Abstract
The objective of this study was to examine heart rate variability (HRV) among sleep stages in obstructive sleep apnea (OSA) patients. The study was retrospective within subjects and examined the sleep stages and HRV in relation to OSA, age, body mass index (BMI), and sex. Data collected during diagnostic polysomnograms were used in this study. There were 105 clinical patients undergoing polysomnography for suspected OSA. We sampled the electrocardiogram (ECG) from wakefulness, stage 2, and REM sleep and analyzed for frequency domain HRV. Sampled epochs were free of apnea and arousals. Heart rate variability decreased with age. Total frequency variability (TF) and low frequency variability (LF) in wakefulness and REM sleep increased as apnea severity increased. Measures of TF, LF, and the LF/HF ratio were greatest in REM sleep. There was less LF and TF in Stage REM sleep in patients with higher BMI. In conclusion, the decrease in HRV with aging is a robust finding that occurs even in a clinical sleep apnea population. However, apnea does not mimic aging effects on the heart because HRV increased as apnea severity increased. The decrease in HRV during REM sleep in the obese apnea patients suggests the possibility of an autonomic dysfunction in this subgroup.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spectral analysis of heart rate variability (HRV) is a reliable noninvasive way to obtain information on parasympathetic and sympathetic cardiac function [1–3]. Low-frequency (LF) oscillations are influenced by sympathetic and vagal activity, while the high frequency (HF) oscillations are under vagal efferent control [4]. For this reason, the LF/HF ratio is generally considered an index of sympathovagal balance, and the total frequency (TF) includes the sum of very low, low, and high frequency power representing a global measure of autonomic activity [5]. Measurement of HRV in patients with OSA is important because metaanalyses of published data demonstrate that reduced HRV is strongly associated with increased risk of total mortality, cardiovascular mortality, and nonfatal cardiovascular events [6]. Numerous studies indicate that reduced heart rate variability predicts cardiac mortality particularly sudden cardiac death [7–11].
Sleep stages and age influence HRV. Decreased HF components and increased LF components characterize HRV in rapid eye movement sleep (REM) with opposite changes in (NREM) sleep [12, 13]. These findings suggest increased sympathetic activity relative to parasympathetic activity in REM sleep in comparison to NREM sleep [14]. However, parasympathetic activity predominates at rest during wakefulness. Additionally, there is an age-related decline in deep sleep that correlates with the nocturnal increase in cardiac parasympathetic nervous system (PNS) activity [15]. Similar to HRV, microneurographically recorded muscle sympathetic nervous system activity decreases during NREM sleep stages and increases during REM sleep [16].
Obstructive sleep apnea (OSA) alters autonomic cardiovascular variability during sleep and wakefulness [17]. Apnea is associated with increased circulating norepinephrine and loss of vagal tone, a combination that might underlie life-threatening arrhythmias [18]. Furthermore, repetitive sympathetic activation and blood pressure surges in response to apnea episodes impair compensatory baroreflex and other cardiovascular reflexes to maintain normal blood pressure accounting for the high incidence of systemic and pulmonary hypertension in patients with OSA [19–23]. Studies indicate that OSA alters HRV by increasing SNS activity or withdrawing PNS innervation altering the circadian rhythm of autonomic activity and resulting in a low HF component, a high LF component, and a high LF/HF ratio of HRV [17, 24–27]. The reduced HF component of HRV may account for the increased morbidity and mortality associated with OSA [28].
The association between apnea severity and HRV during specific sleep stages warrants further investigation. Although it is evident that a sympathovagal dysregulation occurs with OSA patients, it remains unclear how specific sleep stages vary in sensitivity to the effects, and information is limited on how sleep stages, apnea severity, and other variables such as age, sex, and body mass index (BMI) interact and contribute to HRV determined autonomic changes. Few studies examine the unique effects of OSA on HRV in different sleep stages [29, 30] or obtain nocturnal ECG recordings without excluding arousals, apnea, and hypopnea events that would alter HRV data [31, 32]. Therefore, the purpose of this study was to provide additional data by further examining the relationship between apnea severity and the LF and HF components of HRV from arousal free ECG samples taken from wakefulness, stage 2, and REM sleep.
