Abstract
The aim of this study was to establish cardiac damage related to nocturnal ischemia using heart type fatty acid binding protein (h-fabp), which reaches detectable levels in plasma after being released from myocytes in case of ischemia in obstructive sleep apnea syndrome (OSAS) patients without coronary artery disease (CAD). Fifty patients diagnosed with OSAS in our sleep laboratory with polysomnographic analysis (PSG), who did not have any previous history of cardiac disease and in whom CAD was ruled out with myocardium perfusion scintigraphy, were included in the study. Control group comprised 19 volunteers without history of cardiac disease and risk factors in whom OSAS was excluded with PSG analysis. Blood samples were drawn from the patients to examine h-fabp, creatine kinase (CK), creatine kinase-MB (CK-MB), aspartate aminotransferase (AST), troponin I levels before and after sleep. No significant difference was found in CK, CK-MB, AST, Troponin I, and h-fabp levels before and after sleep in patient and control groups (p > 0.05). No significant difference was found between groups in terms of CK, CK-MB, AST, and Troponin I levels before and after sleep, while a significant difference was found between them with regard to h-fabp levels before (p = 0.006) and after sleep (p = 0.022). When arithmetical mean of the fabp levels before and after sleep was taken in the patient group, it was found that mean value of h-fabp was associated with the desaturated period in sleep which was under 80% (p = 0.04). H-fabp seems to be a marker that will enable the detection of cardiac injury in the early asymptomatic period in OSAS patients before development of disease that can be detected by imaging methods. Further studies are required to investigate the relation between the value of h-fabp and the development of cardiac dysfunction in the long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome is a common medical condition with an incidence of 4% and 2% in adult males and females, respectively [1]. It is characterized by complete or partial obstruction of the upper respiratory airways recurring during sleep [2]. Considerable evidence is available in support of an independent association between OSAS and cardiovascular diseases [3]. Moreover, obstructive sleep apnea syndrome (OSAS) patients show increased cardiovascular mortality and morbidity [4].
Nowadays, it is known that OSAS is an independent risk factor for ischemic heart disease (IHD) [5]. In one study, it was shown that coronary artery disease was evident in 50% of patients with clinically significant OSAS [6]. Furthermore, patients with OSAS and IHD show nocturnal ST segment changes that correlate with oxyhemoglobin desaturation and severity of OSAS [7]. Moreover, other investigators have demonstrated ST segment depression during sleep without IHD [8]. The mechanism of ST segment changes is likely to be related to increased myocardial oxygen demand during the post-apneic surge in blood pressure and heart rate at the time when the oxyhemoglobin saturation is at its lowest point [5].
Despite the well-known fact that there are ST segment changes and nocturnal angina due to recurrent hypoxemia episodes occurring during sleep in OSAS patients, these ischemic changes have not been demonstrated by any serological marker yet.
Heart type fatty acid binding protein is a small cytoplasmic protein of 15-kDA weight, which is abundant in the heart and in lower concentrations in the blood and extra-cardiac tissues [9]. It is released from myocytes into the circulation in large amounts in the incidence of acute myocardial ischemia [10]. It appears in the blood as early as 1.5 h after onset of symptoms of infarction, peaks at around 6 h, and returns to baseline values between 12 and 24 h after onset of symptoms. These features of h-fabp make it an excellent potential candidate for detection of acute myocardial infarction [9]. This marker is extremely specific and sensitive to myocardial ischemia [10]. Today, its superiority over other cardiac markers has been shown in the early phase of acute myocardial infarction.
In view of these data, in this study, we aimed at detecting the cardiac damage occurring due to nocturnal ischemia by using h-fabp in patients with OSAS who had no other detected cardiac diseases.
