Abstract
To evaluate sleep-related obstructive breathing events in patients with obstructive sleep apnea–hypopnea syndrome (OSAHS), we developed a technique for digital recording and analysis of esophageal pressure (Pes) and elucidated the Pes parameters. Pes was recorded overnight with a microtip-type pressure transducer in 74 patients with OSAHS. Simultaneously, in all patients digital polysomnography was recorded. The mean nadir end-apneic Pes swing (Pes Nadir) ranged from −20.2 to −147.4 cmH2O, with a mean of −53.6±2.9 cmH2O. Correlation of the mean Pes Nadir indicated a linear relationship with the mean ratio of maximal Pes swing to apnea duration (r2=0.70) and the mean area of the Pes (Pes Area) (r2=0.82). Significant correlations were noted between the mean Pes Nadir and apnea–hypopnea index (AHI, ranging from 7.9 to 109.5 per hour; r2=0.66), minimum SpO2 (r2=0.60), oxygen desaturation index (ODI) of more than 3 (r2=0.65), arousal index (r2=0.54), and between the mean Pes Area and AHI (r2=0.63), minimum percutaneous arterial oxygen saturation (SpO2; r2=0.57), ODI (r2=0.69), and arousal index (r2=0.41). Pes parameters were found to be significant in the evaluation of the severity of the respiratory effort during the sleep-related obstructive breathing events for patients with OSAHS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In obstructive sleep apnea–hypopnea syndrome (OSAHS), upper airway occlusion is caused by an imbalance between the airway dilator muscle tone and respiratory effort [1]. Increasing respiratory effort against the obstructed upper airway during sleep increases the negativity of esophageal pressure (Pes) and prolongs the cessation of airflow before an electroencephalography (EEG) arousal. The arousal that then occurs is associated with a rapid increase in Pes, called Pes reverse, back to resting levels. Postapneic hyperventilation begins after such arousals. Pes reflects pleural pressure. The important feature of the Pes measurement in sleep disordered breathing is that the respiratory effort in sleep-related breathing events can be determined. Pes measurement can detect respiratory effort-related arousals (RERA), central apnea–hypopnea, and Cheyne–Stokes breathing associated with central sleep apnea (CSB-CSA). According to the recommendations of the American Academy of Sleep Medicine (AASM), the measurement of Pes with continuous overnight monitoring provides results that are in good to excellent agreement with those of the standard reference methods of measurement (grade A) and also adequate in terms of the quality of evidence (level 1) for RERA, central hypopnea–apnea, and CSB-CSA [2]. However, there have been no guidelines for using the Pes measurement for OSAHS. Polysomnography (PSG) usually does not include detailed Pes analyses; in addition, analogue analysis of the intensity of the Pes is usually so complicated that only a few sleep laboratories are doing Pes recording for night-sleep studies. In this study, a computational method to integrate Pes overtime and digital analyses of Pes parameters are provided to evaluate the intensity of the respiratory effort during sleep-related obstructive breathing events.
Methods
Study subjects
Seventy-four patients with OSAHS, 63 men and 11 women, were enrolled in this study. Patients with irregular sleep/wake schedules and period of sleep deprivation such as shift workers were eliminated. The patients’ apnea–hypopnea index (AHI) ranged from 7.9 to 109.5 per hour, and the mean AHI was 47.3±2.9 per hour. The patients’ ages ranged from 16 to 70 years, with a mean of 47.1±1.6 years. The average body mass index (BMI) of the patients was 28.1±0.6 kg/m2, ranging from 19.5 to 42.9 kg/m2. Each patient gave informed consent to the protocol, which was approved by the Human Research Committee of our institute.
Polysomnography
An overnight sleep study was carried out using digital PSG (Alice 3, Healthdyne Technologies, Marietta, GA, USA). Briefly, EEG (C4/A1, C3/A2), electrooculography, submental electromyography and electrocardiography using surface electrodes, measurement of airflow at the nose and mouth using a thermistor, measurement of respiratory movements of the rib cage and abdomen by inductive plethysmography, and percutaneous arterial oxygen saturation (SpO2) using a finger pulse oximeter were simultaneously carried out. PSG data were scored manually on a high-resolution monitor using 30-s epochs for staging and arousal detection and 2- or 5-min epochs for respiratory data. Sleep data were scored without visualization of respiratory channels, and respiratory data were scored with arousal data. The predominant sleep stage was scored for each 30-s epoch coded as wake, stage 1, stage 2, stage 3/4 (slow wave sleep), or rapid eye movement (REM) according to the criteria developed by Rechtshaffen and Kales [3]. Arousals were identified according to the criteria of the American Sleep Disorders Association [4]. Apneas were identified as a nearly flat airflow (<25% of baseline, wherein the baseline amplitude is identified during the nearest preceding period of regular breathing with stable oxygen saturation) for at least 10 s. Hypopneas were identified as an airflow or a thoracoabdominal excursion was approximately <70% of baseline for at least 10 s associated with either an oxygen desaturation of >3% or an arousal.