Materials and methods
Subjects
After approval by the Institutional Review Board, we selected 105 patients from a population of 160 patients who had recently completed overnight polysomnography for suspected OSA. We excluded patients under the age of 18, those with frequent preventricular contractions, heart transplant, or severe cardiomyopathy. In addition, we excluded patients who did not have REM sleep or had insufficient artifact and apnea free data to sample for analysis. The study group had a mean age of 49 ± 11 years (range 20–78) and a mean body mass index of 33 ± 7 kg/m2 (range 19–57). There were 70 men and 35 women. Eleven patients (six women) had diabetes. The mean apnea–hypopnea index (AHI) was 30 ± 29 (range 0–129) (Table 1).
Procedures
A polysomnographic system (Sandman®) recorded electroencephalographic, electroocculographic, chin and leg electromyographic, and electrocardiographic activity. We used inductive plethysmography with thoracic and abdominal bands (Respitrace®) along with intercostal EMG and nasal–oral thermistors to measure respiration. Pulse oximetry monitored oxygen saturation. Technicians recorded pre- and postsleep blood pressures. For patients with severe OSA, continuous positive airway pressure (CPAP) was initiated during the last half of the study (“split-night” protocol) resulting in amelioration of the apnea once the optimal CPAP pressure was reached. Therefore, apnea events continued for at least part of the CPAP titration period in these patients.
Experienced technicians visually scored polysomnographic records for sleep stages and apnea events. Obstructive apneas were defined as complete cessation of airflow for a minimum of 10 s while thoracic and abdominal respiratory efforts continued. Hypopnea required a greater than 50% decrease in airflow for at least 10 s with an accompanying O2 desaturation of more than 2% and an arousal. The AHI was calculated for each patient based on the average number of events per hour. A professional boarded by the American Board of Sleep Medicine reviewed the records and scoring.
The polysomnographic system digitized ECG data at 128 Hz, which has been used by other studies to examine HRV [33, 34]. For each patient, we carefully selected three apnea-free, arousal-free, 3–6 min ECG samples—one from wakefulness, one from stage 2 sleep, and one from rapid eye movement (REM) sleep. Power spectral (frequency domain) analysis of the R–R intervals was used to analyze HRV with the Blackman–Tukey method [32]. The beat series derived from ECG was interpolated and resampled, using cubic splines, to obtain equally spaced RR intervals (time series); the sampling rate of the time series was 3 Hz. The frequency resolution was Δf = 0.00146 Hz. The number of points of the spectral analysis was 1,024 samples. The area under the spectral curve from 0.04 to 0.40 Hz represented total power (bpm2). The low frequency (LF) component (between 0.04–0.15 Hz, reflects both sympathetic and parasympathetic tone) and the high frequency (HF) component (between 0.15–0.40 Hz, reflects parasympathetic tone) were calculated [35–38].
Statistical analysis
We used the statistical software packages Systat® and SPSS® to perform a multivariate repeated measures analysis (sleep stages 0, 2, and REM) looking at HRV (beats per minute/second2) in relation to the AHI, age, BMI, and sex for the HRV measures of TF, LF, HF, and LF/HF.
Results
Effects of AHI and sleep stage on heart rate variability
Overall, HRV increased with increasing AHI [F (df=1, 101) = 10.67, p = 0.001]. In Fig. 1, the TF and LF measures demonstrate this increase and less so for the HF measure. TF increased as AHI increased for samples from wakefulness (p < 0.001) and REM sleep (p = 0.026) although increasing AHI had no relationship to TF in stage 2 (p = 0.257). Similar to TF, the LF component increased as AHI increased for samples from wakefulness (p < 0.001) and REM sleep (p = 0.009). Increasing AHI had no significant effect on LF variability in stage 2 (p = 0.236).