Materials and methods
Patients
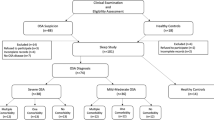
Consecutive patients who had not been diagnosed as having OSAS or coronary artery disease (CAD) in the past, who had been admitted to the sleep laboratory of our clinic between 15.02.06 and 15.06.06 with the presumptive diagnosis of OSAS, were included in the study. All patients underwent a standard questionnaire and physical examination. Patients with the history of cardiac, renal, immunological or other systemic diseases, or those who were on drugs due to disease, were excluded from the study. All patients with the presumptive diagnosis of OSAS and without a previous history of CAD underwent the standard 16-channel polysomnographic analysis. OSAS was diagnosed as apnea–hypopnea index (AHI) ≥ 5 with at least one of the following complaints: (1) excessive daytime sleepiness and (2) witnessed apnea reported by the bed partner or family members. Before polysomnographic analysis, blood samples were drawn for the analyses of CK, CK-MB, AST, Troponin I, and h-fabp at 2100 hours on the day the patient was to be admitted. After sleep, at 9:00 a.m. CK, CK-MB, AST, Troponin I, and h-fabp levels were measured again, and those with AHI ≥ 5 and diagnosed with OSAS underwent electrocardiography, echocardiography, and myocardium perfusion scintigraphy to rule out coronary artery disease. After these investigations, 50 patients in whom OSAS was diagnosed and coronary artery disease excluded were included in the study. Those who did not meet these criteria were excluded from the study. The control group was composed of volunteers who did not have a history of cardiological disease and risk factors. They also underwent polysomnographic analysis, electrocardiography, and echocardiography. Nineteen subjects in whom OSAS and CAD were eliminated were included as control group.
Polysomnography
Sixteen-channel [electroencephalography, electro-oculography, electromyography of the chin and the leg (anterior tibialis), electrocardiography, oxygen saturation (from fingertips), respiratory effort (thoracic, abdominal) and nasal air flow (nasal pressure transducer), body position and tracheal microphone] polysomnography recordings were obtained for 6–8 h with the Embla ®-flaga instrument. Polysomnography recordings were analyzed by a physician experienced in sleeping disorders using Somnologica 3.2 software. Patients with a minimal sleep efficacy of 60% were included. Apnea was defined as complete cessation of airflow for at least 10 s; hypopnea was defined as a 50% decrease in airflow accompanied by development of arousal or at least 4% drop in oxygen saturation. The overall oxygen desaturation time in sleep was considered as the period spent under 4% oxygen saturation when awake. In addition to overall oxygen desaturation time, desaturation periods under 90% and 80% were also recorded. The diagnosis of obstructive sleep apnea syndrome was defined by AHI. All the apneas and hypopneas scored in the study were obstructive in type.
Heart-type fatty-acid-binding protein
The h-fabp quantitative test is based on a solid phase enzyme-linked immunosorbent assay. The assay system utilizes an affinity-purified goat anti-h-fabp antibody for solid phase (microtiter wells) immobilization and anti-h-fabp antibody of the same goat in the antibody–enzyme (horseradish peroxidase) conjugate solution. The test sample is allowed to react simultaneously with the antibodies, resulting in h-fabp molecules being sandwiched between the solid phase and enzyme-linked antibodies. After 60-min incubation at room temperature in an orbital shaker, the wells are washed with distilled water to remove unbound-labeled antibodies. A solution of TMB reagent is added and incubated for 20 min at room temperature, resulting in the development of a blue color. The color development is stopped with the addition of Stop solution, and the color is changed to yellow and measured spectrophotometrically at 450 nm. The concentration of h-fabp is proportional to the color intensity of the test sample. Coefficient of variation for the h-fabp assay was 7–8% for intra-assay and 9.3–12.6% for inter-assay.
Other cardiac markers
Troponin I
VIDAS Troponin I Ultra is an automated quantitative test for use on the VIDAS instruments for determination of human cardiac troponin I in human serum using the enzyme-linked fluorescent assay technique.
CK, CK-MB
Roche automated clinical chemistry analyzer (Roche/Hitachi: ACN 057) is used for determination of CK and CK-MB in human serum.
AST
Roche automated clinical chemistry analyzer (Roche/Hitachi Modular Analytics) is used for determination of AST in human serum.
