Abstract
Purpose
The objective of the study is to test the hypothesis that a level 2 portable sleep device (Embletta X100) is a reliable alternative for standard PSG in surgical patients.
Methods
After hospital ethics approvals, preoperative patients over 18 years old were recruited. The patients for validation underwent standard PSG and Embletta X100 simultaneously in a sleep laboratory before surgery. The other patients received sleep studies with Embletta X100 perioperatively. The correlation analysis and paired Student t test between variables from Embletta and from standard PSG were used to evaluate the accuracy of Embletta. The quality of PSG recordings with Embletta was summarized.
Result
Twenty-one patients completed sleep study on both systems; ten females and ten males, age was 54 ± 11 and BMI was 36 ± 9. There was a significant correlation between the majority of parameters from standard PSG and Embeltta X100 with manual scoring. The inter-rater agreement was substantial to perfect at different AHI cutoffs with a Kappa coefficient of 0.69 to 1. A significant correlation between standard PSG and Embletta X100 with automatic scoring was found only in AHI and a few other parameters. In 385 patients, 1,002 perioperative PSG recordings were carried out with Embletta. Of them, 889(88.7%) were technically good and 90(9%) technically acceptable. Only 23 (2.3%) PSG recordings failed.
Conclusion
Embletta X100, installed by a well-trained sleep technician, is a good alternative when standard PSG was not available or impractical. Manual scoring by a certified PSG technologist is the key for reliable results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a systemic syndrome characterized by episodes of apnea and hypopnea during sleep, due to complete or partial pharyngeal obstruction [1]. From estimations in the general population, moderately severe OSA with an apnea–hypopnea index (AHI) >15 is present in 11.4% of men and 4.7% of women [2, 3]. With the trend of increasing prevalence of obesity and aging of the population, the incidence of clinically significant OSA is expected to increase five- to tenfold over the next decade [4]. There is growing evidence implicating OSA as a causal pathway to the development of cardiorespiratory diseases [5–12]. and it is estimated that nearly 80% of men and 93% of women with moderate to severe sleep apnea are undiagnosed [4].
In-laboratory polysomnography (PSG) is the gold standard for diagnosing OSA [13]. However, in-laboratory PSG is a time-consuming and costly procedure. Furthermore, the growing awareness of sleep apnea has exacerbated the long waiting list in many sleep laboratories [14]. In-laboratory PSG is also not practical in some situations, such as postoperative sleep evaluation. To deal with this issue, a number of portable sleep monitoring devices have been developed [15–20]. To provide evidence-based recommendations on the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients, a clinical guideline has been published by the Portable Monitoring Task Force of the American Academy of Sleep Medicine in 2007 [21–24]. Since then, many new models of portable sleep monitors have been developed.
Embletta with proxy X100 is a new portable sleep study device with ten channels, which is a level 2 diagnostic tool [25]. Although different models of this family have been evaluated and used in research and clinical settings [26–33], there is no available published literature to validate this model of Embletta.
Patients coming to surgery may have increased risk of obstructive sleep apnea. Patients with obstructive sleep apnea are known to have an increased risk of perioperative complications [34–37]. Therefore, preoperative screening for obstructive sleep apnea is essential and the American Society of Anesthesiologists has published a guideline recommending preoperative screening for obstructive sleep apnea [38]. The STOP questionnaire [39], a four-question formatted with yes/no answer has been used as a screening tool in many preoperative clinics. In those surgical patients with the preoperative clinical suspicion of OSA, portable PSG offers an alternative tool in diagnosing OSA. This will expand the realm of anesthesiologists as a perioperative physician. The objective of the study is to test the hypothesis that installed by well-trained technicians, a level 2 portable sleep device (Embletta X100) is a reliable and practical alternative for standard in-laboratory PSG.
Methods
Study subjects
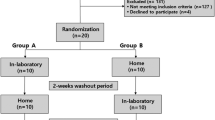
The study was carried out in Toronto Western Hospital and Mount Sinai Hospital, Toronto. Ethics approvals were obtained from both institutions. Patients 18 years or older, who were ASA I–IV and scheduled for elective procedures in general surgery, orthopedics, urology, plastic surgery, gynecology, spine surgery, or neurosurgery were included for the study. Patients who were unwilling or unable to give informed consent, patients previously diagnosed with OSA or any other sleep breathing disorder, or patients who were expected to have abnormal EEG findings (e.g., brain tumor, epilepsy surgery, patients with deep brain stimulator) were excluded. All patients who visited the preoperative clinics for their scheduled surgery and met the inclusion criteria were approached by the research staff. After informed consent was obtained, patients were invited to undergo one to five nights of sleep study using the Embletta X-100.