Procedure for digital recording of the esophageal pressure
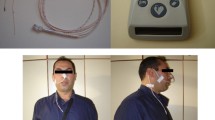
The measurement of Pes with continuous overnight monitoring was performed with a 5F microtip-type pressure transducer (MPC500, Millar, Houston TX, USA). Simultaneously, in all patients digital PSG was recorded. Signals from the pressure transducers were amplified by a signal conditioner (BioAmp, Power Lab, Gladstone, Australia) and converted by a 4-channel analog to digital (A/D) converter (Power Lab/4s, ADI Instrument Pty Ltd, Castle Hill, Australia), then interfaced with the Power Lab and Alice 3 polygraph by means of a transducer control unit TC500 and stored for subsequent analysis in the hard disk of a personal laptop computer. Tracings and data were displayed on the computer and on the screen of Alice 3 PSG. The mesophageal pressure (Pmes) was often simultaneously recorded to identify the obstructive sites by another catheter, the pressure sensor of which was located at the epiglottic tip. Larger pressure differences between the pressure-sensing parts of the catheters reveal the site of obstruction. In the case of respiratory airflow measurement, a snug-fitting oronasal face mask with a pneumotachometer was fitted on the patient overnight. Total airflow was calculated with a laminar flow meter (LFM-317, METABO, Lausanne, Switzerland). SpO2 was monitored with a pulse oximeter (NPB290, Mallinckrodt, St. Louis, MO, USA). These airflow and SpO2 signals were also conveyed directly to the Power Lab system and simultaneously displayed on the computer screen (Fig. 1).
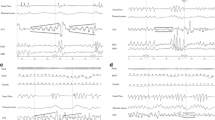
Example of a digital recording in an obstructive sleep apnea patient. The uppermost trace is the tidal volume of airflow (ml/s, pneumotachograph; inspiration downwards); second, esophageal pressure; third, mesophageal pressure (cmH2O; all inspiratory Pes values were negative); and the lowermost is SpO2 (%, pulseoximeter)
Calibration of pressure transducer, patient biocalibrations
Before the Pes measurements, the balance controls were adjusted to obtain the atmospheric pressure (zero) baseline position. After confirming the stability and absence of artifacts in the pressure signals, the transducers were calibrated with a transducer calibration system (X Caliber, Ohmeda Pte Ltd, Singapore) before each study. Positive (+10, +50, +100 mmHg) and negative (−10, −50, −100 mmHg) pressures were applied, then the sensitivity was adjusted to show readings of the same pressure on the computer display. We confirmed that the baseline returned to zero after the positive pressure or vacuum was released. After anesthetizing the nose with 4% xylocaine, the catheter was inserted 35 cm from the nostrils with the pressure-sensing parts of the catheter located in the esophagus in the sitting position. After insertion, the pressure-sensing catheter was able to detect pressures generated by respiratory and cardiac effects. In case the signals did not show these respiratory and cardiac effects, the catheter was correctly positioned by direct pharyngoscopy. Since the reference for all pressure measurements was the atmospheric pressure, all values are given as absolute values and all inspiratory Pes values were negative. In each subject we confirmed that the pressure signals were obtained breath by breath and recorded the corresponding pressure changes on the computer display. Then, the patients performed maximum inhalation from functional residual capacity (FRC). After ventilation at rest several times, the patients performed the maximum inhalation at FRC, did maximum inspiratory maneuver up to total lung capacity (TLC), and then held their breath for 3 to 5 s while in the supine position. The Pes values during maximal inspiratory effort while awake, called PesMax [5], were measured in each patient.
Digital analyses of Pes parameters
Between 80 and 100 obstructive breathing events demonstrating the severity of the respiratory effort were selected for each patient during non-rapid eye movement (NREM) sleep and the possible interference from REM sleep was excluded. Pes parameters were digitally analyzed using Chart software (ADI instrument Pty Ltd, Castle Hill, Australia). Figure 2 schematically represents the Pes analysis procedure. The upper trace shows the Pes increases in four apnea events, and the lower trace is a reverse image of the upper part simplified into a line graph. The integration of Pes increases in one obstructive breathing event (Pes Area) was digitally calculated from this line graph. The maximal negative pressure swing (Max) was identified and also the maximal end-apneic pressure swing, from zero to this inspiratory Max, called Pes Nadir in this study. The nadir is determined by measurement of the peak through differences in the waveforms on a breath-to-breath basis. Pes Duration means the time from the minimal negative pressure swing (Min) to the next Min. The rate of increase in Pes, the ratio of Pes Nadir to the duration of the apnea in seconds, is expressed as Ratio Pes.
