Abstract
Purpose
Sleep apnea–hypopnea syndrome and its chronic intermittent hypoxia component may cause multi-system-targeted injury. The latest finding shows that liver is one of the injured organs. The purpose of the study is to observe the dynamic process of the influence that chronic intermittent hypoxia plays on rat liver enzyme, hepatic histology, and ultrastructure based on lipid disorders.
Methods
A total of 72 male Wistar rats were randomly divided into three groups. The control group was fed with a regular chow diet, the high fat group with a high fat diet, and the high fat plus intermittent hypoxia group with a high fat diet with a 7-h/day intermittent hypoxia treatment. Changes were observed in rat liver enzyme, hepatic histology, and ultrastructure of the three groups on the third, sixth, and ninth weeks, respectively. The liver paraffin sections were detected with myeloperoxidase.
Results
The liver function and structure of the control group were found to be normal; the liver enzyme level of the high fat group was significantly higher than that of the control group on the sixth and ninth weeks; and the liver enzyme level of the high fat plus intermittent hypoxia group was significantly higher than that of the control group and the high fat group on the third, sixth, and ninth weeks (all P < 0.01). Observed by a light microscope and a transmission electron microscope, the high fat group and the high fat plus intermittent hypoxia group were all characterized by nonalcoholic fatty liver disease: the high fat group was characterized by simple fatty liver on the third and sixth weeks and by steatohepatitis on the ninth week; the damage of the high fat plus intermittent hypoxia group was significantly more severe than that of the high fat group in all the monitoring points, characterized by steatohepatitis on the sixth week and by obvious liver fibrosis on the ninth week; the myeloperoxidase level of the high fat plus intermittent hypoxia group was significantly higher than that of the control group and the high fat group (all P < 0.01).
Conclusions
Under the conditions of high fat and intermittent hypoxia, the injury to the liver function, hepatic histology, and ultrastructure is more severe than that of the high fat group. The injury mainly was characterized by nonalcoholic fatty liver disease and becomes more severe with increased exposure time. Oxidative stress may play an important role in the mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep apnea–hypopnea syndrome (SAHS) is a common disorder with a potential danger. Studies on SAHS have progressed through stages from epidemiological investigation, study on pathogenesis, to the current study on the mechanism of multiple organ damage. Regarding the systems injured by SAHS, people at first have noticed its close relationship with cardiovascular and cerebrovascular disease. Clinical and epidemiological studies have proven that SAHS is an independent risk factor for hypertension, coronary artery disease, congestive cardiac failure, stroke, etc. [1–4]. However, the injury that SAHS has on an organism goes beyond the cardio–cerebral–vascular system and includes the respiratory system, urogenital system, endocrine system, etc. [5]. The latest research in recent years shows that SAHS and its chronic intermittent hypoxia (CIH) component may cause chronic liver injury, which is mainly manifested by histologic features of nonalcoholic fatty liver disease (NAFLD) [6–13]. In 2003, Chin et al. found that 1/3 of obese SAHS patients had elevated transaminases [6]. In 2005, Tanne et al. reported that severe obstructive sleep apnea is a risk factor for steatohepatitis, independent of obesity [7]. In 2007, Savransky et al. proved in an animal experiment based on lipid disorders that CIH can promote the progression from simple fatty liver to steatohepatitis [8, 9]. Although more and more studies report that there is a definite link between SAHS and NAFLD, the correlation between them is at an early stage. Further research is needed as there are only a few animal experimental studies on SAHS leading to chronic liver injury. This study established a human SAHS-simulated CIH animal model to (1) dynamically observe the influence that CIH plays on rat liver enzyme, hepatic histology, and ultrastructure based on lipid disorders, (2) monitor the evolution of liver function and structural injury of the rats subjected to CIH, and (3) detect the indicators of liver oxidative stress to probe the mechanism of SAHS liver injury.