For the patients recruited for validation study, they underwent standard PSG and Embletta X100 simultaneously in a sleep laboratory before surgery. The other patients received sleep studies with Embletta X100 preoperatively at home. Some patients had sleep studies with Embletta X100 on postoperative nights 1, 3, 5, and 7. In patients with positive PSG indicating clear pathology, their family physicians were notified so that the patients could be referred to sleep physicians regarding further clinical management.
The standard in-laboratory PSG
A one-night, in-laboratory PSG was conducted before surgery at Toronto Western Hospital Sleep Research Laboratory. During the overnight PSG study, every patient went to bed at his/her usual bedtime. Collection of continuous sleep architectural data was accomplished using a standard EEG montage consisting of an electroencephalogram, electro-oculogram, submental electromyogram and electrocardiogram using surface electrodes. Ancillary channels were used to specifically record respiratory parameters including respiratory effort by thoracoabdominal excursion, respiratory inductive plethysmography, and oronasal airflow by nasal airflow pressure. Oxygen saturation was measured with a pulse oximeter.
One certified PSG technologist with 10 years experience scored all the sleep records under the supervision of a sleep physician (C.M.S.). The certified technologist was blinded to the results of the sleep study conducted with Embletta X100 and clinical information of the patients. Sleep stages and the AHI were scored according to the standard criteria [40].
Ambulatory PSG
Full-night unattended PSG recording with Embletta X100 were performed at the patient’s home preoperatively and on postoperative nights 1, 3, 5, or 7 in hospital or at home when feasible. The PSG recording montage consisted of two electroencephalographic channels (C3 and C4), left or right electroculogram, chin muscle electromyograms, nasal cannula (pressure), thoracic and abdominal respiratory effort bands, body-position sensor, and pulse oximetry. The device measures the oxyhemoglobin saturation at a rate of three samples per second.
At bedtime, the device was hooked up to the patients by well-trained PSG technicians. The overnight recording was unattended. The patients were taught how to dismantle the device, which was picked up by the same sleep technician or study coordinator the following morning. The patients were asked to keep a sleep diary (Appendix 1). The sleep technician or study coordinator picking up the device ensured that the sleep diary was completed.
One certified PSG technologist with more than 8 years experience scored recordings from Embletta. He was blinded to the clinical information and the results of in-laboratory PSG. The scoring of recordings from Embletta was carried out in two ways: manual scoring and automatic scoring. Somnologia Studio 5.0 was the scoring platform for both scoring methods. In manual scoring (manual-scoring), the PSG recording was manually scored epoch by epoch by the PSG technologist, according to the manual published in 2007 by American Academy of Sleep Medicine [40]. In automatic scoring (auto-scoring), the PSG recording was scored fully with Somnologia Studio 5.0.
Data analysis
Sample size estimation
According to a previously published study to validate an Embletta model with nasal pressure transducer, thoraco-abdominal movement detection, oximeter, and body position detection [24], the correlation coefficient between AHI from standard PSG and Embletta was 0.98. If we consider that the null Pearson coefficient is 0.3, the expected Pearson correlation coefficient is 0.8, alpha = 0.05, power = 0.9 and with two sides; the estimated sample size will be 20.
The demographic data is presented with descriptive statistics, mean ± SD was used for continuous data with normal distribution, median (inter-quartile range, IQR) for continuous data with skewed distribution, and frequency (%) for categorical data. The correlation analysis and paired Student t test between variables from Embletta with manual scoring or automatic scoring and the corresponding variables from standard laboratory PSG were used to evaluate the accuracy of Embletta with manual or automatic scoring. Bland Altman analysis for AHI and the agreement between two methods at different AHI cutoffs was also analyzed. Descriptive statistics with frequency of different quality category of PSG recording with Embletta was summarized across the different types of PSG.
