Abstract
Automatic positive airway pressure (auto-PAP) devices, used in the treatment of patients with obstructive sleep apnea (OSA), may not function optimally in the presence of an air leak. We set out to determine the magnitude of air leak that prevents auto-PAP devices from responding to respiratory events of OSA in a bench model. We simulated apnea, flow limitation, obstructive hypopnea, nonobstructive hypopnea, and snoring events of OSA with an artificial airway and a loudspeaker in a bench model connected to a commercially available auto-PAP device. Four auto-PAP devices were tested, but two of the “older-generation” devices (Tranquility and Virtuoso) did not respond to events of OSA that involved changes in flow contour; hence, we studied the effects of air leak and humidifier in the two “newer-generation” auto-PAP devices only (GoodKnight 418P, Autoset-T). When the air leak was progressively increased from baseline levels recommended by the manufacturer to levels seen clinically—5 to 7, 10, and 30 l/min—the GoodKnight 418P decreased pressure response by 56% (5.6±1.8 cm H2O, p=0.04). The pressure response of the Autoset-T, however, did not change from baseline during similar levels of air leak. The GoodKnight performed appropriately when the air leak was within 20 l/min, but the corresponding value for the Autoset was higher at 50 l/min. For both devices combined, air leak caused the pressure to drop between the device and the airway: 2.8±0.3 cm H2O at 30 l/min of air leak (p<0.001). Air leaks cause auto-PAP devices to underestimate the pressure required to treat events of OSA and to overestimate the pressure delivered at the upper airway. Physicians should be aware of performance limitations of auto-PAP devices in the presence of an air leak.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most effective treatment for patients suffering from obstructive sleep apnea (OSA) is continuous positive airway pressure (CPAP) [1, 2]. In order to determine the effective treatment pressure, however, a technician manually adjusts CPAP during an overnight polysomnogram performed in the sleep laboratory. Such attended polysomnograms, however, are expensive, labor-intensive, and lengthen wait times for other patients who require diagnostic studies [3, 4]. Automatic positive airway pressure (auto-PAP) devices, however, can help identify the effective treatment pressure without the need for a nighttime technician or laboratory space [5–10].
How reliable are auto-PAP devices in determining effective treatment pressure for treatment of patients with OSA? Clinical studies suggest that auto-PAP devices may under- or overestimate the effective treatment pressure [11, 12]. Alternatively, some clinical studies have demonstrated auto-PAP-derived pressures to be comparable to that determined by the technician during overnight polysomnography [8]. Conceivably, the discrepancies between these studies could arise from the different algorithms used by the various auto-PAP devices used in these studies. Commercially available auto-PAP devices have been reported to differ in how they detect and respond to flow- (apnea, hypopnea, airflow limitation) and pressure-based (snoring) events of OSA [5, 13–15]. Specifically, Farre and colleagues were the first to report differences in the performance of various auto-PAP devices when an air leak was introduced [13]. However, the investigators did not report the magnitude of the air leak or systematically adjust such air leak. Such knowledge of the level of air leak above which these devices fail—a “threshold value”—may allow the physician to discard such data when estimating the effective treatment pressure. Such a method may result in more accurate estimation of effective treatment pressure. In addition, knowing the relative susceptibility of different auto-PAP devices to an air leak is useful information.
Humidifiers can improve a patient’s tolerance of CPAP [16, 17]. When used with an auto-PAP device, however, the humidifier may act as a capacitor and dampen the pressure oscillations that occur during snoring. Additionally, turbulence in the water may confound detection of the flow-based events (apnea, hypopnea, and flow-limitation). Currently, the effect of humidifiers on the performance of auto-PAP devices is not known.
We set out to determine the magnitude of air leak that prevents auto-PAP devices from responding to the respiratory events of OSA. A secondary aim of the study was to study the effect of a humidifier on the performance of auto-PAP devices. We evaluated the performance of the devices in a bench rather than a clinical study because it is difficult to control the level of air leak across patients and devices in the latter setting.
Methods
Model
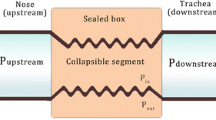
Four auto-PAP devices were tested under baseline conditions without an air leak or humidifiers: GoodKnight 418P (Tyco Healthcare, St. Louis, MO, USA), Autoset-T (ResMed), Tranquility (Healthdyne), Virtuoso (Respironics, Murrysville, PA, USA). An auto-PAP device was connected to an artificial airway (Starling resistor), which was, in turn, connected to an artificial lung (Michigan Instruments, Grand Rapids, MI, USA) driven by a mechanical ventilator (Puritan Bennett 7200, Tyco; Fig. 1). All the auto-PAP devices were tested in a “therapeutic mode,” that is, they were used to detect and respond to events of OSA.