Materials and methods
Animals and experimental groups
A total of 72 male Wistar rats weighing 260–280 g were purchased from the Center of Experimental Animal Academy of Military Medical Sciences. All animals were allowed to acclimatize for a week on normal diet before grouping. The rats were then randomly divided into three groups of 24: group A, the control group, fed a regular diet; group B, the high fat group, fed a high fat diet (3% cholesterol, 0.2% propylthiouracil, 0.3% sodium cholate, 10% lard, 2% yolk powder); and group C, the high fat plus intermittent hypoxia group, fed a high fat diet, which is of similar feed formulation as group B, and exposed to intermittent hypoxia. The study was approved by the Tianjin Medical University Animal Care and Use Committee.
Experimental design
The three groups were respectively placed into the same Plexiglas chambers, 7 h/day during the animals’ diurnal sleep period for days. The chamber of group C was periodically filled with nitrogen and compressed air by computer-controlled timed solenoid valves. This cycle was repeated every 60 s. The process consisted of filling the chamber with nitrogen for the first 30 s and filling it again with compressed air for another 30 s. The gas flow rate was adjusted to make the nadir concentration of oxygen 4∼6% during every cycle and then gradually increased to 21%. To study the change of blood oxygen saturation, four additional rats were anesthetized with 1.5% isoflurane, the right femoral artery was cannulated, and the blood oxygen saturations in the three hypoxia reoxygenation cycle at different timing points were monitored. It was observed that the average nadir arterial oxygen saturation of the rats is 66% (60∼75%), which gradually rises to the level of 97% (94–100%). The pressure inside the chamber was at atmospheric level. The chambers of groups A and B were periodically filled with compressed air. The rats were bred conventionally indoors after 7 h. They were allowed free access to water. On the third, sixth, and ninth weeks, eight rats were randomly selected, fasted for 8 h, and killed by exsanguinations, and blood samples were collected for biochemical analysis. The liver was surgically removed and weighed.
Biochemical assays
Serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were measured by the Clinical Chemistry Laboratory of Tianjin Medical University General Hospital using a standard automatic clinical analyzer (Hitachi 7170A, Tokyo, Japan).
Light microscopy
Liver tissue specimens were fixed in 10% buffered formalin solution, progressively dehydrated, and embedded in paraffin for sectioning. The sections (6-µm thick) were stained with hematoxylin and eosin (HE) and Masson’s trichrome. Histological assessment of tissue morphology was performed by using an Olympus light microscope (Olympus BX50, Tokyo, Japan) and was evaluated blindly by a single pathologist. Liver tissue steatosis, hepatocellular ballooning, lobular inflammation, and necrosis are observed by HE. The collagen fibers in Masson’s trichrome are dyed blue to observe the level of liver fibrosis. To quantify the histological changes, the NAFLD scoring system designed by Brunt et al. was used [14, 15] (Table 1).
Transmission electron microscopy
Hepatic tissue was taken from the left lobe of the liver in the same position, washed by physiological saline, and cut into 1 mm×1 mm×1 mm tissue pieces. The specimens were immediately fixed with a solution containing 2.5% glutaraldehyde paraformaldehyde, postfixed in 1% osmium tetroxide solution, dehydrated progressively by ethanol, embedded by EPON812 epoxy resin, cut into ultrathin sections with 50–70 nm thickness, dyed with uranyl acetate and lead citrate, and observed through a JEDL-100CX transmission electron microscope.
Immunohistochemistry
The level of myeloperoxidase (MPO) in hepatic tissue paraffin section was performed in the Pathology Department of Tianjin Medical University General Hospital. Immunohistochemistry was performed using the commercially available non-biotin HRP detection system kit (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) according to the manufacturer’s instructions. Briefly, tissue sections (6-µm thick) were cut from formalin-fixed, paraffin-embedded livers. Sections were deparaffinized and rehydrated by passage through a graded series of ethanol and distilled water. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 for 10 min at room temperature. After incubation at 37°C for 45 min with polyclonal rabbit anti-human myeloperoxidase antibody, sections were washed with PBS. A rabbit anti-IgG polymer was used at 37°C for 45 min. The sections were counterstained with hematoxylin, dehydrated, and mounted.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical analyses were made by using one-way ANOVA and LSD procedures. P values lower than 0.05 were considered to be statistically significant. Statistical analysis was performed using SPSS statistical software (SPSS for Windows 11.5).