Results
Validation of Embletta
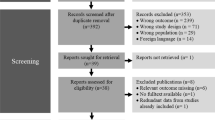
Twenty-four patients were recruited for the validation study. Of them, three patients did not successfully complete both in-laboratory PSG and Embletta PSG: standard in-laboratory PSG not done due to a misunderstanding between technicians in one patient, Embletta did not start recording in one patient, and Embletta only recorded for 40 min in another patient. Twenty-one patients who had complete sleep study with good quality of PSG recordings on both systems were included in the final data analysis. There were ten females and 11 males. The average age was 54 ± 11. Body mass index was 36 ± 9 kg/m2.
Table 1 summarizes the results of correlation analysis and paired Student t test in sleep parameters from in-laboratory standard PSG and Embletta with manual scoring. Except for the central apnea index, mixed apnea index, average duration for apnea hypopnea episodes, longest duration for apnea–hypopnea episodes, and average wake SaO2; all the corresponding parameters analyzed from the two methods are significantly correlated. This suggests that results from the Embletta are good predictors for the results of laboratory PSG. However, at the same time, there was a significant difference of absolute value between the two methods in sleep latency, sleep stage 1, AHI, NREM AHI, respiratory arousal index and spontaneous arousal index. For example, AHI from Embletta with manual scoring was higher than that from laboratory PSG: 11.5 (18.7) vs. 8.1(18.3) in median (IQR). The mean difference is 2.3 ± 4.7 (mean ± SD) or 1.2(2.6) (median(IQR)), p = 0.038. The relationship for AHI was further demonstrated in Fig. 1a and b.
a Scatter plot of apnea-hypopnea index (AHI) recorded by laboratory standard PSG and Embletta with manual scoring. b Bland-Altman analysis of apnea hypopnea index (AHI) recorded by laboratory standard PSG and Embletta with manual scoring. The difference was calculated as AHI from Embletta (manual scoring) minus AHI from laboratory PSG
The inter-rater agreement between the laboratory PSG and Embletta with manual scoring at AHI > 5, AHI > 10, and AHI > 15 as cutoffs was almost perfect with Kappa coefficient = 1 for AHI > 5 and AHI > 15, and 0.811(0.566–1.000) for AHI > 10. The inter-rater agreement at AHI > 30 was substantial with Kappa coefficient = 0.69 (95% CI: 0.29–1.00; Table 2).
Table 3 shows the comparison between parameters from laboratory PSG and Embletta with automatic scoring. A significant correlation was observed in sleep period time, AHI, REM AHI, NREM AHI, obstructive apnea index, hypopnea index, and lowest SpO2. There was no correlation between any sleep architecture parameters from two methods. There was no significant difference in REM AHI, NREM AHI, obstructive apnea index, average SpO2 and lowest SpO2 from both methods. The difference of AHI (AHIEmblettaAuto – AHILab) was −3.7 ± 7.7 (mean ± SD ) or −0.9(7.0) (median(IQR)). The relation of AHI from two methods was further demonstrated in Fig. 2a and b. The inter-rater agreement between laboratory PSG and Embletta with autoscoring was moderate to substantial at different AHI cutoffs (Table 2).
a Scatter plot of apnea-hypopnea index (AHI) recorded by laboratory standard PSG and Embletta with automatic scoring. b Bland-Altman analysis of apnea hypopnea index (AHI) recorded by laboratory standard PSG and Embletta with automatic scoring. The difference was calculated as AHI from Embletta (automatic scoring) minus AHI from laboratory PSG
The quality of Embletta PSG
In 385 patients, 1,002 perioperative PSGs were carried out with Embletta: preoperative—385, postoperative night 1—298, night 3—208, night 5—56, night7—55. There were 204 females and 181 males. The average age was 59 ± 13 years and body mass index was 39 ± 5 kg/m2.