The pressure in the external chamber of the Starling resistor (P star ) was adjusted to simulate the flow pattern of the events of OSA while connected between an artificial lung and an auto-PAP device. Different levels of leak were introduced by using a variable aperture between the device and the artificial lung (Leak). The difference in flow measured close to the Starling resistor (V) and at the outlet of the auto-PAP device (v′) yielded the magnitude of leak. Pressure was measured close to the device (P device ), just distal (P airway ) and proximal (P thorax ) to the Starling resistor, reflecting the pressure delivered by the auto-PAP device, oral pressure, and intrathoracic pressure, respectively
Flow was measured at two locations with heated pneumotachographs (Hans Rudolf Inc., Kansas City, MO, USA): between the artificial airway and the leak aperture (V, Fig. 1) and close to the auto-PAP device (v′, Fig. 1). The difference in flow measured at the artificial airway and the device yielded the magnitude of leak that was introduced. Different levels of air leak were introduced, using a variable aperture located between the auto-PAP device and the artificial airway. For the experiments testing the humidifier, the humidifier was connected to the auto-PAP device as is customary. Pressure was measured by transducers (MP±45, Validyne, Northbridge, CA, USA) connected both distal and proximal to the Starling resistor, thus mimicking the pressure in the upper airway (P airway) and intrathoracic cavity (P thoracic), respectively. Pressure measured in the external chamber of the Starling resistor (P star) was used to verify that the pressures generated in the external chamber of the artificial airway were not different across different devices tested. Pressure was also measured close to the device and reflected the pressure response of the device without the effect of leak—namely, the pressure that the auto-PAP registered in the microprocessor.
The artificial airway consisted of an inner collapsible elastic tube fixed within an outer rigid cylinder. Air was injected into the space between the outer rigid and inner collapsible elastic tube favoring airway collapse. Different levels of airflow limitation (partial collapse) or even apnea (complete collapse) events of OSA were mimicked by varying the amount of air injected into this space (Fig. 2). By design, apneas, obstructive hypopneas, and mild flow limitation were achieved by injecting 4, 3, and 2 ml of air, respectively (Fig. 2). Nonobstructive hypopneas that are seen with periodic breathing were achieved by changing the settings on the ventilator that drove the artificial lung: simultaneously, tidal volume was decreased from 500 to 200 ml and inspiratory flow rate (in the sinusoidal waveform) was reduced from 60 to 20 l/min (Fig. 2). Before beginning the experiments, the reversibility of flow limitation in the bench model was attempted using a conventional CPAP machine (Respironics; Fig. 3). Such a validation study was performed by introducing obstructive hypopnea and then subjecting the airway to manual increments in pressure until the flow contour and magnitude were normalized. Snoring—pressure waves of 30 Hz and 1.5 cm H2O amplitude—was simulated by a loudspeaker that was introduced between the pneumotachograph and the humidifier.
Flow pattern was altered to mimic the events of obstructive apnea. Baseline consisted of a tidal volume of 0.5 l and respiratory rate of 16 breaths per minute. Induced events consisted of nonobstructive hypopneas, obstructive hypopneas, mild flow limitation (not shown), or apnea, with events lasting 15 s
Tracings of inspiratory flow and pressure measured proximal to (P thorax ) and distal to (P airway ) the Starling resistor in the artificial lung model. Representative breaths obtained during flow limitation (CPAP level of 0 cm H20) and after administration of incremental levels of CPAP (4 to 12 cm H20). Note that the contour of inspiratory flow progressively improves from that of severe airflow limitation (when no CPAP was administered) to that of a normal convex-upward contour at a pressure level of 12 cm H2O
Protocol
The auto-PAP devices (Autoset-T, GoodKnight 418P, Tranquility, and Virtuoso) were set at a pressure range of 4–18 cm H2O. The lower limit was 3 cm H2O for the Virtuoso. Because one of the devices has a 15-min dormant period (Tranquility), all devices were subjected to baseline “breathing” conditions for 15 min before events of sleep apnea were introduced. Such baseline breathing conditions constituted a sinusoidal flow contour (Fig. 2), tidal volume of 500 ml, a peak inspiratory flow rate of 60 l/min, and respiratory rate of 16 per minute. Each device was tested against a given event of OSA during a 5-min run. Mild airflow limitation was then introduced for a 15-s duration and repeated every 30 s during the 5-min run. The final pressure reached by the device at the end of the run was taken to represent the pressure response of the device to mild airflow limitation. Between runs, the devices were turned off to prevent any “memory” effects from confounding the experiment. The events were introduced in a random manner for each device.