The study complied with the declaration of Helsinki and was approved by the local research ethics committee. All subjects signed written informed consent to participate in the study.
Statistical analyses
The analysis of the data was carried out using the SPSS 11.5 software. Continuous variables were shown as mean ± standard deviation or median (min–max), while categorical variables were expressed with percent percentage. The Shapiro–Wilks test was used to investigate whether or not continuous variables showed parametric distribution, and the Student’s t test or the Mann–Whitney test were utilized to assess whether or not there was a significant difference between groups in terms of measured characteristics. When there were more than two groups, the significance of the difference between groups in terms of continuous variables was investigated using the Kruskall–Wallis test. If the difference was significant, to establish the group or groups causing the difference and for multiple comparison, the Kruskal–Wallis multiple comparison test was used. Bonferroni correction was made for evaluations within groups. The Wilcoxon rank test was used to determine whether the difference between repeated measurements was significant or not. The significance of the linear correlation between continuous variables was evaluated with the Spearman correlation test. The chi-square or Fisher’s exact test was used for categorical comparisons. A p value of <0.05 was considered to be statistically significant.
Results
Overall, 69 cases were included in the study. Nineteen cases with AHI < 5 were included as the control group and 50 patients diagnosed with OSAS as the patient group. The mean age of the cases was 48.02 ± 9.5. The patient and the control groups were matched for age, sex, smoking history, and body mass index. None of the patients had a history of coronary artery disease, congestive heart failure, cardiomyopathy, hypertension, diabetes mellitus, hyperlipidemia, chronic obstructive lung disease, or any other systemic diseases. The demographic characteristics of the cases are illustrated in Table 1.
When the cases in the patient group were classified into three groups according to their AHI status, 16 (32%) had mild OSAS (AHI = 5–14.9), 13 (26%) had moderate (AHI = 15–29.9), and 21 (42%) had severe OSAS (AHI ≥ 30).
A statistically significant difference was observed between the patient and the control groups in terms of the number of apnea and hypopnea, overall oxygen desaturation period, and desaturation periods spent under 90% and 80% (p < 0.05).
Subjects in the patient and the control groups showed no significant differences within the group regarding the levels of CK, CK-MB, AST, troponin I, and h-fabp before and after sleep (p > 0.05; Table 2). While no significant difference was found between the patient and control groups with regard to CK, AST, CK-MB, troponin I levels, a significant difference was found in both groups between h-fabp levels before (p = 0.006) and after sleep (p = 0.022; Table 3). H-fabp values were significantly higher in the patient group than in the control group before and after sleep. In the analysis carried out by taking the arithmetic mean of the h-fabp values before and after sleep, the mean value was found to be related to the duration of desaturation spent under 80% (p = 0.04). However, no significant relation was found between the mean h-fabp value and AHI, the number of apnea and hypopnea episodes, the amount of oxygen desaturation, the overall oxygen desaturation period, and the desaturation period spent under 90% (p > 0.05).
When the patients were classified into three groups according to their AHI, no statistically significant difference was found between groups in terms of h-fabp levels (p > 0.05). Similarly, when the groups were compared separately with the control group with regard to h-fabp levels, no statistically significant relation was found (p > 0.05).
Electrocardiographic, echocardiographic, and myocardial perfusion scintigraphy findings of all subjects included in the study were within the normal range.
Discussion
There is yet no marker that will predict cardiovascular mortality and morbidity or that will indicate an increased risk of cardiac disease in patients with OSAS. In the present study, we obtained promising results in that h-fabp could be a possible candidate for this marker.