The quality of 1002 PSG recordings was summarized in Table 4. The majority of PSG recordings (88.7%) were technically good, which is defined as more than 4 h recording with good quality on all channels. Nine percent of PSG recordings were technically acceptable, which is defined as more than 4 h recording with defect on one or two channels, but the AHI was still viewed as reasonably reliable. This includes the PSG recordings with no thorax and/or abdominal effort recording, no EOG, heavy EKG artifact on EEG, no EMG, or only one channel of EEG. Only 23 (2.3%) PSG recordings with Embletta failed, including 6 (0.6%) without EEG recording, 4 (0.4%) with battery failure within 4 h and 13 (1.3%) with electrodes removed by patients within 4 h. The PSG recordings on nights 5 and 7 after surgery had a higher rate of PSG recordings with good quality. The scoring of AHI was viewed as reliable in 98.2% of home PSG recordings.
Discussion
This study demonstrated that the parameters of sleep architecture, sleep respiratory events, arousals, and pulse oxygen saturation level from manually scored PSG recording with Embletta X100 were highly correlated with the those from in-laboratory standard PSG . However, the AHI was overestimated by Embletta X100 by 2.3. It was also noted that the significant correlation between the parameters from Embletta X100 with automatic scoring and standard PSG was found in sleep period time, AHI, REM AHI, NREM AHI, obstructive apnea index , hypopnea index, and lowest SpO2. The automatic scoring by Somnologia Studio 5.0 is not reliable, especially for the sleep architecture. Out of a total of 1,002 recordings with Embletta X100, 88.7% of PSG recordings was technically good for sleep architecture and AHI scoring. The scoring of AHI was reliable in another 9% of recordings with minor technical defects. The major limitation for the study is that the validation sample size was not big enough to do predictive analysis and receiver–operator characteristic (ROC) analysis.
Based on these results, when it is installed by a well-trained technician, Embletta X100 has a high rate of good-quality recordings and can be used as an alternative when in-laboratory standard PSG is not available. The recordings should be scored by a certified PSG technologist and reviewed by a physician trained in sleep medicine. Total automatic scoring by Somnologia Studio 5.0 is not reliable, especially regarding the sleep architecture. However, the absolute value from Embletta is not equal to the value from standard PSG. Some adjustments may be needed.
The major focus of previous studies on portable sleep devices is the ability of the devices to detect sleep breathing disorder [16, 22, 41, 42]. Tiihonen and colleagues reported that 77.2% of PSG recording with a type 3 Embletta was technically good and 80.8% of recordings was diagnostically acceptable in technician-assisted installation [22]. Our study comprehensively compared major sleep parameters between two systems. This provides valuable information on the accuracy of portable sleep study device on the sleep architecture parameters. Compared with a previous study [22], the rate of technically good and diagnostically acceptable recordings was higher in our study, 88.7% and 97.7%, respectively. We trace this to the well-trained PSG technicians, education and cooperation of patients, and continuous feedbacks from the scoring technologist. The PSG recordings done on the 5th and 7th night after surgery had a higher percentage of good quality recordings. This indicated that patient's experience with the system could increase the success rate of unattended PSG recording. The patients on the first and third postoperative night had significant pain and this may reduce the level of compliance and recording quality.
The high correlation between the results from manually scored PSG recordings using Embletta X100 and laboratory standard PSG suggests that if Embletta X100 is used properly, it could provide useful information of sleep physiology, including sleep stages, sleep respiratory events, arousals, and oxygen saturation level. However, we could not find a significant correlation in central sleep apnea and mixed sleep apnea detected by Embletta X100 and laboratory PSG. Since the frequency of central apnea and mixed sleep apnea in the study patients was very low (close to 0), we may need a bigger sample size or a study in patients with higher frequency of central sleep apnea to further evaluate the ability of Embletta on detecting central sleep apnea.
Although the difference of AHI between Embletta with manual scoring and laboratory-standard PSG was statistically significant, it is comparable with previously published studies on other portable devices, [16, 22, 41, 42]. There were several possible reasons for the difference. The most significant one is that the scoring platform is different. Embletta recording was scored on Somnologia Studio 5.0 and the standard PSG was scored on Sandman version 7.2. The other possible causes include the difference between PSG technologists and different sensitivity of nasal flow sensor and oximeter. Collon demonstrated that there was a significant variation in AHI and sleep stages from the same PSG recordings scored by different PSG technologists [43]. The difference suggests that validation and possible adjustment is necessary before adopting a portable system.
