarily, the PT group showed a slight increase in pain at the end of treatment. It is important to notice that the amount of mandibular protrusion did not differ between the groups, which allow us to infer that the amount of mandibular protrusion had no influence in the final results. This data confirms the efficacy of ST in OSAS/TMD patients treated with a MAD. As yet, the available literature does not report that exercise aids the treatment of OSAS for patients with concomitant TMD.
Important studies have assessed pain as a factor for poor compliance to MAD; these studies showed that in a period of 3–7 months of follow-up, compliance rates vary from 68% to 100% [1, 45]. As for compliance to the MAD therapy in this investigation, no significant difference was found between the groups in the baseline conditions (76% vs. 88% in the PT and ST groups, respectively). However, after MAD advancement, higher adherence in the ST group was observed (68% vs. 92% for PT and ST, respectively). It can thus be inferred that mandible exercises most likely leads to higher compliance to the use of a MAD in OSAS patients.
Although the results of the current investigation are significant and might change indications and add exercises to the management of patients in MAD therapy, clinical trials poses challenges and some limitations to the study must be taken into account. First, the number of patients was small, and the RDC/TMD diagnosis was carried out by only one examiner. Larger samples are necessary, and ideally, two investigators would evaluate intra- and inter-observer biases. Second, because of the nature of the intervention, the patients would have been aware of the study allocation and may have inadvertently unblended the investigators. As the main outcome measures in this study were subjective, the potential for investigator bias is an important consideration. Third, because there is no objective instrument to assess MAD compliance, we have recorded compliance subjectively by means of a diary, which has been shown to overestimate the hours of use. Another limitation of the present study was that exercises were not supervised; adherence to the exercise protocol was also recorded only by means of a diary. The follow-up period of 120 days is relatively short and thus, it is not possible to draw conclusion about the long-term effectiveness of intervention. Finally, the MAD advancement protocol used in the present study does not match the traditional protocol. Our patients started at 50% advancement, instead of the 60% to 75% advancements stated in the literature. We believe that this was not important to our results, as we had a controlled placebo group. The exclusion of patients with mandibular protrusion of less than 5 mm or limited mouth opening would have excluded patients with more significant forms of TMD and limits the generalizability of the results. In addition, this study did not recruit obese patients or those with severe OSAS further limiting the generalizability of the findings for these patients. However, further studies are necessary to evaluate the ST under traditional advancement protocols.
The fact of MAD being contraindicated in presence of TMD is still controversial. There is one paper in the literature [49] stating short-term benefits of MAD on bruxism, which is commonly associated with TMD. Despite of this, to our knowledge, most papers available in Pubmed, have stated that moderate to severe TMJ problems or an inadequate protrusive ability may be contraindications to MAD therapy [3, 10, 45]. These conflicting positions might be related to the term TMDs, which we understand is not specific. Although, in this research field the RDC criteria are until now, in our opinion, the best research tool available. Still, we believe that once better assessments tools are available further studies are necessary. The fact that we have evaluated only one type of MAD also configures a limitation of this study, and therefore similar studies with most commonly used appliances such as Klearway, Herbist, and PM Positioner are necessary for our data to be generalized.
Despite some limitations, the results of the present study allow us to suggest that OSAS patients who are referred for MAD therapy require a specific assessment of TMD. Our results also demonstrate that the contraindication of MAD for the treatment of OSAS should be reexamined and that mandibular exercises, when adopted as ST, can be influential in decreasing pain and increasing MAD compliance in the 120 days of treatment in this study. Although the positive result of ST in patients treated in this study, severe functional limitations for TMD should be evaluated carefully. These conditions have yet to be primarily treated for TMD.