Results
Weight change
The weight of the rats in group A increased noticeably as the monitoring period extends, the weight of the rats in group B did not increase after the sixth week, and the increase of the weight of the rats in group C was relatively small. There were significant differences in the compared changes of the weights among the three groups and every individual group at different monitoring points (on the third, sixth, and ninth weeks; F = 35.088, P < 0.01) (Fig. 1a): weight gain = weight at the end of experiment − weight at the beginning of experiment.
Serum biochemistry
There were significant differences in the compared changes of blood fat index in TC and LDL-C (Fig. 1b, c) and liver enzymes in ALT and AST (Fig. 1d, e) among the three groups and every individual group at monitoring points, with separate F values of 67.064 and 120.706 and 96.681 and 94.238, respectively, all P < 0.01.
General observation of liver specimens
Comparing the groups at identical monitoring points, the livers of the rats in groups B and C were bigger than those in group A at the end of the third week, the liver capsule was tense, and the liver appeared yellowish and greasy. At the end of the sixth and ninth weeks, the livers of the rats in groups B and C appeared khaki and smooth in surface without any nodules. There were statistically significant differences in the compared liver body weight ratios among the three groups and every individual group at different monitoring points (F = 23.965, P < 0.01; Fig. 1f): liver body weight ratio (LBR) = liver weight/body weight × 100%.
Light microscopy findings
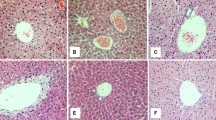
The liver tissues were found to be normal in group A (Fig. 2a, b). The liver tissues in group B on the third week showed only slight (grade 1) steatosis, mainly distributed in zone I hepatocytes; on the sixth week, steatosis was at grade 2, hepatocellular swelling at grade 1 without inflammatory cell infiltration or fibrosis (Fig. 2c). On the ninth week, steatosis was at grade 2∼3, with microvesicular steatosis, hepatocellular swelling at grade 1∼2, occasional inflammatory cell infiltration (focal necrosis), slight collagen fibers hyperplasia in liver portal area, without perisinusoidal fibrosis (Fig. 2d). The liver tissues were abnormal in group C: on the third week, steatosis was at grade 1; on the sixth week, steatosis was at grade 2, hepatocellular swelling at grade 1∼2, and there were occasional inflammatory cell infiltration, periportal fibrosis, and no perisinusoidal fibrosis observed; on the ninth week, steatosis was at grade 3, with microvesicular steatosis, hepatocellular swelling at grade 2, and inflammatory cell infiltration (focal necrosis) at grade 2∼3 (Fig. 2e). Periportal fibrosis was more severe than that of group B on the ninth week and that of group C on the sixth week (Fig. 2f), with perisinusoidal fibrosis. No hypoxic hepatitis appeared in group C at every monitoring point. No signs of bridging fibrosis and liver cirrhosis were noted.
Histological section of rat livers. a Group A (the control group), 6 weeks, the rat normal liver cell HE, ×200. b Group A (the control group), 9 weeks, the rat normal liver cell Masson’s trichrome, ×200. c Group B (the high fat group), 6 weeks, steatosis of liver cells, cell swelling HE, ×200. d Group B (the high fat group), 9 weeks, mild hyperplasia in periportal collagen fibers Masson’s trichrome, ×100. e Group C (the high fat plus intermittent hypoxia group), 9 weeks, steatosis of liver cells, cell swelling, inflammatory cell infiltration, focal necrosis (arrow) HE, ×200. f Group C (the high fat plus intermittent hypoxia group), 9 weeks, hyperplasia in periportal collagen fibers, stained blue Masson’s trichrome, ×200
Transmission electron microscopy findings
No abnormal liver tissue ultrastructure was found in group A (Fig. 3a). In group B, at the end of the third week, slight injury in liver tissue, mainly manifested by mild steatosis, was observed; at the end of the sixth week, the amount of liver cell cytoplasm lipid droplets increased obviously (Fig. 3b), and liver sinusoid and the Disse space enlarged. At the end of the ninth week, accumulation of liver cell cytoplasm lipid droplets, lipid droplets fusion, and decreased cytoplasmic organelles were observed; hepatocellular nuclei became smaller for being squeezed; liver sinusoid and the Disse space enlarged; and collagen fiber proliferation, sinus endothelial cells degeneration, Kupffer cells proliferation, and edema were noted. Regarding the liver tissue ultrastructure in group C, the liver tissue injury was more severe than that in group B at identical monitoring points. At the end of the third week, liver steatosis was more severe than