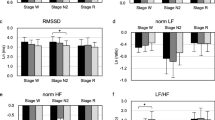
Total, low frequency (LF) and high frequency (HF) heart rate variability in relation to age and sleep stage. Units on the y-axis are beats per minute2 (bpm2). Significant decreases in variability occurred with increasing age for total frequency variability in all sleep stages: wakefulness (p = 0.002), stage 2 (p = 0.022), and REM sleep (p < 0.001). LF decreased with increasing age for all stages: wakefulness (p < 0.001), stage 2 (p = 0.007), and REM sleep (p < 0.001). HF decreased with increasing age only in REM sleep (p = 0.010)
The measure of HF (parasympathetic) was least sensitive to increasing apnea severity by increasing only during wakefulness (p = 0.044). The LF/HF ratio also increased with increasing AHI only in wakefulness (p = 0.025) indicating a relative increased sympathetic role for the TF increase.
Independent of AHI, the LF/HF ratio was the only measure that distinguished the three stages from each other, i.e., wakefulness from stage 2 from REM sleep (Table 2). Stage 2 had the lowest ratio, followed by wakefulness and then by REM sleep reflecting a relative sympathetic dominance of HRV in REM sleep and the least contribution to HRV in stage 2 sleep (Table 2).
As might be expected (Table 2), TF was greatest in REM sleep, followed by wakefulness and stage 2 sleep, which did not differ. LF was also greatest in REM sleep followed by wakefulness and stage 2, which did not differ. HF was greatest in stage 2, which significantly differed from REM and wakefulness. REM and wakefulness did not differ. For the LF/HF ratio, REM sleep was the greatest followed by wakefulness and stage 2 again reflecting greater sympathetic activity in REM and wakefulness sleep. All stages differed significantly.
Secondary measures
Age
TF decreased with increasing age in all categories: wakefulness (p = 0.002), stage 2 (p = 0.022), and REM sleep (p < 0.001). LF also decreased with increasing age for all categories: wakefulness (p < 0.001), stage 2 (p = 0.007), and REM sleep (p < 0.001). HF decreased with increasing age only in REM sleep (p = 0.010). The ratio of LF/HF was not significantly related to age in any stage [F (df=1,100) = 2.495, p = ns] (Fig. 2).
Total, low frequency (LF) and high frequency (HF) heart rate variability in relation to apnea event frequency and sleep stage. Units on the y-axis are beats per minute2 (bpm2). AHI is apnea hypopnea index. Significant increases in variability occurred with increasing apnea frequency for total variability during wakefulness (p = 0.001, and REM sleep (p = 0.026) and for LF in wakefulness (p < 0.001) and REM sleep (p = 0.009)
Sex
The sex of the patient was not significantly related to HRV [F (df=1,100) = 3.176, p = 0.078]. The tendency was for males to have a greater HRV perhaps related to their higher AHI [34 ± 30 vs 21 ± 22, t (df=35,70) = −2.25, p = 0.027]. There was no interaction with wakefulness or sleep [F (df=2,200) = 1.387, p = 0.252], with HRV frequency measures [F (df=3,300) = 0.956, p = 0.414], or three-way interaction [wakefulness or sleep, HRV and sex, F (df=6,600) = 1.283, p = 0.263].
Blood pressure and AHI
Average evening blood pressure (BP) was 137/77 and the average morning BP was 139/79. The AHI positively correlated with postsleep systolic BP (r = 0.30, p = 0.02 with Bonferroni correction). No other BP reading correlated with the AHI (Table 1).
BMI
There was a significant three-way interaction among REM sleep, the HRV measure, and BMI [F (df=6,600) = 2.78, p = 0.011]. This reflected a lower LF and TF in REM sleep in higher BMI patients. Otherwise, BMI was not related to HRV (Fig. 3).
Separate analyses of diabetics (n = 11) and those patients on cardiovascular medications (n = 12) indicated that these subgroups did not differ from other subjects on the HRV measures. Those with CPAP initiation during the polysomnographic study (n = 21) also did not differ from other subjects [HRVtot: stage 0, no CPAP 6.3 ± 6.3; CPAP 7.3 ± 6.1; stage 2, no CPAP 6.4 ± 6.1; CPAP 6.2 ± 4.5; stage REM, no CPAP 11.5 ± 10.8;CPAP 10.0 ± 7.7; F (df=1,98) = 0.088, p=ns] (Fig. 4).