Similar runs for other respiratory events that occur in patients with OSA, viz., obstructive hypopnea (Fig. 4), nonobstructive hypopnea, and apnea, were performed. These runs were repeated for all devices. We noticed that only the newer-generation auto-PAP devices (GoodKnight 418P and Autoset-T) responded to such flow-based events of OSA and that the older-generation devices (Virtuoso and Tranquility) did not (Fig. 5). Although the latter two devices did react vigorously to “snoring” (pressure-based) events of OSA, they did not respond to the flow-based events during the 5-min runs; hence, we found it counterproductive to perform further experimentation testing the effect of air leak or humidifier on these older-generation auto-PAP devices. Therefore, the rest of this paper describes the performance of the two newer-generation auto-PAP devices only.
Representative tracings of flow proximal to (P thorax ) and distal (P airway ) to the Starling resistor during induction of obstructive hypopneas. Note that the pressure increment in the external chamber of the Starling resistor (P star ) (closed arrow) induces the obstructive hypopnea with flattened inspiratory flow. Subsequently, the auto-PAP device responds to such a stimulus with a 1-cm H2O increase in airway pressure (open arrow)
Response of four auto-PAP devices to apnea events are shown. The apnea events resulted in pressure response from the newer-generation devices—Autoset (open circle) and GoodKnight (closed squares). However, the older-generation devices—Tranquility (open triangles) and Virtuoso (closed triangle)—did not react to such flow-based events of OSA
All of the baseline runs were performed with air leaks produced by the exhalation valves recommended by the manufacturer (5–7 l/min). For each device, multiple runs were repeated at progressively greater levels of leak (10 and 30 l/min) introduced by the variable aperture. Similar runs were performed with and without the inline humidifier. All signals were digitized and stored for analysis on a personal computer (Windaq, Akron, OH, USA). Paired t tests, one-way ANOVA, and two-way ANOVA were performed where appropriate. A p value less than 0.05 was considered to be statistically significant.
Results
For flow-based events, during baseline conditions—in the absence of a humidifier and with manufacturer-recommended levels of leak—the GoodKnight responded with greater final pressures than the Autoset: a difference of 2.1±0.4 cm H2O (p=0.03). Similarly, for pressure-based events, during baseline conditions, the GoodKnight responded more vigorously than the Autoset: a difference of 1.1±0.2 cm H2O (p=0.04). For baseline conditions, the greatest difference in the pressure between the two devices was in response to repetitive apneas—3.5 cm H2O.
Effect of air leak
The Autoset continued to respond to events of OSA at levels of leak up to 50 l/min, while the GoodKnight failed to respond at air leaks of 30 l/min (Fig. 6). The average final pressure achieved by the GoodKnight in response to all of the tested events of OSA at a leak of 30 l/min was 5.6±1.8 cm H2O (56%) less than the response achieved in the absence of leak (p=0.04, one-way ANOVA). There was no change in pressure response of the Autoset with or without observed levels of air leak (p=0.9; one-way ANOVA) (Fig. 7). At air leak of 30 l/min, the GoodKnight tended to respond with a lower final pressure than the Autoset in response to the events of OSA (two-way ANOVA, p=0.06) (Fig. 7).
Pressure response of the Autoset-T (left) and the GoodKnight 418P (right) auto-PAP devices to apnea when air leak was at baseline (closed squares), 10 l/min (open circle), and 30 l/min (closed triangle). Note that while the Autoset responds with the same pressure increment at all levels of leak, the GoodKnight fails to respond at an air leak level of 30 l/min
Pressure response of the auto-PAP devices at different levels of air leak is represented as a percent change from baseline level of air leak. Each symbol represents the average of the pressures achieved in response to the events of OSA. The pressure response of the GoodKnight device decreases with increases in air leak (p=0.04; one-way ANOVA) and thereby tends to respond with lower pressures when compared to the Autoset (p=0.06; two-way ANOVA)
The pressure at the artificial airway was lower than that registered at the device as the magnitude of air leak was increased (Fig. 8). For both devices combined, the final pressure was lower at the artificial airway than at the auto-PAP: by 0.6±0.1 cm H2O at a leak of 5–7 l/min, by 1.0±0.3 cm H2O at a leak of 10 l/min, and by 2.8±0.3 cm H2O at a leak of 30 l/min (p<0.001, one-way ANOVA).