that in group B. There were also a large amount of liver cell cytoplasm lipid droplets, lipid droplets combined into irregular big lipid droplets, and small nuclei with irregular shapes, and the liver sinusoid and the Disse space enlarged. Also noted were extreme hyperplasia in liver cell sinus surface microvillus, sinus endothelial cell degeneration, Kupffer cells proliferation, and myelin figures in sinus. At the end of the sixth week, the amount of liver cell cytoplasm lipid droplets increased obviously, and there were increased myelin figures, irregularly shaped nuclei (Fig. 3c), serious injury in sinus endothelial cell, cytoplast aggregation, irregular enlargement of cell union, liver sinusoid and the Disse space enlargement, and collagen fiber proliferation. On the ninth week, liver tissue injury was the most serious, full of lipid droplets in the liver cell cytoplasm, greatly different in size; irregularly shaped nuclei for being squeezed, and small and few cell organs. There were also obvious enlargement in liver sinusoid, scattered activated platelets, and obvious enlargement of the Disse space, containing large bundles of collagen fibers (Fig. 3d).
Ultrastructure of rat livers. a Group A (the control group) rat normal liver cell ultrastructure electron microscope ×7,500. b Group B (the high fat group), 6 weeks, a large amount of lipid droplets in liver cell cytoplasm, some lipid droplets fused into clusters electron microscope ×4,950. c Group C (the high fat plus intermittent hypoxia group), 6 weeks, a large amount of lipid droplets in liver cell cytoplasm, visible myelin figures in some lipid droplets (arrow), small and irregular cell nucleus electron microscope ×9,900. d Group C (the high fat plus intermittent hypoxia group), 9 weeks, huge collagen fiber bundle (arrow) in a Disse space electron microscope ×9,900
Comparison of hepatic histological changes among the three groups
The hepatic histology in group A was found normal. The rats in group B were found to have steatosis at the end of the third week, which became more severe with increased exposure time, and at the end of the ninth week, steatohepatitis was observed. In group C, the rats were only found to have steatosis at the end of the third week, steatohepatitis was observed at the end of the sixth week, and liver tissue injury was most serious at the end of the ninth week. Diffuse steatosis, obvious cell swelling, inflammatory cell infiltration, focal necrosis, and periportal and perisinusoidal fibrosis were found. See Table 2 for the liver tissue changes of the three groups.
Immunohistochemistry
MPO-positive expression shows the cytoplasm to be brown-yellow in color and negative cells blue. For the quantitative analysis, the MPO-positive cells were counted under ×200 magnification in ten randomly chosen fields. The results were expressed as the mean percentage of MPO-positive cells. On the ninth week, the MPO-positive expression rates in the liver tissue of the three groups are 2.41 ± 0.84, 40.31 ± 6.86, and 70.73 ± 12.09, respectively, F = 145.35, P < 0.01 (Fig. 4).
The myeloperoxidase (MPO) expression of rat livers. MPO immunohistostaining: a the control group, b the high fat group, and c the high fat plus intermittent hypoxia group. d On the ninth week, the MPO-positive expression rates in the liver tissue of the three groups were statistically significantly different, P < 0.01
Discussion
This study has dynamically observed the influence of the CIH on Wistar rat liver histopathology based on lipid disorders. The result shows that after being exposed to a high fat diet plus intermittent hypoxia for 3 weeks steatosis was observed among rats. After 6 weeks, features of steatohepatitis such as hepatocellular swelling, inflammatory cell infiltration, and focal necrosis within the hepatic lobule were exhibited; after 9 weeks, the liver tissue injury became more severe and steatosis became diffused, and obvious cell swelling, inflammatory cell infiltration, focal necrosis, and periportal and perisinusoidal fibrosis were found. While rats fed simply with high fat diet have shown steatohepatitis after 9 weeks, they are obviously slighter than the rats in the high fat plus intermittent hypoxia group. Our data indicate that in histopathology the SAHS injury to the liver is mainly manifested by NAFLD, including the process from simple fatty liver to steatohepatitis and hepatic fibrosis. The intermittent hypoxia of sleep apnea promotes progression from simple fatty liver to steatohepatitis and hepatic fibrosis. The results of the present study are consistent with the established literature [6–9]. No liver cirrhosis was observed at the end of the experiment, which may due to the not-so-sufficient exposure time.