References
Gotsopoulos H, Chen C, Qian J, Cistulli PA (2002) Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med 166:743–748
Chan AS, Lee RW, Cistulli PA (2007) Dental appliance treatment for obstructive sleep apnea. Chest 132:693–699
Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W (2006) Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 29:244–262
Hoffstein V (2007) Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath 11:1–22
Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Owens J, Pancer JP (2006) Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 29:240–243
Bloch KE, Iseli A, Zhang JN, Xie X, Kaplan V, Stoeckli PW, Russi EW (2000) A randomized controlled crossover trial of two appliances for sleep apnea treatment. Am J Respir Crit Care Med 162:246–251
Fritsch KM, Iseli A, Russi EW, Bloch KE (2001) Side effects of mandibular advancement devices for sleep apnea treatment. Am J Respir Crit Care Med 164:813–818
Johnston CD, Gleadhill IC, Cinnamond MJ, Gabbey J, Burden DJ (2002) Mandibular advancement appliances and obstructive sleep apnoea: a randomized clinical trial. Eur J Orthod 24:251–262
Almeida FR, Bittencourt LR, de Almeida CI, Tsuiki S, Lowe AA, Tufik S (2002) Effects of mandibular posture on obstructive sleep apnea severity and the temporomandibular joint in patients fitted with an oral appliance. Sleep 25:507–513
Petit FX, Pépin JL, Bettega G, Sadek H, Raphaël B, Lévy P (2002) Mandibular advancement devices: rate of contraindications in 100 consecutive obstructive apnea patients. Am J Respir Crit Care Med 166:274–278
Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA (2001) A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med 163:1457–1461
Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA (2002) Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med 166:860–864
Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA (2007) A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 132:806–814
Marklund M, Franklin KA (2007) Long-term effects of mandibular repositioning appliances on symptoms of sleep apnoea. J Sleep Res 16:414–420
Cooper BC, Kleinberg I (2007) Examination of a large patient population for the presence of symptoms and sings of temporomandibular disorders. Cranio 25:114–126
Selaimen CM, Jeronymo JC, Brilhante DP, Grossi ML (2006) Sleep and depression as risk indicators for temporomandibular disorders in a cross-cultural perspective: a case-control study. Int J Prosthodont 19:154–161
Oliveira AS, Dias EM, Contato RG, Berzin F (2006) Prevalence study of signs and symptoms of temporomandibular disorders in Brazilian college students. Braz Oral Res 20:3–7
Conti PC, Ferreira PM, Pegoraro LF, Conti JV, Salvador MC (1996) A cross-sectional study of prevalence and etiology of signs and symptoms of temporomandibular disorders in high school and university students. J Orofac Pain 10:254–262
Okeson JP (2008) General considerations in the treatment of temporomandibular disorders. In: Okeson JP (ed) Management of temporomandibular disorders and occlusion, 6th edn. Mosby, St. Louis, pp 358–361
Reissmann DR, John MT, Schierz O, Wassell RW (2007) Functional and psychosocial impact related to specific temporomandibular disorder diagnoses. J Dent 35:643–650
John MT, Reissmann DR, Schierz O, Wassell RW (2007) Oral health-related quality of life in patients with temporomandibular disorders. J Orofac Pain 21:46–54
Michelotti A, Steenks MH, Farella M, Parisini F, Cimino R, Martina R (2004) The additional value of a home physical therapy regimen versus patient education only for the treatment of myofascial pain of the jaw muscles: short-term results of a randomized clinical trial. J Orofac Pain 18:114–125
Michelotti A, de Wijer A, Steenks M, Farella M (2005) Home-exercise regimes for the management of non-specific temporomandibular disorders. J Oral Rehabil 32:779–885
Nicolakis P, Erdogmus B, Kopf A, Djaber-Ansari A, Piehslinger E, Fialka-Moser V (2000) Exercise therapy for craniomandibular disorders. Arch Phys Med Rehabil 81:1137–1142
Nicolakis P, Erdogmus B, Kopf A, Ebenbichler G, Kollmitzer J, Piehslinger E, Fialka-Moser V (2001) Effectiveness of exercise therapy in patients with internal derangement of the temporomandibular joint. J Oral Rehabil 28:1158–1164
Nicolakis P, Erdogmus B, Kopf A, Nicolakis M, Piehslinger E, Fialka-Moser V (2002) Effectiveness of exercise therapy in patients with myofascial pain dysfunction syndrome. J Oral Rehabil 29:362–368