Pressure measured at the upper airway (P airway, left) and auto-PAP device (P device, right) for the Autoset-T in response to repetitive apneas during baseline levels of leak recommended by manufacturer [5 or 7 l/min, (closed squares), 10 l/min (open circles), and 30 l/min (closed triangles) of air leak]. Note that the pressure measured at the airway is progressively lower at the upper airway than at the device with increasing levels of air leak
As anticipated, for a given leak aperture setting, the magnitude of air leak increased as the airway pressure was increased by the auto-PAP device (Fig. 9). In Fig. 9, the measured air leak is plotted against the pressure response of the two devices for flow-based events of OSA (obstructive hypopnea, nonobstructive hypopnea, and apneas). At air leaks less than 20 l/min, the GoodKnight continued to respond to the events of OSA (left and middle panels, Fig. 9). However, at air leaks greater than 30 l/min, the GoodKnight failed to respond—the pressure level remained at 4 cm H2O (right panels, Fig. 9). In contrast, however, the Autoset continued to perform at air leaks of 50 l/min in response to repetitive events of OSA excepting nonobstructive hypopneas (right upper and right lower panels, Fig. 9).
Measured air leak is plotted against the pressure response of two auto-PAP devices for flow-based events of OSA: obstructive hypopnea (top), nonobstructive hypopneas (middle), and apneas (bottom). Such plots are also shown for different aperture openings that caused a leak of 5 or 7 l/min (left), 10 l/min (middle), or 30 l/min (right) at a starting pressure of 4 cm H2O. Note that as the auto-PAP devices respond to the events with an increase in pressure the level of air leak for a given aperture opening increases in a linear manner. Unlike the GoodKnight device, regardless of the level of air leak, the Autoset device does not respond to hypopneas that lack flow limitation
Nonobstructive hypopneas
During baseline conditions, the GoodKnight responded to nonobstructive hypopneas with an increase in pressure from 4 to 7 cm H2O—a 75% increment—whereas the Autoset failed to respond (left middle panel, Fig. 9).
Effect of humidifier
The final pressure reached by the GoodKnight in response to flow-based events with or without the humidifier was not different: 6.9±2.0 and 7.9±1.9 cm H2O, respectively (p=0.7). Similarly, the final pressure reached by the Autoset in response to flow-based events was not different with or without the humidifier: 5.6±1.2 and 6.1±1.3 cm H2O, respectively (p=0.8). In response to pressure-based events (snoring), however, both devices achieved lower final pressures with the humidifier than without: 4.1±0.1 vs 6.2±0.4 for the Autoset (p<0.01), and 4.1±0.1 vs 6.3±0.5 for the GoodKnight (p<0.01).
Discussion
This is the first study to systematically examine the effect of varying levels of air leak and the addition of humidifier on the performance of auto-PAP devices. There are some general observations. First, in the preliminary study of four devices, only the two newer-generation devices detected and reacted to the flow-based events of OSA. The old devices—Virtuoso (Respironics) and Tranquility (Healthdyne)—did not detect or respond to flow-based events of OSA. The old devices responded primarily to pressure-based events of OSA. Second, an air leak of 30 l/min or more can cause the GoodKnight to respond less vigorously—56% less than baseline—but the Autoset can perform well even at air leaks of 50 l/min. Third, air leak causes the pressure at the upper airway to drop significantly lower than that delivered—and therefore registered—by the auto-PAP device. Fourth, the humidifier attenuates the auto-PAP response to snoring, but not flow-based events of OSA. Fifth, the GoodKnight increased the pressure in response to nonobstructive hypopneas (hypopneas with a normal “convex” inspiratory flow contour), but the Autoset did not.
