Abstract
Information on obstructive sleep apnea–hypopnea syndrome (OSAHS) in Japan has been limited. The purposes of this clinical study were to evaluate the demographic characteristics of Japanese OSAHS patients and to assess how demographic factors are associated with OSAHS severity. We analyzed 3,659 OSAHS patients who underwent polysomnographic evaluation between January 2000 and December 2004 at 11 hospitals in Niigata Prefecture, Japan. Data consisted of apnea–hypopnea index (AHI) and demographic characteristics, including sex, age, and body-mass index, for statistical analysis. Levels of obesity were classified according to the WHO criteria. The male-to-female patient ratio for OSAHS was 4.6, and male patients presented more severe OSAHS than female patients. High AHI and a high proportion of moderate to serious OSAHS (AHI ≥ 15) were found among the patients in their 30s, as well as female patients in their 70s and male patients in their 80s. The AHI and the proportion of moderate-to-serious OSAHS (AHI ≥ 15) were greater in patients classified as underweight than in normal weight patients. In conclusion, there is a higher male predominance in the prevalence of OSAHS, and in both sexes, the results suggest different pathophysiological mechanisms of deteriorating OSAHS between adults under age 55 and adults 55 years or over. In addition, underweight patients exhibit more severe OSAHS than normal weight patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea–hypopnea syndrome (OSAHS) is a disorder characterized by repeated apneas and/or hypopneas and is known to increase the risk of hypertension, cardiovascular diseases, stroke, and mortality [1–4]. Waking after apnea or hypopnea disrupts the quality of sleep at night. Chronic sleep disturbance causes daytime sleepiness and decreased concentration associated with increased risk of motor-vehicle accidents [5, 6] and occupational accidents [7]. For these reasons, OSAHS is an issue of great concern in both clinical medicine and public health.
The severity of OSAHS is an important aspect of the disorder. The apnea–hypopnea index (AHI) reflects the severity of OSAHS. A person with AHI greater than or equal to 5 is diagnosed as having OSAHS, and a patient with AHI of 15 or over is considered to have a disorder associated with significant morbidities and should be treated with continuous positive airway pressure or surgery [8].
Epidemiologic studies have found obesity to be a critical factor associated with the development or severity of OSAHS [2]. While most previous studies had been conducted in white populations, a few studies of Asian populations have suggested that the contribution of obesity to OSAHS may differ between whites and Asians [9], indicating the need for further epidemiologic and clinical research.
The aims of this clinical study were to examine the demographic characteristics of Japanese OSAHS patients and to assess how demographic factors, including sex, age, and body-mass index (BMI), are associated with severity of OSAHS.
Materials and methods
Patients
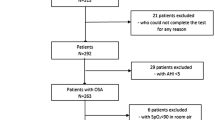
We targeted a total of 4,561 patients who were suspected to suffer from SAHS and underwent polysomnographic evaluation between January 2000 and December 2004 at 11 hospitals in Niigata Prefecture (population, 2.5 million), Japan. The 11 hospitals were the only hospitals in which polysomnography was available in Niigata Prefecture, a geographically defined area [10], and consequently almost all suspected SAHS patients in Niigata Prefecture were examined during the study period. Many of the 4,561 patients were referred to the selected hospitals from other hospitals and clinics in Niigata Prefecture. Of the 4,561 patients suspected of having SAHS, 3,788 were diagnosed with the sleep-disordered breathing with a comprehensive polysomnographic examination. Of the 3,788 SAHS patients, 60 patients were diagnosed with central SAHS (CSAHS), which is distinguished from OSAHS by a different pathophysiological mechanism, and excluded from the study. Of the 3,728 OSAHS patients, 50 patients under the age of 16, 18 patients whose age was unknown, and an abnormally undersized patient, were also excluded for the same reason. Ultimately, 3,659 OSAHS patients aged 16 and over were statistically analyzed for the study. The protocol for this study was approved by the Ethics Committee of Niigata University School of Medicine.