When coming to inter-rater agreement between Embletta X-100 and laboratory PSG in terms of diagnosing OSA at different cutoffs of AH, it was almost perfect to recognize all patients with OSA (Kappa Coefficient=1). Although the performance of Embletta to identify the patients with severe OSA was not as perfect as to recognize all patients with OSA, it is acceptable and better than the performance of electrocardiography to detect the pathologic Q-wave in myocardial infarction patients ( Kappa Coefficient = 0.64) [44]
Our results also support that fully automatic scoring by Somnologia Studio 5.0 is not as reliable, especially on sleep stages. However, there was also a significant correlation between results from the automatic scoring of Embletta PSG and standard laboratory PSG in AHI, REM AHI, NREM AHI and lowest SpO2. This indicates that when manual scoring by a certified technologist is not available, AHI and lowest SpO2 from the automatic scoring with Somnologia Studio 5.0 could be used. But caution must be taken.
In conclusion, Embletta X100 installed by a well trained sleep technician is a good alternative when standard PSG was not available or impractical. Manually scoring by a certified PSG technologist is the key for reliable results. There is a potential of significant difference between two systems, so that validation is necessary.
References
(1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667-689
Bixler EO, Vgontzas AN, Ten HT, Tyson K, Kales A (1998) Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 157:144–148
Bixler EO, Vgontzas AN, Lin HM, Ten HT, Rein J, Vela-Bueno A, Kales A (2001) Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163:608–613
Young T, Evans L, Finn L, Palta M (1997) Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 20:705–706
Bresnitz EA, Goldberg R, Kosinski RM (1994) Epidemiology of obstructive sleep apnea. Epidemiol Rev 16:210–227
Simonds AK (1994) Sleep studies of respiratory function and home respiratory support. BMJ 309:35–40
Hung J, Whitford EG, Parsons RW, Hillman DR (1990) Association of sleep-apnea with myocardial-infarction in men. Lancet 336:261–264
Doherty LS, Kiely JL, Swan V, McNicholas WT (2005) Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 127:2076–2084
Leung RS, Bradley TD (2001) Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 164:2147–2165
Moruzzi P, Sarzi-Braga S, Rossi M, Contini M (1999) Sleep apnoea in ischaemic heart disease: differences between acute and chronic coronary syndromes. Heart 82:343–347
Schafer H, Koehler U, Ploch T, Peter JH (1997) Sleep-related myocardial ischemia and sleep structure in patients with obstructive sleep apnea and coronary heart disease. Chest 111:387–393
Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM (2008) Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31:1071–1078
(1997) Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep 20:406-422
Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J (2004) Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med 169:668–672
Santos-Silva R, Sartori DE, Truksinas V, Truksinas E, Alonso FF, Tufik S, Bittencourt LR (2009) Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep 32:629–636
Yin M, Miyazaki S, Ishikawa K (2006) Evaluation of type 3 portable monitoring in unattended home setting for suspected sleep apnea: factors that may affect its accuracy. Otolaryngol Head Neck Surg 134:204–209
Popovic D, King C, Guerrero M, Levendowski DJ, Henninger D, Westbrook PR (2009) Validation of forehead venous pressure as a measure of respiratory effort for the diagnosis of sleep apnea. J Clin Monit Comput 23:1–10
Westbrook PR, Levendowski DJ, Cvetinovic M, Zavora T, Velimirovic V, Henninger D, Nicholson D (2005) Description and validation of the apnea risk evaluation system: a novel method to diagnose sleep apnea-hypopnea in the home. Chest 128:2166–2175
Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP (2004) A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep 27:1560–1566
Ayas NT, Pittman S, MacDonald M, White DP (2003) Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med 4:435–442
Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R (2007) Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable monitoring task force of the American academy of sleep medicine. J Clin Sleep Med 3:737–747
Tiihonen P, Hukkanen T, Tuomilehto H, Mervaala E, Toyras J (2009) Evaluation of a novel ambulatory device for screening of sleep apnea. Telemed J E Health 15:283–289
Bridevaux PO, Fitting JW, Fellrath JM, Aubert JD (2007) Inter-observer agreement on apnoea hypopnoea index using portable monitoring of respiratory parameters. Swiss Med Wkly 137:602–607
Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, Douglas NJ (2003) Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J 21:253–259
Littner MR (2005) Portable monitoring in the diagnosis of the obstructive sleep apnea syndrome. Semin Respir Crit Care Med 26:56–67
Endeshaw YW, White WB, Kutner M, Ouslander JG, Bliwise DL (2009) Sleep-disordered breathing and 24-hour blood pressure pattern among older adults. J Gerontol A Biol Sci Med Sci 64:280–285
Ruberto M, Caputi M, Fiorentino G, Vessella W, Liotti F (2007) Obstructive sleep apnea syndrome (OSAS) and work. G Ital Med Lav Ergon 29:836–838
Smith LA, Chong DW, Vennelle M, Denvir MA, Newby DE, Douglas NJ (2007) Diagnosis of sleep-disordered breathing in patients with chronic heart failure: evaluation of a portable limited sleep study system. J Sleep Res 16:428–435
Zvartau NE, Conrady AO, Sviryaev YV, Rotari OP, Merkulova NK, Kalinkin AL, Shlyakhto EV, Bagrov AY (2006) Marinobufagenin in hypertensive patients with obstructive sleep apnea. Cell Mol Biol (Noisy-le-grand) 52:24–27
Abdelghani A, Roisman G, Escourrou P (2007) Evaluation of a home respiratory polygraphy system in the diagnosis of the obstructive sleep apnea syndrome. Rev Mal Respir 24:331–338
Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V (2007) Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 9:251–257
Shen QB, Xu DL, Lin S, Lai WY (2006) Sleep-disordered breathing and left ventricular remodeling in patients with chronic heart failure. Nan Fang Yi Ke Da Xue Xue Bao 26:486–489
Endeshaw YW, Katz S, Ouslander JG, Bliwise DL (2004) Association of denture use with sleep-disordered breathing among older adults. J Public Health Dent 64:181–183
Gupta RM, Parvizi J, Hanssen AD, Gay PC (2001) Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc 76:897–905
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM (2008) Validation of Berlin questionnaire and ASA checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology 108:822–830
Hwang D, Shakir N, Limann B, Sison C, Kalra S, Shulman L, De Corla SA, Greenberg H (2008) Association of sleep-disordered breathing with postoperative complications. Chest 133:1128–1134
Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F (2009) Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth 56:819–828
Gross JB, Bachenberg KL, Benumof JL, Caplan RA, Connis RT, Cote CJ, Nickinovich DG, Prachand V, Ward DS, Weaver EM, Ydens L, Yu S (2006) Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 104:1081–1093
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM (2008) STOP questionnaire a tool to screen obstructive sleep apnea. Anesthesiology 108:812–821
Iber C, Ancoli-Israel S, Chesson A Jr, Quan S The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification
Ayappa I, Norman RG, Seelall V, Rapoport DM (2008) Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med 4:26–37
Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, Douglas NJ (2003) Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J 21:253–259
Collop NA (2002) Scoring variability between polysomnography technologists in different sleep laboratories. Sleep Med 3:43–47
Snoey ER, Housset B, Guyon P, ElHaddad S, Valty J, Hericord P (1994) Analysis of emergency department interpretation of electrocardiograms. J Accid Emerg Med 11:149–153
Author information
Authors and Affiliations
Corresponding author
Additional information
Sources of financial support for the work (including institutional support)
University Health Network Foundation, Physician Services Incorporated Foundation, ResMed Foundation, and Respironic Foundation. University of Toronto for Dr. Chung and Dr. Shapiro.
Appendix 1 Sample of Sleep Diary
Appendix 1 Sample of Sleep Diary
Sleep Diary
Name _____________________ PSG ID____________
Op Date _______________ PSG Date ______________
Regular Bed Time___________
_____________________________________________
_______________________________________________
CPAP: Yes/No Oxygen Therapy: Yes/No
Bedtime (Lights Off):
Wash Room Time:
Wakeup Time in morning (Lights On):
Did you have a good sleep last night? Yes / No
Did any unusual thing happen last night? Yes / No
If yes, please provide some detail:
Comments:
_____________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
_______________________________________________
Embletta No: Proxy No:
PSG Technician’s Comments:
Rights and permissions
About this article
Cite this article
Chung, F., Liao, P., Sun, Y. et al. Perioperative practical experiences in using a level 2 portable polysomnography. Sleep Breath 15, 367–375 (2011). https://doi.org/10.1007/s11325-010-0340-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-010-0340-9