Determination of effective pressure
Currently, auto-PAP devices may be used to determine the effective treatment pressure for patients with OSA [3, 4]. Typically, after a 2-week period of use, the information stored in these devices—reflecting the pressure levels achieved—is downloaded, and the effective pressure determined as the 95th percentile of the pressures administered [5, 18]. In bench studies, however, investigators have shown that different auto-PAP devices achieved different pressure levels when subjected to the same events of OSA [13, 14]. None of these investigators, however, had systematically adjusted the air leaks or examined the performance of the devices in the presence of a humidifier. Knowledge of a “threshold level” of air leak above which performance of such devices deteriorates will allow physicians to analyze only data that were acquired within such leak levels. Based on this experiment, the levels of air leak below which data can be safely included during identification of treatment pressure is 50 l/min for Autoset and 20 l/min for GoodKnight. Conceivably, the effective pressure determined in such a manner would be more accurate.
Besides quantifying and identifying the threshold of air leak within which auto-PAP devices remain reliable, there are other important differences between our study and that of Farre and colleagues [13]. While Farre and colleagues measured pressure at one location—close to the device—we measured pressure both at the device and at the airway. Air leak that occurs between the device and the upper airway would be expected to cause a pressure drop between the two sites. Such a difference will result in the auto-PAP device overestimating the pressure delivered at the upper airway since the pressure delivered by the device is what is registered in the microprocessor memory. Moreover, the difference between the pressure registered in the device and that measured at the airway seems to increase in proportion to the magnitude of air leak. Differences in pressure can reach levels up to 3 cm H2O in the current study, but it is also true that such differences may be greater if the auto-PAP device were to administer much higher pressures.
The bench model in the study by Farre and colleagues [13] was not designed to respond to changes in the airway pressure effected by the auto-PAP device. This is contrary to real-life situations wherein for a certain transmural pressure, as long as body position and sleep stage remain constant, increments in airway pressure would be expected to at least begin to rectify the obstructive events (Fig. 3). Lastly, we compared the effect of hypopneas—with and without flow limitation—on the performance of auto-PAP devices, whereas Farre and colleagues studied hypopneas with flow limitation only. Knowledge of such differences between auto-PAP devices has the following important clinical implication.
Central apneas and nonobstructive hypopneas, which are part and parcel of periodic breathing, can be present in a number of patients with OSA. Moreover, in many patients with severe OSA, periodicity in breathing may develop at high levels of CPAP due to unstable control of breathing [19–21]. In our study, in the absence of leak or in-line humidifier, hypopneas with normal inspiratory flow contours elicited a 75% increase in airway pressure from the GoodKnight but elicited no increase from the Autoset (middle row, Fig. 9). When such nonobstructive hypopneas (the hypopneas of periodic breathing) elicit increments in pressure, frank central apneas may result in patients [20]. Studies have shown that patients with OSA may develop central apneas when CPAP level is increased by auto-PAP devices [19, 22]. Besides disrupting sleep, in two of six patients, such central apneas resulted in oxygen desaturations and arrythmias, leading to study termination [19]. Although, such propagation of apneas was not demonstrated in the current bench study, theoretically, such a response of an auto-PAP device to nonobstructive hypopneas may be expected to induce central apneas in patients with OSA who have a predisposition to develop central apneas at higher levels of CPAP. Notably, clinical studies of auto-PAP devices have been performed on carefully selected patients, and patients with central sleep apnea and congestive heart failure were often excluded [3, 6–8, 10, 11]. Alternatively, such increments in pressure may be preferable in response to nonobstructive hypopneas—with concomitant oxygen desaturations—that occur during rapid eye movement sleep.
Some devices detect the presence or absence of cardiac artifacts in the flow tracing or impedance measurements to distinguish an occluded airway from a patent airway. The assumption is that the airway is patent during a central apnea. However, Badr and colleagues have demonstrated that the airway may remain collapsed during central apneas [22]. Hence, noninvasive methods of discriminating central and obstructive apneas may not be accurate. In our bench model, cardiac artifacts were obviously not present, and thus both devices reacted to these apneas. While the response of the Autoset device appeared to plateau, the GoodKnight continued to increase pressure in an unrelenting manner (Fig. 6). Such increments in CPAP levels, by both devices, despite the persistence of apneas may contribute to breathing instability in some patients [20].
Snoring and humidifier
Most of the auto-PAP devices react to pressure-based events, viz., snoring. Ayappa and colleagues have shown that decreases in effective CPAP levels can result in snoring in up to 30% of occasions [24]. In our study, in-line humidifiers attenuated the response of both auto-PAP devices to snoring by approximately 2 cm H2O. The clinical significance of such a small, yet statistically significant, difference in pressure can be debated. However, the clinical use of auto-PAP indefinitely, or a 2-week period for determining effective pressure level, is much longer than the 5-min runs in the current study. Such long periods of clinical use may magnify the impact of such seemingly small pressure differences observed in the current study.