Diagnostic procedure
All patients underwent overnight polysomnographic evaluation (Sandman, Nellcor Puritan Bennett, Ottawa, Ontario, Canada, in four hospitals; Somnostar Pro, SensorMedics, Yorba Linda, CA, USA, in four hospitals; Somnostar Alpha, SensorMedics, Yorba Linda, CA, USA, in three hospitals; E series, Compumedics, Abbotsford, Victoria, Australia, in one hospital), which included electroencephalogram (EEG), electromyogram, electrooculogram, electrocardiogram, airflow by oronasal thermistor, chest and abdominal wall movements, oxygen saturation (SaO2) by pulse oximeter, snoring sounds by tracheal microphone, and body position. Patients were examined by a trained clinical technologist at each hospital using a standardized procedure. Sensors were attached to a patient before going to sleep at each hospital, and real-time data were collected overnight until the patient awoke in the morning. Using the Chicago Criteria [11], apnea was defined as the complete absence of oronasal airflow for at least 10s, and hypopnea was defined as a <50% decrease in oronasal airflow accompanied by a 3% fall in SaO2 from baseline or an EEG arousal from sleep. Sleep-disordered breathing was assessed with the AHI, and lowest SaO2 levels and cumulative percentage of sleep time with an SaO2 level less than 90% (CT90%). A person with an AHI greater than or equal to 5 is generally diagnosed as having OSAHS. Patients with 5 ≤ AHI <15, 15 ≤ AHI<30, and AHI greater than or equal to 30 were classified as having mild, moderate, and serious OSAHS, respectively. Polysomnographic data of all patients obtained from each hospital were collected and entered in the database used for this study.
Other information
Information on sex, age, height, and weight was added to the database from patient medical charts. BMI was calculated by dividing weight (kilograms) by the square of height (square meters) for each patient. Levels of obesity were classified according to the WHO criteria [12] as follows: “underweight”, BMI < 18.5; “normal weight”, 18.5 ≤ BMI < 25; “preobese”, 25 ≤ BMI ≤ 30; class I, 30 ≤ BMI < 35; class II, 35 ≤ BMI < 40; and class III, BMI ≥ 40. We further divided “normal weight” into “lower normal weight”, 18.5 ≤ BMI < 21.75, and “upper normal weight”, 21.75 ≤ BMI < 25, because too many (56%) OSAHS patients were classified as normal weight.
Statistical methods
Means and standard deviations were used to characterize continuous variables. AHI scores were skewed to higher values, and thus they were logarithmically transformed for statistical tests. Sex was coded as a dichotomous variable with “0” for male and “1” for female. Student t tests were performed to test for statistically significant differences between the mean values of two groups. The chi-square test was used to test independence of categorical data. Multiple linear regression analysis was used to test associations between log-transformed AHI as an outcome variable and predictor variables, including sex, age (a continuous variable), and BMI (a continuous variable). Statistical tests were performed using the SAS statistical package (release 8.02, SAS Institute, Cary, NC, USA). P-values of less than 0.05 were considered to be statistically significant.
Results
Demographic characteristics and AHI by sex are shown in Table 1. Male patients were significantly younger and had significantly higher average values in height, weight, and log-transformed AHI than female patients. The male-to-female patient ratio was 4.6. The database was incomplete, with some missing values for height, weight, and BMI. AHI ranged from 5 to 158 events per hour. Mean values of lowest SaO2 and CT90% were 77.3% (SD 12.9, median 81.0) and 16.0% (SD 24.9, median 4.0), respectively. Mean values of apnea and hypopnea episodes were 25.7 events per hour (SD 24.7, median 16.6) and 8.6 events per hour (SD 9.0, median 6.4), respectively.
Distributions of patients’ age by sex are shown in Fig. 1. The mode of the distributions was the 50- to 59-year age group in both sexes. The age distribution of females tended to shift to the right, and there were fewer female patients under 50 years of age than those aged 50 years or over. The percent frequency distributions of male and female patients were significantly different (χ 2 = 126.0, df = 8, P < 0.0001). Distributions of patients’ BMI by sex are shown in Fig. 2. The distributions for males tended to have higher kurtosis than females, and the percent frequency distributions of male and female patients were significantly different (χ 2 = 67.2, df = 6, P < 0.0001).