The study has observed the influence of CIH on the ultrastructure of the liver cell. Based on lipid disorders, CIH may cause obvious steatosis of the liver cells, the deformation and irregularity of the liver cell nucleus, the shrinkage of the cytoplasmic organelles, necrosis of sinus endothelial cells, irregular enlargement of cell union, and enlargement of liver sinusoid and the Disse space with large bundle collagen fiber hyperplasia. The observations are consistent with ultrastructural features of NAFLD.
Serum transaminase is a sensitive indicator of liver cell injury. The present study shows that after 3 weeks of high fat food intake and intermittent hypoxia ALT and AST increase; after 9 weeks, the levels of ALT and AST are four times higher than that of the control group and two times higher than that of the high fat group, which is consistent with the findings of Savransky et al. [9]. Our data have shown evidence that CIH may promote injury of the liver. The injury mechanism has a close relationship with CIH resulting to histological changes. Abnormal liver enzymes are one of the basic clinical manifestations of NAFLD.
In our experiment, with the lengthening of the exposure time of intermittent hypoxia, the levels of ALT began to increase on the third week. On the sixth week, the peak was reached. During the sixth and ninth weeks, ALT levels maintained in a state of plateau (although the value of ALT tended to decrease on the ninth week, there was no statistical difference compared to the condition of the sixth week, P > 0.05). However, the levels of AST continued to increase from the third week to the ninth week. The fact that the changes of ALT and AST are not fully consistent maybe related to their different patterns of distribution in hepatocytes. In hepatocytes, ALT mainly exists outside the mitochondria. However, about 80% of AST exists inside the mitochondria. As shown in our experiment, chronic intermittent hypoxia can cause obvious damage to the mitochondria of hepatocytes. Especially on the ninth week of the experiment, the transmission electron microscope shows that the cytoplasm of hepatocytes is full of lipid vesicle and the organelle is small both in size and number, with little mitochondria. It is due to the fact that the damage to the mitochondria of hepatocytes is continually getting more serious, causing the value of AST to increase from the third week to the ninth week.
The liver is a hypoxia-sensitive organ. A sustained severe hypoxia or ischemia can lead to hypoxic hepatitis. The term hypoxic hepatitis is due to acute ischemia and hypoxia of the liver which will lead to acute liver cell degeneration, necrosis, and liver damage, often caused by congestive heart failure, respiratory failure, and shock by a variety of reasons. It is histologically characterized as various degrees of centrilobular liver cell necrosis. In the study, the rats exposed to CIH have not shown histological changes of hypoxic hepatitis. According to recent data, the histological evidence of SAHS leading to liver injury mostly focuses on NAFLD, but there are a few case studies reporting that serious SAHS leads to the histological changes of hypoxic hepatitis. In a prospective study of 142 cases of hypoxic hepatitis, Henrion et al. have found that two of them were caused by severe SAHS with serious obesity [16]. Oxygen arterial saturation was very low, less than 35% in both patients, and there was no evidence of heart failure and shock. The causes are speculated as follows: intermittent hypoxia component of sleep apnea is different from serious sustained hypoxia on injury to the liver. The former may cause NAFLD, while the latter may cause hypoxic hepatitis. CIH of sleep apnea is characterized by repetitive cycles of hypoxia and reoxygenation during sleep, but patients with extreme severe SAHS cannot recover to normal blood oxygen saturation after hypoxia, which will form a serious sustained hypoxia and thereby cause hypoxic hepatitis.