Device settings
It should be noted that the devices in the current study were used at their default settings. Conceivably, certain devices can have their responsiveness to hypopneas or central apneas disabled, but the sleep physician may not be aware of such options. Other settings, such as percentage of drop in flow that constitutes a hypopnea, settling time, changes in the length of tubing, type of mask, and presence or absence of an in-line humidifier, may need to be specified for some of these devices. Currently, the impact of such settings on the performance of these devices, and whether sleep physicians are aware of such settings, is unknown.
Critiques and limitations
The current study evaluates two auto-PAP devices in a bench model, and caution is advised in extrapolating these results to clinical situations or other devices. First, aspects of the auto-PAP algorithm that were not tested here—rate of pressure increase or decrease, pressure level at which events were detected, and type and number of events previously detected—may interact in a complex manner and attenuate the effects attributable to air leak alone. Such interplay of factors may be one reason why the auto-PAP devices do not perform differently in clinical studies [5, 25]. However, these studies were performed in highly selected patients. Patients with significant nasal congestion or uvulopalatopharyngoplasty—and possibly greater air leak due to mouth breathing—were excluded in many studies [3, 6–8, 10, 11]. Second, manufacturers continually upgrade these devices. For example, a recent version of GoodKnight (420E) responds to inspiratory flow limitation but not to nonobstructive hypopneas. However, such nuances in algorithms may create significant differences in tolerance and safety [19], and the sleep physician needs to be apprised of this. Third, after preliminary study of four devices, only two newer-generation devices were chosen because, at the time of the study, these were the only devices that detected and reacted to the flow-based events of OSA. Other devices, such as the Virtuoso (Respironics) and Tranquility (Healthdyne), did not detect or respond to flow-based events of OSA.
Fourth, the pressure response characteristics of a device in response to events of OSA may not be directly related to superior performance of the device. For example, at face value one could assume that the Autoset-T is superior to the GoodKnight because the former responded in the presence of air leak of 30 l/min, whereas the latter did not (Fig. 6). Conversely, the GoodKnight may be assumed to perform better because of its vigorous response to 13 cm H2O in contrast to the “plateau” of 9 cm H2O reached by the Autoset-T in the absence of significant air leak (left panel, Fig. 6). Fifth, the final pressure response was chosen as the end point—instead of the normalization of inspiratory flow contours—because the duration of each run was brief and sufficient time was not allowed for normalization of the inspiratory flow contours. Similarly, we chose not to vary the shapes of the inspiratory flow contours—as in real life—as this was not a focus of this particular study. Sixth, the duration of time that the devices were tested during each run was only 5 min. The conclusions of this study cannot be extrapolated from the “brief” bench experiments to the clinical realm. In particular, the older devices may function by detecting only pressure-based events while not sensing or responding to flow-based events of OSA. However, clinical studies that systematically quantify these effects and the effects of air leaks and their influence on patient tolerance and compliance are needed. Lastly, our model produced only a limited number of inspiratory flow limitation patterns as compared to other such models that are capable of producing a larger variety of flow configurations that more closely mimic events produced by patients with sleep apnea [13].
Conclusion
In summary, the presence of an air leak allows auto-PAP devices to underestimate the effective treatment pressure for patients with OSA and overestimate the pressure delivered at the upper airway. The net effect of such opposing influences appears to favor underestimation of the effective treatment pressure in this bench study. In conclusion, physicians prescribing these auto-PAP devices should be familiar with the performance of the devices they prescribe [26]. We speculate that physicians may screen the downloaded data for air leak and, based on this information, they could truncate the data at appropriate air leak levels that are peculiar to each device. Conceivably, this may help them estimate the effective treatment pressure with greater accuracy.