Post hoc correlations between apnea event duration and the LF component indicated small negative and nonsignificant correlations of −0.06, −0.09, and −0.05 in wakefulness, stage 2, and REM sleep, respectively.
Discussion
Heart rate variability in our study population demonstrated a complex relationship to apnea frequency, sleep stage, BMI, and age. Overall, HRV increased as apnea severity increased, particularly for LF and TF measures. Sympathetic nervous system activation, reflected by the increase in LF, accounted for much of the increased total variability. These results are similar to other studies that have found increased LF components of HRV increases in OSA patients [29, 31, 39] and are consistent with an elevated sympathetic drive. Although this sympathetic activation might contribute to negative consequences such as increasing blood pressure, the increased sympathetic activity could help protect against prolonged apnea and oxygen desaturations by lowering the sleep arousal threshold and thus facilitate an earlier termination of apnea events. However, although there was a negative correlation between apnea duration and LF activity, the correlation was small and not significant. As Fig. 1 illustrates, considerable variability exists in the relationship between the AHI and LF measures. Not all high apnea index patients have high LF. We expect that apnea patients with the least HF and LF components of HRV are at the greatest risk for sudden death similar to that which occurs in other low HRV situations [24–28].
Heart rate variability is not uniform across sleep stages. REM sleep is more sensitive to apnea-induced alterations in LF and TF than stage 2 sleep. Because we carefully sampled from epochs not overlapping with arousal or apnea events, this should have minimized the apnea events contribution to changes in LF and HF components of HRV. Furthermore, measures of HRV during REM sleep in non-OSA patients indicate that particularly the LF component increases in REM sleep [28, 40, 41]. In this respect, nonapnea subjects respond similarly to apnea patients. The exclusion of patients without REM sleep allowed us to compare HRV between stage 2 and REM within patients, one of the goals of the study. In addition, patients with apnea severe enough to distort sleep, e.g., eliminate REM sleep, might have had heart rate variability patterns qualitatively different from those who had REM sleep and less severe apnea. Thus, exclusion of patients without REM sleep potentially resulted in a clearer data set at the expense of reducing the range of HRV values.
There was a selective and likely important relationship between BMI and HRV. Only in REM sleep of the higher BMI patients was there a suggestion that autonomic impairment existed. This decrease in LF and TF components of HRV in REM might reflect the beginnings of autonomic dysfunction in the heavy apnea patients. Therefore, those high BMI patients with little LF and TF components of HRV response to apnea could be at the greatest risk for sudden cardiac death and might be candidates for aggressive intervention. However, at least one small study found no relationship to BMI [17].
Because we obtained ECG samples from patients undergoing a sleep study, some patients were on CPAP while others were not. Ideally, we would have studied all patients under the same condition. However, those 21 patients on CPAP in a “split-night” protocol did not differ from other patients on HRV measures. This is not surprising because patients used CPAP at optimal pressure for a relatively brief period during the last half of the night’s titration period. Therefore, we have no evidence that CPAP use in some patients affected the overall results. One study comparing the effects of CPAP at 3 and 9 months on HRV in 13 patients with moderate-to-severe OSA found that CPAP did not change the conventional indices of heart rate variability [42]. Ideally, a long-term prospective study would monitor HRV and follow cardiovascular consequences in CPAP-treated patients.