The pathogenesis and progression of NAFLD has been postulated as the result of a “two-hit” process [17, 18]. The insulin resistance associated with obesity, diabetes, hyperlipidemia, and other factors causes hepatic fat accumulation and forms hepatic steatosis as well as simple fatty fiver, which is the “first hit”. Although the liver with steatosis easily develops into steatohepatitis, in some individuals, steatosis, irrespective of its etiology, never progresses to steatohepatitis. In other words, the process is not inevitable. It depends on the existence of the “second hit”. The “second hit” comes from the increase of the reactive oxygen species (ROS) caused by other causes. ROS-induced oxidative stress is capable of initiating enough lipid peroxidation to overcome the normal cellular defense mechanisms, producing necroinflammation and forming steatohepatitis.
The reported association between CIH and liver injury is consistent with the “two-hit” hypothesis explaining the pathogenesis of NAFLD. For the insulin resistance resulting from dyslipidemia and intermittent hypoxia [19], the treated rats start fat accumulation in liver cells and form hepatic steatosis, which is the “first hit”. SAHS is an oxidative stress disorder [20]. Numerous researches have demonstrated that repeated hypoxia/reoxygenation process of SAHS is similar to ischemia/reperfusion injury in that it can produce large amounts of reactive oxygen species (ROS) and thereby lead to oxidative stress status [20–23]. ROS may induce hepatic lipid peroxidation, which can lead to hepatocyte degeneration and necrosis, inflammation, and liver fibrosis [24]. In order to prove that CIH can increase liver oxidative stress, we monitored the MPO level of hepatic tissues of rats exposed to CIH. MPO is an important peroxidase produced in the azurophilic granule of neutrophils. Under physiological conditions, MPO catalyzes the reaction between chlorine and hydrogen peroxide and produces hypochlorous acid and various free radicals. Overexpression of MPO is an important index of oxidative stress of the body [25]. As revealed by our study, CIH increases the expression of MPO in liver tissue, which indicates the increase of liver lipid peroxidation. So the oxidative stress caused by CIH constitutes the “second hit”, which leads to the progression of simple fatty liver into steatohepatitis and liver fibrosis. The existence of fatty liver is the basis of the progress in which the CIH leads to liver injury. The study of Savransky et al. also revealed that intermittent hypoxia alone did not lead to significant liver injury, while steatosis caused by high cholesterol feed constituted the “first fit”. Based on this, CIH, as the “second hit”, caused the liver to progress from simple fatty liver to steatohepatitis and liver fibrosis [8, 9]. From this, we conclude that the insulin resistance of SAHS patients caused by obesity, lipid metabolism disorder, and intermittent hypoxia becomes the basic mechanism of fatty liver, and the oxidative stress caused by CIH of sleep apnea constitutes the “second hit”, causing the progression of simple fatty liver into steatohepatitis and liver fibrosis.
The blood lipid test results of this study show that there is no significant difference for the control group at every monitoring point in TC and LDL-C. The high fat group and the high fat plus intermittent hypoxia group started to increase from the third week and reached the peak on the sixth week, but went down on the ninth week even lower than the level on the third week. The reasons may include as follows: (1) reduction in endogenous lipid synthesis: injury of liver function and structure affects the liver lipid anabolism; (2) the reduction of exogenous lipid intake: as the liver injury gets more severe, the rats in the two groups have shown obvious signs of anorexia and the amount of food intake is reduced; and (3) inducible enzyme metabolism. Weight change also shows that the weight of the rats in the high fat plus intermittent hypoxia group and the high fat group increased slower on the ninth week. Nonetheless, the NAFLD of rats in the two groups progressively develops, which demonstrates that the change of blood fat does not block the development of NAFLD. Another limitation of the study is the frequency of the intermittent hypoxia in the experiment being 60 times per hour, which matches severe SAHS clinical features, but the study does not refer to liver injury under the conditions of moderate and slight intermittent hypoxia. The influence on liver injury by different hypoxia conditions needs further research.
In conclusion, this study has observed the dynamic evolution in which rat liver injury develops from simple fatty liver into steatohepatitis and finally into liver fibrosis under the conditions of high fat feed plus CIH. It suggests that CIH has a natural relationship with liver injury, which is mainly characterized by NAFLD; CIH leads to the strengthening of the response of liver oxidative stress and supports the assumption that CIH causes liver injury through the oxidative stress pathway. Thus, we speculate that obstructive sleep apnea (OSA) patients have potential hepatic injuries, especially those with hyperlipidemia. OSA may act as one of the causes for the initiation and development of NAFLD. OSA patients should be advised to pay enough attention to changes in liver function.