References
American Thoracic Society (1994) Indications and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndromes. Official statement adopted March 1994. Am J Respir Crit Care Med 150:1738–1745
Sullivan CE, Issa FG, Berthon-Jones M, Eves L (1981) Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1:862–865
Berry RB, Parish JM, Hartse KM (2002) The use of auto-titrating continuous positive airway pressure for treatment of adult obstructive sleep apnea. An American Academy of Sleep Medicine review. Sleep 25:148–173
Littner M, Hirshkowitz M, Davila D et al (2002) Practice parameters for the use of auto-titrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome. An American Academy of Sleep Medicine report. Sleep 25:143–147
Senn O, Brack T, Matthews F, Russi EW, Bloch KE (2003) Randomized short-term trial of two autoCPAP devices versus fixed continuous positive airway pressure for the treatment of sleep apnea. Am J Respir Crit Care Med 168:1506–1511
Planes C, D’Ortho MP, Foucher A, Berkani M, Leroux K, Essalhi M, Delclaux C, Quera-Salva MA, Lofaso F (2003) Efficacy and cost of home-initiated auto-nCPAP versus conventional nCPAP. Sleep 26:156–160
Hudgel DW, Fung C (2000) A long-term randomized, cross-over comparison of auto-titrating and standard nasal continuous airway pressure. Sleep 23:645–648
Lloberes P, Ballester E, Montserrat JM et al (1996) Comparison of manual and automatic CPAP titration in patients with sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 154:1755–1758
Teschler H, Berthon-Jones M (1998) Intelligent CPAP systems: clinical experience. Thorax 53:S49–S54
Meurice JC, Marc I, Series F (1996) Efficacy of auto-CPAP in the treatment of obstructive sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 153:794–798
Ficker JH, Wiest GH, Lehnert G, Wiest B, Hahn EG (1998) Evaluation of an auto-CPAP device for treatment of obstructive sleep apnoea. Thorax 53:643–648
Berkani M, Lofaso F, Chouaid C et al (1998) CPAP titration by an auto-CPAP device based on snoring detection: a clinical trial and economic considerations. Eur Respir J 12:759–763
Farre R, Montserrat JM, Rigau J, Trepat X, Pinto P, Navajas D (2002) Response of automatic continuous positive airway pressure devices to different sleep breathing patterns: a bench study. Am J Respir Crit Care Med 166:469–473
Lofaso F, Leroux K, Quera-Salva MA, Mrove G, D’Ortho MP, Isabey D, Louis B (2002) Snoring detection during auto-nasal CPAP. Eur Respir J 19:108–112
Lofaso F, Lorino AM, Duizabo D, Najafi Zadeh H, Theret D, Goldenberg F, Harf A (1996) Evaluation of an auto-nCPAP device based on snoring detection. Eur Respir J 9:1795–1800
Massie CA, Hart RW, Peralez K, Richards GN (1999) Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest 116:403–408
Wiest GH, Lehnert G, Bruck WM, Meyer M, Hahn EG, Ficker JH (1999) A heated humidifier reduces upper airway dryness during continuous positive airway pressure therapy. Respir Med 93:21–26
Levy P, Pepin JL (1998) Auto-CPAP: an effective and low-cost procedure in the management of OSAS? Eur Respir J 12:753–755
Juhasz J, Schillen J, Urbigkeit A, Ploch T, Penzel T, Peter JH (1996) Unattended continuous positive airway pressure titration. Clinical relevance and cardiorespiratory hazards of the method. Am J Respir Crit Care Med 154:359–365
Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W (2001) Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 163:1181–1190
Meza S, Mendez M, Ostrowski M, Younes M (1998) Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol 85:1929–1940
Badr MS, Toiber F, Skatrud JB, Dempsey J (1995) Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 78:1806–1815
Boudewyns A, Van de Heyning P, De Backer W (1998) Appearance of central apnoea in a patient treated by auto-CPAP for obstructive sleep apnoea. Respir Med 92:891–893
Ayappa I, Norman RG, Hosselet JJ, Gruenke RA, Walsleben JA, Rapoport DM (1998) Relative occurrence of flow limitation and snoring during continuous positive airway pressure titration. Chest 114:685–690
Ayas NT, Patel SR, Malhotra A et al (2004) Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 27:249–253
Parthasarathy S, Habib M, Quan SF (2005) How are automatic positive airway pressure and related devices prescribed by sleep physicians? A web-based survey. J Clin Sleep Med 1:27–34
Acknowledgements
This study was supported by the Richard M. Bane Trust. The corresponding author has received lecture fees of $1,500 from Respironics, Inc. in the year 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coller, D., Stanley, D. & Parthasarathy, S. Effect of air leak on the performance of auto-PAP devices: a bench study. Sleep Breath 9, 167–175 (2005). https://doi.org/10.1007/s11325-005-0032-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-005-0032-z