Thus far, many investigations have been carried out to examine the effect of hypoxic events on the cardiac system that occurs all throughout the night. Previous studies have consistently noted nocturnal myocardial ischemia in patients with OSAS. More than a third of OSAS patients with CAD manifest signs of nocturnal ischemia [11]. OSAS occurs commonly in patients with coronary artery disease and is associated with nocturnal angina and nocturnal ST-T segment depression [5]. There is uncertainty as to whether OSAS causes nocturnal myocardial ischemia in the absence of coronary artery disease, but in the study of Alonso-Fernández et al. [12], patients with OSAS show a higher frequency of nocturnal ST segment depression episodes in comparison with control subjects. In addition, they also found that the number of ST segment depressions per hour throughout the day were higher in OSAS patients than in the snoring and the control group. They also stated that ST segment changes were related to sympathetic tonus and fragmentation of sleep. In some studies [7, 13], no evidence of nocturnal ischemic episodes in such patients has been reported; in another study [14], 30% of the patients showed ST segment depression during the night, and CPAP treatment significantly reduced the total duration of these events. Moreover, sleep apnea was found in nine of ten subjects with severely disabling angina pectoris and nocturnal angina [15].
In their study aiming to evaluate whether troponin T can be used as a marker of nocturnal ischemic events observed in OSAS patients, Gami et al. [16] demonstrated that in severe OSAS patients with accompanying CAD, troponin T was not able to predict nocturnal ischemic events. All patients included in their study had cardiac troponin T levels under 0.01 ng/ml.
In the current study, we aimed to investigate the impact of hypoxic events on the cardiac system, repeating throughout the night in patients with only OSAS and no other cardiac or systemic disease. For this purpose, we used h-fabp, which is an extremely sensitive and specific marker of myocardial ischemia. Taking release kinetics of the h-fabp into consideration, we drew blood samples from the patients included in the study to examine h-fabp levels before and after sleep. Contrary to our expectations, we did not find any significant difference in h-fabp levels before and after sleep in the patient group (p > 0.05). However, in comparison with the control group, h-fabp levels were significantly higher in the patient group before and after sleep.
In addition, there was a significant relation in the patient group between the mean h-fapb levels and the desaturated period which was under 80% desaturation. The fact that in OSAS patients, who did not have any cardiac disease detected by electrocardiography, echocardiography, or even myocardium perfusion scintigraphy, h-fabp, a sensitive and specific marker, was found to be significantly higher than that in the control group suggests that myocardial ischemia, caused by apnea/hypopnea and resultant hypoxia, leads to an increase in h-fabp levels. The significant relation between the mean h-fabp level and time spent under 80% desaturation supports our hypothesis. Furthermore, while no relation was found in the OSAS patients between the mean h-fabp values and the number of apnea and hypopnea episodes and AHI index, a relation was found between the mean h-fabp values and the desaturated period which was under 80% desaturation, which stresses the importance of the depth and duration of hypoxia in the development of cardiac diseases.
The lack of a difference in the patient group between h-fabp levels before and after sleep suggests that the ischemic process induced by hypoxia continues throughout the day.
Different pathophysiological mechanisms underlie the difference between cardiovascular disease and sleep apnea syndrome. Oxygen desaturation is the strongest stimulus, explaining the observed acute cardiovascular responses [17]. In patients with OSAS, baroreceptor and chemoreceptor dysfunctions developing on the basis of hypoxia, release of nocturnal endothelin, and endothelial dysfunction give rise to an increase in sympathetic activity [18]. The increase in sympathetic activity is maintained in OSAS patients throughout the day [19]. However, the disorder in cardiac autonomic functions continues throughout 24 h in OSAS patients [20]. Hypoxic events in association with repeated apnea induce oxidative stress in the vascular endothelium similar to reperfusion injury. In addition to increased sympathic activity and oxidative stress, inflammation and other metabolic factors also contribute to the development of cardiovascular disease in OSAS patients [5]. These alterations are not restricted to nighttime in OSAS patients. Many humoral and neuronal mechanisms are induced in relation to nocturnal hypoxia, and their effects continue all day. The cardiovascular system adapts to hypoxic events repeated every night by increasing the sympathetic tone and decreasing the parasympathetic tone [17]. In conclusion, many pathological processes are initiated in OSAS patients, which we believe are triggered by apnea and consequent hypoxia, and last all day. We believe that excessive sleepiness in OSAS patients who do not receive treatment and apneic episodes experienced throughout the day contribute to this continuity. In one study, it was shown that daytime sleep attacks accompanied by oxygen desaturation in patients with moderate to severe OSAS may contribute to the occurrence of traffic or cardiovascular accidents [21]. The reason for h-fabp levels being higher in OSAS patients than in the control group while not demonstrating any difference before and after sleep, may be explained by the aforementioned factors. When we consider the release kinetics of h-fabp, blood sample drawn at 9:00 a.m. after sleep does not adequately mirror the hypoxic effect occurring in the early morning. Because, h-fabp released in the early hours of the morning reaches detectable levels in the blood within 1–2 h and peak levels in 6 h. This may be another reason for the lack of difference between the h-fabp values before and after sleep. Therefore, it would be a possible future direction to assess h-fabp levels later in the morning to investigate the cardiovascular affects of sleep apnea.