References
Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O’Connor GT, Boland LL, Schwartz JE, Samet JM (2001) Sleep-disordered breathing and cardiovascular disease cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 163:19–25
Lavie L (2004) Sleep apnea syndrome, endothelial dysfunction, and cardiovascular morbidity. Sleep 27:1053–1055
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353:2034–2041
McNicholas WT, Bonsignore MR, Management Committee of EUCOST ACTION B26 (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29:156–178. doi:10.1183/09031936.00027406
Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053
Chin K, Nakamura T, Takahashi K, Sumi K, Ogawa Y, Masuzaki H, Muro S, Hattori N, Matsumoto H, Niimi A, Chiba T, Nakao K, Mishima M, Ohi M, Nakamura T (2003) Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med 114:370–376. doi:10.1016/S0002-9343(02)01570-X
Tanne F, Gagnadoux F, Chazouilleres O, Fleury B, Wendum D, Lasnier E (2005) Chronic liver injury during obstructive sleep apnea. Hepatology 41:1290–1296. doi:10.1002/hep.20725
Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, Polotsky VY (2007) Chronic intermittent hypoxia predisposes to liver injury. Hepatology 45:1007–1013. doi:10.1002/hep.21593
Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY (2007) Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293:G871–877. doi:10.1152/ajpgi.00145.2007
Norman D, Bardwell WA, Arosemena F, Nelesen R, Mills PJ, Loredo JS, Lavine JE, Dimsdale JE (2008) Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. Sleep 31:121–126
Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ (2007) Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol 41:918–921. doi:10.1097/01.mcg.0000225692.62121.55
Mishra P, Nugent C, Afendy A, Bai C, Bhatia P, Afendy M, Fang Y, Elariny H, Goodman Z, Younossi ZM (2008) Apnoeic–hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int 28:1080–1086. doi::10.1111/j.1478-3231.2008.01822.x
Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, Steele KE, Schweizter MA, Clark JM, Torbenson MS, Schwartz AR (2009) Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med 179:228–234
Brunt E, Janney C, Di Bisceglie A, Neuschwander-Tetri B, Bacon B (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321. doi:10.1002/hep.20701
Henrion J, Colin L, Schapira M, Heller FR (1997) Hypoxic hepatitis caused by severe hypoxemia from obstructive sleep apnea. J Clin Gastroenterol 24(4):245–249
Day CP, James OFW (1998) Steatohepatitis: a tale of two “hits”? Gastroenterology 114:842–845
Day CP, Saksena S (2002) Non-alcoholic steatohepatitis: definitions and pathogenesis. J Gastroenterol Hepatol 17:377–384. doi:10.1046/j.1440-1746.17.s3.31.x, http://www3.interscience.wiley.com/doiinfo.html
Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS (2002) Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 165:670–676. doi:10.1164/rccm.2103001
Lavie L (2003) Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7:35–51. doi:10.1053/smrv.2002.0261
Lavie L, Vishnevsky A, Lavie P (2004) Evidence for lipid peroxidation in obstructive apnea. Sleep 27:123–128
Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM (2005) Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172:915–920. doi:10.1164/rccm.200504-560OC
Suzuki YJ, Jain V, Park AM, Day RM (2006) Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med 40:1683–1692. doi:10.1016/j.freeradbiomed.2006.01.008
Solis Herruzo JA, Garcia Ruiz I, Perez Carreras M, Muñoz Yagüe MT (2006) Non-alcoholic fatty liver disease. From insulin resistance to mitochondrial dysfunction. Rev Esp Enferm Dig 98:844–863
Winterbourn CC, Kettle AJ (2004) Reactions of superoxide with myeloperoxidase and its products. Jpn J Infect Dis 57:S31–S33
Acknowledgments
The authors thank Ms. Hai-xian Yang and Ms. Yu-tong Gao of Tianjin Medical University for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Feng, Sz., Tian, Jl., Zhang, Q. et al. An experimental research on chronic intermittent hypoxia leading to liver injury. Sleep Breath 15, 493–502 (2011). https://doi.org/10.1007/s11325-010-0370-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-010-0370-3