In conclusion, the results confirm the findings of decreased TF, LF, and HF components of HRV with aging and extend this to a clinical sleep apnea population. By selecting epochs from stage 2 sleep, we showed that the decrease in LF and TF components of HRV with aging is not related to the loss of stage 4 sleep alone. We also confirmed increased sympathetic nervous system activity in apnea patients in an apparent dose response manner even when apnea events are excluded from the sampled ECG. The TF and LF components of HRV increased with the severity of the apnea for men and women and in epochs of REM sleep and wakefulness. The increase was most pronounced in REM sleep. Obese apnea patients had decreased LF and TF components of HRV during REM sleep suggesting autonomic dysfunction, which might increase their risk of mortality. Thus, analysis of HRV offers an additional way to understand the relationship between cardiovascular functioning and sleep apnea. Because data from this study indicate a decrease in HRV during REM sleep in obese patients, it is important to determine if this represents an early marker for autonomic neuropathy in these patients. If so, the availability of digitized ECG signals and computer programs to analyze HRV should add benefit to polysomnography in sleep apnea patients. Spectral measures of HRV during sleep have the potential to determine a patient’s sympathovagal balance, identify at risk patients, and monitor response to any treatment. Furthermore, assessment of intervention effects, such as exercise, weight loss, CPAP, and cardiovascular drugs, may be possible by monitoring HRV.
References
Risk MR, Bril V, Broadbridge C et al (2001) Heart rate variability measurement in diabetic neuropathy: review of methods. Diabetes Technol Ther 3:63–76
Klieger RE, Bigger JT, Bosner MS et al (1991) Stability over time of variables measuring heart rate variability in normal subjects. Am J Cardiol 68:626–630
Bootsma M, Swenne CA, Van Bolhuis HH et al (1994) Heart rate and heart rate variability as indexes of sympathovagal balance. Am J Physiol 266:H1565–H1571
Pomeranz B, Macaulay RJ, Caudill MA et al (1985) Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 248:H151–H153
Malliani A, Pagani M, Lombardi F (1994) Physiology and clinical implications of variability of cardiovascular parameters with focus on heart rate and blood pressure. Am J Cardiol 73:3C–9C
Vinik AI, Maser RE, Mitchell B et al (2003) Diabetic autonomic neuropathy. Diabetes Care 26:1553–1579
Makikallio TH, Huikuri HV, Makikallio A et al (2001) Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J Am Coll Cardiol 37:1395–1402
Algra A, Tijssen JG, Roelandt JR et al (1993) Heart rate variability from 24-hour electrocardiography and the 2-year risk for sudden death. Circulation 88:180–185
Molgaard H, Sorensen KE, Bjerregaard P (2001) Attenuated 24-h heart rate variability in apparently healthy subjects, subsequently suffering sudden cardiac death. J Am Coll Cardiol 37:1395–1402
Boveda S, Galinier M, Pathak A et al (2001) Prognostic value of heart rate variability in time domain analysis in congestive heart failure. J Interv Card Electrophysiol 5:181–187
Vanoli E, Adamson PB, Ba-Lin Pinna et al (1995) Heart rate variability during specific sleep stages: a comparison of healthy subjects with patients after myocardial infarction. Circulation 91:1918–1922
Bonnet MH, Arand DL (1997) Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol 102:390–396
Cajochen C, Pischke J, Aeschbach D et al (1994) Heart rate dynamics during human sleep. Physiol Behav 55:769–774
Trinder J, Kleiman J, Carrington M et al (2001) Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res 10:253–264
Crasset V, Mezzetti S, Antoine M et al (2001) Effects of aging and cardiac denervation on heart rate variability during sleep. Circulation 103:84–88
Okada H, Iwase S, Mano T et al (1991) Changes in muscle sympathetic nerve activity during sleep in humans. Neurology 41:1961–1966
Narkiewicz K, Montano N, Cogliati C et al (1998) Altered cardiovascular variability in obstructive sleep apnea. Circulation 98:1071–1077
Garcia-Rio F, Racionero MA, Pino JM et al (2000) Sleep apnea and hypertension: the role of peripheral chemoreceptors and the sympathetic nervous system. Chest 117:1417–1425
Sanner BM, Doberauer C, Konermann M et al (1997) Pulmonary hypertension in patients with obstructive sleep apnea syndrome. Arch Intern Med 157:2483–2487