Furthermore, there are some limitations of h-fabp assays. H-fabp exists in high concentrations in the heart only. However, this protein is not totally cardiac-specific and occurs in the other tissues, although at much lower concentrations. It occurs in skeletal muscles in concentrations varying between 0.05 and 0.2 mg/g wet weight of tissue, depending on the muscle fiber type studied [22, 23]. It has also been reported in very low concentrations in tissues such as the kidney, aorta, testes, mammary glands, placenta, brain, adrenal glands, adipose tissue, and stomach [9, 24]. However, the detection of h-fabp in these tissues does not necessarily mean its presence in all cells of that tissue [9]. By way of addition, surgery (both cardiac and non-cardiac) and renal insufficiency cause elevation of h-fabp concentration [9, 25]. None of the patients included in this study had history of surgery and renal insufficiency. The normal ranges reported for h-fabp in plasma and serum are assay- and method-dependent. Tanaka et al. [26] has reported the normal range for h-fabp to be 0.0–2.8 μg/l; Wodzig et al. [27] reported 0.3–5 μg/l as the normal limit; and Tsuji et al. [28] used 3 μg/l (normal range 0.0–0.6 μg/l). One study used a cutoff concentration of 19 μg/l (mean ± 2 SD of controls) [29]. In addition, 6.2 ng/ml was used to define a significant elevation of h-fabp by other investigators [22, 26, 30]. Thus, a standard cutoff value for h-fabp assays has not been established yet. The reason for this may be that a relatively small number of clinical studies have been performed on h-fabp.
This is the first study that evaluates the association between h-fabp and the risk of cardiac damage in patients with OSAS. Therefore, further clinical studies are required to find a cutoff value for h-fabp assay in OSAS patients.
In conclusion, h-fabp seems to be a marker that will enable the detection of cardiac injury in the early asymptomatic period in OSAS patients before development of disease that can be detected by imaging methods. Further studies are required to investigate the relation between the value of h-fabp and the development of cardiac dysfunction in the long term.
References
Young T, Palta M, Dempsey J et al (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328(17):1230–1235
Lattimore J-DL, Celermajer DS, Wilcox I (2003) Obstructive sleep apnea and cardiovascular disease. J Am Coll Health 41:1429–1437
McNicholas WT, Bonsignore MR (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29(1):156–178 (Jan)
Marin JM, Carrizo SJ et al (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365(9464):1046–1053 (Mar 19–25)
Parish JM, Somers VK (2004) Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc 79(8):1036–1046 (August)
Andreas S, Schultz R, Werner GS, Kreuzer H (1996) Prevalence of obstructive sleep apnea in patients with coronary artery disease. Coron Artery Dis 7:541–545
Peled N, Abinader EG, Pillar G, Sharif D, Lavie P (1999) Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol 34(6):1744–1749 (Nov 15)
Hanly P, Sasson Z et al (1993) ST-segment depression during sleep in obstructive sleep apnea. Am J Cardiol 71(15):1341–1345
Alhadi HA, Fox KA (2004) Do we need additional markers of myocyte necrosis: the potential value of heart fatty acid binding protein. Q J Med 97:187–198
Nakata T et al (2003) Human Heart type fatty acid binding protein as an early diagnostic and prognostic marker in acute coronary syndrome. Cardiology 99:96–104