Distributions of patients’ body-mass index (BMI) by sex. Obesity was evaluated based on the WHO classification of BMI (underweight, <18.5; lower normal, 18.5 ≤ BMI < 22.25; upper normal, 22.25 ≤ BMI < 25; preobese, 25 ≤ BMI < 30; class I, 30 ≤ BMI < 35; class II, 35 ≤ BMI < 40; and class III, BMI ≥ 40). The mode of the distributions was in the “preobese” group in both sexes
The numbers of OSAHS patients diagnosed at each of the three levels of AHI by sex are shown in Table 2. An AHI greater than or equal to 15 was found in 74.8% of male patients, in 58.0% of female patients, and in 71.8% of patients overall. The percent frequency of patients with AHI greater than or equal to 30 was higher in males than in females, whereas the proportion of patients with AHI less than 15 was significantly lower for males than females (χ 2 = 103.2, df = 2, P < 0.0001). Absolute numbers of OSAHS patients grouped according to the three levels of AHI and age group by sex are shown in Table 3. The proportion of patients with AHI greater than or equal to 15 tended to be lower in the 16- to 19-year age group (60.0%) than the other age groups (70.9–100%) among male patients (χ 2 = 37.6, df = 16, P = 0.0017), and lower in the 16- to 19-year age group (20.0%) and 80- to 89-year age group (25.0%) than the other age groups (40.9–69.9%) among female patients (χ 2 = 26.5, df = 14, P = 0.0223). The frequency of moderate-to-serious OSAHS (AHI ≥ 15) did not increase with increasing years of age. The numbers of OSAHS patients according to the three levels of AHI and obese class by sex are shown in Table 4. The proportion of patients with AHI greater than or equal to 15 increased with higher obese class with the exception of the “underweight” group for both male and female patients.
Associations between demographic variables and AHI as a continuous variable were analyzed. Mean values of log-transformed AHI according to age groups were plotted by sex in Fig. 3. In both sexes, separate peaks in the log-transformed AHI occurred before and after the 50- to 59-year age group. Among males, these peaks occurred in the 30- to 39- and 80- to 89-year age groups, and peaks appeared among female patients in the 30- to 39- and 70- to 79-year age groups. Mean values of log-transformed AHI according to obese class were plotted by sex in Fig. 4. Beginning with the “upper normal” group, the log-transformed AHI increased as obese class rose in both sexes. Multiple linear regression analysis showed that sex (β = 0.394, R 2 = 0.035, P < 0.0001), age (β = 0.00509, R 2 = 0.003, P < 0.0001), and BMI (β = 0.0662, R 2 = 0.138, P < 0.0001) were independently associated with the log-transformed AHI.
Mean values of log-transformed apnea–hypopnea index (AHI, events/hour) in 10-year age groups were plotted by sex. Overall, the mean values of log-transformed AHI were higher in male patients than in female patients. Two peaks of the log-transformed AHI are seen before and after the 50- to 59-year age group in both sexes
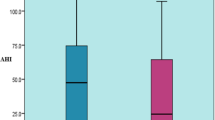
Mean values of log-transformed apnea–hypopnea index (AHI, events/hour) in obese classes were plotted by sex. Obesity was evaluated using the WHO classifications for BMI (underweight, <18.5; lower normal, 18.5 ≤ BMI < 22.25; upper normal, 22.25 ≤ BMI < 25; preobese, 25 ≤ BMI < 30; class I, 30 ≤ BMI < 35; class II, 35 ≤ BMI < 40; and class III, BMI ≥ 40). The log-transformed AHI rises as obese class becomes higher beginning from the upper normal group in both sexes
Because two peaks of the log-transformed AHI were seen before and after the 50- to 59-year age group (Fig. 3), we supposed that patients above and below 55 years of age might have different pathophysiological mechanisms and thus chose to analyze the relative contributions of sex, age, and BMI to the log-transformed AHI for the two groups separately. The results of multiple linear regression analysis are shown in Table 5. The R 2 value of BMI for this was 2.6 times larger than that for patients’ age 55 years and over.
Discussion
This study revealed interesting demographic findings in relation to AHI. We found two peaks in the log-transformed AHI and in the proportion of moderate-to-serious OSAHS (AHI ≥ 15) associated with age. These findings suggest that different pathophysiological mechanisms may be at work in patients under and over 55 years of age. Obesity is known to be an important causal factor of OSAHS [2]. Our data showed that the contribution of BMI to AHI was lower in older patients than in younger patients, suggesting that OSAHS in the elderly may be associated less with BMI and more with other unknown factors as compared with younger populations. Tishler et al. [13] similarly found that the effects of BMI on the incidence of sleep-disordered breathing diminish with increasing age.