Siverberg DS, Oksenberg A (1997) Essential hypertension and abnormal upper airway resistance during sleep. Sleep 20:794–806
Bixler EO, Vgontzas AN, Lin HM et al (2000) Association of hypertension and sleep-disordered breathing. Arch Intern Med 160:2289–2295
Nieto FJ, Young TB, Lind BK et al (2000) Association of sleep disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 283:1829–1836
Peppard, PE, Young T, Paltra M et al (2000) Prospective study of the association between sleep-disordered breathing and hypertension. New Engl J Med 342:1378–1384
Noda A, Yasuma F, Okada T et al (1998) Circadian rhythm of autonomic activity in patients with obstructive sleep apnea syndrome. Clin Cardiol 21:271–276
Baharav A, Kotagal S, Rubin BK et al (1999) Autonomic cardiovascular control in children with obstructive sleep apnea. Clin Auton Res 9:345–351
Salo TM, Jula AM, Piha JS et al (2000) Comparison of autonomic withdrawal in men with obstructive sleep apnea syndrome, systemic hypertension, and neither condition. Am J Cardiol 85:232–238
Wiklund U, Olofsson BO, Franklin K et al (2000) Autonomic cardiovascular regulation in patients with obstructive sleep apnoea: a study based on spectral analysis of heart rate variability. Clin Physiol 20:234–241
Bauer T, Ewig S, Schafer H et al (1996) Heart rate variability in patients with sleep-related breathing disorders. Cardiology 87:492–496
Khoo, MC, Kim TS, Berry, RB (1999) Spectral indices of cardiac autonomic function in obstructive sleep apnea. Sleep 22:443–450
Penzel T, Kantelhardt JW, Grote et al (2003) Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans Biomed Eng 50:1143–1151
Roche F, Gaspoz JM, Court-Fortune I et al (1999) Screening of obstructive sleep apnea syndrome by heart rate variability analysis. Circulation 100:1411–1415
Keyl C, Lemberger P, Dambacher M et al (1996) Heart rate variability in patients with obstructive sleep apnea. Clin Sci 91:56–57
Bigger JTJr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN (1992) Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 85(1):164–171 (Jan)
Bigger JT Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN (1992) Correlations among time and frequency domain measures of heart period variability two weeks after acute myocardial infarction. Am J Cardiol 69(9):891–898 (Apr 1)
Blackman RB, Tukey JW (1959) Measurement of power spectra from the point of view of communications engineering. Dover, New York
Berger RD, Saul JP, Cohen R (1989) Transfer function analysis of autonomic regulation. I Canine atrial rate response. Am J Physiol 256:H142–H152
Saul JP, Berger RD, Chen MH et al (1989) Transfer function analysis of autonomic regulation. II Respiratory sinus arrhythmia. Am J Physiol 256:H153–H161
Saul JP, Berger RD, Albretch P et al (1991) Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol 261:H1231–H1245
Dworschak M, Maurer JT, Haschemian T, Rapp H-J, Waschke KF (2001) The use of spectral measures of heart rate variability to differentiate between male snorers and patients with sleep apnoea syndrome. Anaesthesia 56:424–428
Elsenbruch S, Harnish MJ, Orr WC (1999) Heart rate variability during waking and sleep in healthy males and females. Sleep 22:1067–1071
Berlad II, Shlitner A, Ben-Haim S et al (1993) Power spectrum analysis and heart rate variability in Stage 4 and REM sleep: evidence for state-specific changes in autonomic dominance. J Sleep Res 2:88–90
Khoo MC, Belozeroff V, Berry RB, Sassoon CS (2001) Cardiac autonomic control in obstructive sleep apnea: effects of long-term CPAP therapy. Am J Respir Crit Care Med 164:807–812
Acknowledgement
This study was done at Eastern Virginia Medical School and Sentara Norfolk General Hospital. It was supported by NIH award HL03652-01A1, the Sleep Research Fund of the Eastern Virginia Medical School Foundation, and The Strelitz Diabetes Institute Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Educational objective: To review and expand the knowledge of heart rate variability in sleep apnea patients.
Rights and permissions
About this article
Cite this article
Reynolds, E.B., Seda, G., Ware, J.C. et al. Autonomic function in sleep apnea patients: increased heart rate variability except during REM sleep in obese patients. Sleep Breath 11, 53–60 (2007). https://doi.org/10.1007/s11325-006-0083-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-006-0083-9