Schafer H, Koehier U, Ploch T et al (1997) Sleep related myocardial ischemia and sleep structure in patients with obstructive sleep apnea and coronary heart disease. Chest 111:387–393
Alonso-Fernández A, García-Río F et al (2005) Cardiac rhythm disturbances and ST-segment depression episodes in patients with obstructive sleep apnea–hypopnea syndrome and its mechanisms. Chest 127:15–22
Andreas S, Hajak G, Natt P et al (1991) ST segmental changes and arrhythmias in obstructive sleep apnea. Pneumologie 45(9):720–724
Hanly P, Sasson Z, Zuberi N et al (1993) ST-segment depression during sleep in obstructive sleep apnea. Am J Cardiol 71:1341–1345
Franklin KA, Nilsson JB, Sahlin C et al (1995) Sleep apnoea and nocturnal angina. Lancet 345:1085–1087
Gami AS, Svatikova A, Wolk R et al (2004) Cardiac troponin T in obstructive sleep apnea. Chest 125(6):2097–2100
Pepin JL, Levy P (2002) Pathophysiology of cardiovascular risk in sleep apnea syndrome (SAS). Rev Neurol (Paris) 158(8–9):785–797
Narkiewicz K, Somers VK (2003) Sympathetic nerve activity in obstructive sleep apnea. Acta Physiol Scand 177:385–390
Somers VK, Dyken ME, Clary MP, Abboud FM (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96:1897–904
Aydin M, Altin R (2004) Cardiac autonomic activity in obstructive sleep apnea. Tex Heart Inst J 31(2):132–136
Noda A, Ito R, Okada T et al (1998) Twenty-four-hour ambulatory oxygen desaturation and electrocardiographic recording in obstructive sleep apnea syndrome. Clin Cardiol 21(7):506–510 (Jul)
Yoshimoto K, Tanaka T, Somiya K et al (1995) Human heart-type cytoplasmic fatty acid-binding protein as an indicator of acute myocardial infarction. Heart Vessels 10:304–309
Zschiesche W, Kleine AH, Spitzer E, Veerkamp JH, Glatz JF (1995) Histochemical localization of heart-type fatty acid-binding protein in human and murine tissues. Histochem Cell Biol 103:147–156
Azzazy HME, Pelsers MMAL, Christenson RH (2006) Unbound free fatty acids and heart-type fatty acid-binding protein: diagnostic assays and clinical applications. Clin Chem 52:19–29
Gorski J, Hermens WT, Borawski J, Mysliwiec M, Glatz JF (1997) Increased fatty acid-binding protein concentration in plasma of patients with chronic renal failure. Clin Chem 43:193–195
Tanaka T, Hirota Y, Sohmiya K, Nishimura S, Kawamura K (1991) Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clin Biochem 24:195–201
Wodzig KW, Pelsers MM, Van der Vusse GJ, Roos W, Glatz JF (1997) One-step enzyme-linked immunosorbent assay (ELISA) for plasma fatty acid-binding protein. Ann Clin Biochem 34:263–268
Tsuji R, Tanaka T, Sohmiya K et al (1993) Human heart-type cytoplasmic fatty acid-binding protein in serum and urine during hyperacute myocardial infarction. Int J Cardiol 41:209–217
Kleine AH, Glatz JF, Van Nieuwenhoven FA, Van der Vusse GJ (1992) Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol Cell Biochem 116:155–162
Ohkura Y, Asamaya K, Ishii H et al (1995) Development of sandwich enzyme-linked immunosorbent assay fort he determination of human heart type fatty acid-binding protein in plazma and urine by using two different monoclonal antibodies specific for human heart fatty acid binding protein. J Immunol Methods 178:99–111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oktay, B., Akbal, E., Firat, H. et al. Evaluation of the relationship between heart type fatty acid binding protein levels and the risk of cardiac damage in patients with obstructive sleep apnea syndrome. Sleep Breath 12, 223–228 (2008). https://doi.org/10.1007/s11325-007-0167-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-007-0167-1