In this study, we propose a few hypotheses on factors that might explain OSAHS in elderly people. First, coexisting central apnea/hypopnea may contribute to OSAHS as some patients diagnosed with OSAHS by polysomnography have central and mixed apnea/hypopnea events. Bixler et al. [14] showed that central events are observed exclusively in middle-aged and elderly people (reflecting a normal aging process). When we compared the proportion of patients with 10% or more nonobstructive events in polysomnographic data, patients 55 years and older had a significantly higher proportion (P = 0.0022) than those less than 55 years (36.4 vs 31.6%, data not shown in the “Results” section). Therefore, latent central apnea/hypopnea may influence the severity of OSAHS in the elderly. Secondly, a relative increase in mouth breathing to nasal breathing in the elderly may contribute to progression of OSAHS. Gleeson et al. [15] reported that an age-related decrease in nasal ventilation due to nasal obstruction was associated with apneic episodes during sleep. Finally, Worsnop et al. [16] reported that falls in ventilation and activities of upper airway muscle functions and a rise in upper airway resistance during a transition from wakefulness to sleep are greater in older men than in younger men, and these age-related changes in upper airway physiology may be possible candidates for the “unknown” factor associated with OSAHS in the elderly. Further research is necessary to isolate factors contributing to OSAHS in the elderly.
Asians are generally slimmer than North American and European whites, and Asian OSAHS patients are reported to be less obese than white patients [17]. Nevertheless, our data showed that BMI remained a leading factor associated with high AHI. We also showed that the “underweight” group had higher AHI and a higher proportion of moderate-to-serious OSAHS (AHI ≥ 15) patients of both sexes than the normal weight groups. This finding may be unique to Asian OSAHS patients, as nonobese Asian patients have been reported to exhibit relative narrowing of the upper airway due to craniofacial bony structure and the soft tissue of the naso- and oro-pharynx, which may be associated with the development of OSAHS [18, 19].
Among the demographic variables analyzed in this study, BMI can be lowered through lifestyle change, which in turn has the potential of lowering the AHI of OSAHS patients. A recent cohort study observed that weight loss in sleep-disordered patients decreased their AHI [20]. Our data suggest that weight loss could decrease the proportion of OSAHS patients with AHI greater than or equal to 15 in any obese-class group, with the exception of “underweight” and “normal weight” groups.
It is also known that OSAHS patients are predominantly male. In this study, the male-to-female patient ratio was 4.6:1, and male patients experienced more severe OSAHS than female patients, i.e., the proportion of OSAHS patients with AHI of 15 or above was significantly higher in males (74.8%) than in females (58.0%). Previous studies reported male-to-female patient ratios of 8:1 or more in clinic populations, and lower ratios of 2–3:1 in community samples [21–23], suggesting that there may be a disparity between clinic and community populations. The true male-to-female patient ratio in the Japanese population may be lower than that obtained in this study, although accurate techniques were used to diagnose patients with OSAHS.
The age distribution of male OSAHS patients approximated a normal distribution with a mode of 50–59 years, yet most (80%) female patients were 50 years of age or older. This finding has been reported by several researchers. Young et al. [24] reported that changes in sex hormone economy during the menopausal transition increased incidence of sleep-disordered breathing in perimenopausal women. Shahar et al. [25] found that the incidence of sleep-disordered breathing in postmenopausal women who received hormone replacement therapy (HRT) was half of that in postmenopausal women who did not receive HRT. These data suggest that decreased estrogen levels may explain the increase of OSAHS patients among postmenopausal women.
A strength of this study was the use of full polysomnographic examination as the standard diagnostic tool of OSAHS. Moreover, we identified almost all OSAHS cases within a geographically defined area. Therefore, the subjects in this study were correctly classified, and selection bias was minimized. However, some limitations were observed in the study. First, we were not able to collect data for all OSAHS patients in Niigata Prefecture because there may have been patients with OSAHS who did not visit hospitals or clinics during the study period. For example, given the disparity between clinic and community populations, we may have underestimated female OSAHS patients and thus may have overestimated the male-to-female ratio. In addition, elderly OSAHS patients are less likely to visit hospitals or clinics, and thus we may have missed more elderly OSAHS patients as compared with younger patients. In our data, there were fewer elderly OSAHS patients compared to middle-aged patients even when a distribution of the population of Niigata Prefecture is taken into account. We calculated the period prevalence of OSAHS for males in their 30s, 40s, 50s, 60s, 70s, and 80s that would be calculated as 294, 423, 435, 412, 262, and 66 /100,000, respectively, although the prevalence of OSAHS has been reported to be similar between the middle-aged and the elderly ones in population-based studies [2]. This problem is a major limitation of the clinical study. There were also limitations in diagnostic procedure, which could lead to misclassification in the case definition. We used thermistors instead of nasal pressure transducers for polysomnography, and quality control over multiple centers was difficult to achieve. Finally, this study did not evaluate risk factors other than the specified demographic characteristics for OSAHS. Well-designed, population-based epidemiologic studies are needed to address these limitations.