Yoda T, Sakamoto I, Imai H, Honma Y, Shinjo Y, Takano A, Tsukahara H, Morita S, Miyamura J, Yoda Y, Sasaki Y, Tomizuka K, Takato T (2003) A randomized controlled trial of therapeutic exercise for clicking due to disk anterior displacement with reduction in the temporomandibular joint. Cranio 21:10–16
Yoda T, Sakamoto I, Imai H, Ohashi K, Hoshi K, Kusama M, Kano A, Mogi K, Tsukahara H, Morita S, Miyamura J, Yoda Y, Ida Y, Abe M, Takano A (2006) Response of temporomandibular joint intermittent closed lock to different treatment modalities: a multicenter survey. Cranio 24:130–136
American Academy of Sleep Medicine (2005) The international classification of sleep disorders, 2nd ed. Diagnostic and coding manual. American Academy of Sleep Medicine, Westchester, IL
Dworkin SF, LeResche L (1992) Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 6:301–355
Pereira FJ Jr, Favilla EE, Dworkin SF, Huggins K (2004) Research diagnostic criteria for temporomandibular disorders (RDC/TMD): formal translation to Portuguese. J Bras Clin Odontol Integr 8:384–395
Kosminsky M, Lucena LBS, Siqueira JTT, Pereira FJ Jr, Góes PSA (2004) Cultural adaptation of the “Researche diagnostic criteria for tempodomandibular disorders: axis II” questionnaire. J Bras Clin Odontol Integr 8:51–61
Fletcher EC, Luckett RA (1991) The effect of positive reinforcement on hourly compliance in nasal continuous positive airway pressure users with obstructive sleep apnea. Am Rev Respir Dis 143:936–941
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
McHorney CA, Ware JE Jr, Raczek AE (1993) The MOS 36-item short form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31:247–263
Rechtschaffen A, Kales A (1968) A manual of standardized technology, techniques and scoring system for sleep stages of human subjects. Brain Information Service/Brain Research Institute/UCLA, Los Angeles, CA
American Academy of Sleep Medicine (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep 22:667–689
American Sleep Disorders Association (1992) EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15:173–184
Travel JG, Simons DG (1983) Myofascial pain and dysfunction: the trigger point manual. Williams and Wilkins, Baltimore
Shadaba A, Battagel JM, Owa A, Croft CB, Kotecha BT (2000) Evaluation of the Herbst mandibular advancement splint in the management of patients with sleep-related breathing disorders. Clin Otolaryngol Allied Sci 25:404–412
Bates CJ, McDonald JP (2006) Patients’ and sleeping partners’ experience of treatment for sleep-related breathing disorders with a mandibular repositioning splint. Br Dent J 200:95–101
Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, Pierce RJ (2004) Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 170:656–664
Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, Mackay TW, Douglas NJ (2002) Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med 166:855–859
Chervin RD, Aldrich MS (1999) The Epworth sleepiness scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 52:125–131
Clark GT (1998) Mandibular advancement devices and sleep disordered breathing. Sleep Med Rev 2:163–174
Cunali PA, Almeida FR, Santos CD, Valdrighi NY, Nascimento LS, Dal-Fabbro C, Tufik S, Bittencourt LRA (2009) Prevalence of temporomandibular disorders in obstructive sleep apnea patients referred for oral appliance therapy. J Orofac Pain 23:339–344
McGown AD, Makker HK, Battagel JM, L’Estrange PR, Grant HR, Spiro SG (2001) Long-term use of mandibular advancement splints for snoring and obstructive sleep apnoea: a questionnaire survey. Eur Respir J 17:462–466
Ueda H, Almeida FR, Chen H, Lowe AA (2009) Effect of 2 jaw exercises on occlusal function in patients with obstructive sleep apnea during oral appliance therapy: a randomized controlled trial. Am J Orthod Dentofacial Orthop 135:430e1–430e7
Landry ML, Rompré PH, Manzini C, Guitard F, de Grandmont P, Lavigne GJ (2006) Reduction of sleep bruxism using a mandibular advancement device: an experimental controlled study. Int J Prosthodont 19(6):549–556
Acknowledgements
The authors would like to thank the Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP, number 2006/04488-4), the FAPESP-Centros de Pesquisa, Inovação e Difusão (CEPID), and the Associacao Fundo de Incentivo a Psicofarmacologia (AFIP) for their support and funding. Sergio Tufik and Lia Rita Azeredo Bittencourt are principal investigators of FAPESP and researchers of Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cunali, P.A., Almeida, F.R., Santos, C.D. et al. Mandibular exercises improve mandibular advancement device therapy for obstructive sleep apnea. Sleep Breath 15, 717–727 (2011). https://doi.org/10.1007/s11325-010-0428-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-010-0428-2