Based on this study of 3,659 Japanese OSAHS patients, we conclude that: (1) the male-to-female patient ratio was 4.6:1, (2) different pathophysiological mechanisms of developing OSAHS are active in adults under 55 years as compared with adults 55 years and older, and (3)OSAHS in this population is more severe in underweight patients than in normal weight patients.
References
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 283:1829–1836
Young T, Peppard PE, Gottleib DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165:1217–1239
Arzt M, Young T, Finn L, Skatrud JB, Bradley TD (2005) Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 172:1447–1451
Marine JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnea-hypopnea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–4053
Young T, Blustein J, Finn L, Palta M (1997) Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 20:608–613
George CF (2004) Sleep:5 Driving and automobile crashes in patients with obstructive sleep apnea/hypopnea syndrome. Thorax 59:804–807
James PK, Norman E, Sarkis D, Michale H, Michale L (1994) Cardiopulmonary disorders of sleep. In: Wake up America: a national sleep alert. A report of national commission on sleep disorders research, pp 10–25
Lobe DI, Gay PC, Strohl KP, Pack AI, White DP, Collop NA (1999) Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest 115:863–866
Kim J, In K, Kim J, You S, Kang K, Shim J, Lee S, Lee J, Lee S, Park C, Shin C (2004) Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med 170:1108–1113
Nakamura K, Yamamoto M, Yamazaki O, Kawashima Y, Muto K, Someya T, Sakurai K, Nozoe S (2000) Prevalence of anorexia nervosa and bulimia nervosa in a geographically defined area in Japan. Int J Eat Disord 28:173–180
AASM Task Force (1999) Sleep-related breathing disorders in adults: recommendation for syndrome definition and measurement techniques in clinical research. Sleep 22:667–689
Report of WHO Consultation (2000) Obesity: Preventing and managing the global epidemic. WHO Technical Report Series, Geneva 894:1–253
Tishler PV, Larkin EK, Schluchter MD, Redline S (2003) Incidence of sleep-disordered breathing in an urban adult population. JAMA 289:2230–2237
Bixler EO, Vgontzas AN, Have TT, Tyson K, Kales A (1998) Effects of age on sleep apnea in men: I. prevalence and severity. Am J Respir Crit Care Med 157:144–148
Gleeson K, Zwillich CW, Braier K, White DP (1986) Breathing route during sleep. Am Rev Respir Dis 134:115–120
Worsnop C, Kay A, Kim Y, Trinder J, Pierce R (2000) Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol 88:1831–1839
Li KK, Powell NB, Kusida C, Riley RW, Adornato B, Guilleminault C (1999) A comparison of Asian and white patients with obstructive sleep apnea syndrome. Laryngoscope 109:1937–1940
Kubota Y, Nakayama H, Takada T, Matsuyama N, Sakai K, Yoshizawa H, Nakamata M, Satoh M, Akazawa K, Suzuki E, Gejyo F (2005) Facial axis angle as a risk factor for obstructive sleep apnea. Intern Med 44:805–810
Yu X, Fujimoto K, Urushibata K, Matsuzawa Y, Kubo K (2003) Cephalometric analysis in obese and nonobese patients with obstructive sleep apnea syndrome. Chest 124:212–218
Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T (2005) Progression and regression of sleep-disordered breathing with changes in weight: The Sleep Heart Health Study. Arch Intern Med 165:2408–2413
Redline S, Kump K, Tisher PV, Browner I, Ferrette V (1997) Gender difference in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 149:722–726
Young T, Hutton R, Finn L, Badr S, Palta M (1996) The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med 156:2445–2451
Young T, Peppard PE (2005) Clinical presentation of OSAS: gender does matter. Sleep 28:293–295
Young T, Finn L, Austin D, Peterson A (2003) Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 167:1165–1166
Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O’Connor GT, Rapoport DM, Robbins JA (2003) Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 167:1186–1192
Acknowledgement
The authors wish to acknowledge the valuable support of Drs Hideo Sato (Nagaoka Chuou General Hospital), Jouji Toyama, Haruhiko Nakajima, Shin-ichi Kinebuchi (Joetsu General Hospital), and Kunihiko Sakai (Division of Respiratory Medicine, Nishi-Niigata Chuo National Hospital) in data collection for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohdaira, F., Nakamura, K., Nakayama, H. et al. Demographic characteristics of 3,659 Japanese patients with obstructive sleep apnea–hypopnea syndrome diagnosed by full polysomnography: associations with apnea–hypopnea index. Sleep Breath 11, 93–101 (2007). https://doi.org/10.1007/s11325-006-0087-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-006-0087-5