Parameters of the esophageal pressure. A section of a recording shows how the esophageal pressure (Pes) readings were made. The upper trace shows Pes increases in four apnea events, and the lower trace is a reverse image of the upper part simplified into a line graph. The right upper column shows digitally analyzed parameters of each Pes increase. Min Minimal negative pressure swing; Max maximal negative pressure swing; Pes Nadir (Pes Min Value in the upper right column), nadir end-apneic Pes swing; Pes Duration (Sel Duration in the upper right column), the time from a Min to the next Min; Pes Area (Pes Area Int Lowest in the upper right column), the integration of Pes increase in one obstructive breathing event
Statistical analysis
Data are expressed as means ± SE. The differences between the means of variables in this study were analyzed using unpaired Student’s t test. Correlations between parameters were evaluated using Spearman’s correlation coefficient. A p value less than 0.05 was considered to be significant.
Results
The Pes Nadir in apnea events of the 74 patients with OSAHS ranged from −10 to −168.5 cmH2O, and in hypopnea events ranged from −10 to −85.7 cmH2O. The mean Pes Nadir ranged from −20.2 to −147.4 cmH2O, with a mean of −53.6±2.9 cmH2O.
Figures 3 and 4 show the correlations between Pes Parameters. The correlations of the mean Pes Nadir indicated a linear relationship with the mean Ratio Pes (r2=0.70, p<0.01) and the mean Pes Area (r2=0.82, p<0.01) (Fig. 3). In contrast, although negative pressure increases with apnea time, there were no correlations between the mean Pes Duration and the mean Pes Nadir (r2=0.17), mean Pes Area (r2=0.10) (Fig. 4).
The next series of studies was performed to elucidate the direct relationship between the Pes parameters and polysomnographic parameters by correlation analyses. We used the Pes Nadir and the Pes Area for representative parameters demonstrating the intensity of sleep-related obstructive breathing events. The results of the correlation between the Pes Nadir, the Pes Area, and AHI are shown in Fig. 5. The AHI had a relatively moderate correlation both with the Pes Nadir (r2=0.66, p<0.01) and the Pes Area (r2=0.63, p<0.01). However, for an individual patient whose AHI was 105.0 per hour, the mean Pes Nadir was only −35.2 cmH2O. On the other hand, the AHI with the largest mean Pes Nadir (−147.4 cmH2O), was only 54.7 per hour. In the case of patients with OSAHS, only the AHI does not always reflect the severity of the disease. Figure 6 shows the correlations between the Pes Nadir, the Pes Area and the minimum SpO2. The minimum SpO2 is considered to be a marker of the intensity of the sleep-related obstructive breathing events and this correlated moderately both with the mean Pes Nadir (r2=0.60, p<0.01) and the mean Pes Area (r2=0.57, p<0.01). Because the minimum SpO2 rarely goes below 50% (only three of 74 patients in the present study), these correlations did not indicate a linear relationship. On the other hand, ODI showed relatively linear correlations both with the mean Pes Nadir (r2=0.65, p<0.01) and with the mean Pes Area (r2=0.69, p<0.01) (Fig. 7). Moreover, a moderate correlation was noted between the arousal index and the mean Pes Nadir (r2=0.54, p< 0.01) and the mean Pes Area (r2=0.41, p<0.05) (Fig. 8).
Discussion
We revealed that digital recording of the Pes with a Power Lab system enabled detailed Pes analyses in patients with OSAHS. The Pes Nadir, the Area Pes, and the Ratio Pes were closely correlated with each other, and significant correlations were noted between Pes parameters AHI, minimum SpO2, ODI, and arousal index. Upper airway resistance can usually be studied by the change in the wave pattern shown by the inspiratory airflow using nasal pressure cannula monitoring. This study, however, allows one to see the actual relationships of the Pes changes directly as they occur.
Pes values and parameters
Since the reference for all pressure measurements was the atmospheric pressure, all values are given as absolute values and all inspiratory Pes values were negative. There is no absolute level of esophageal pressure that is known to be abnormal. During normal respiration PES deflections are usually less than −10 cmH2O in normal subjects during sleep. However, this normative cutoff is based on clinical experience. Further studies are necessary to establish and validate a particular cutoff value.
Although we were not able to demonstrate direct evidence that these Pes parameters are independent of each other, Pes parameters analyzed in this study are plausibly important for understanding the severity of OSAHS. Pes Nadir reflects the maximum instantaneous energy of the respiratory effort; on the other hand, Pes Area reflects the total amount of energy of the respiratory effort in each obstructive breathing event. Both should be recognized as representative Pes parameters demonstrating the intensity of the obstructive breathing events during sleep.
The intensity of the respiratory effort changes with age or the lung capacity. The Pes value measured during maximal inspiratory effort while awake is called PesMax [5]. The Pes Nadir of any patient may depend on his or her own PesMax value. As for Pes Nadir, it might be desirable to estimate by percent of PesMax. Pes Duration, defined as the time from Pes Min to Pes Min, is also determined by the portion of apnea where the Pes swings are falling after apnea onset. This may be determined by the ventilatory overshoot during arousal, etc. The measurement of the Pes Area this time did not include postapneic area exactly. This may explain why there was no correlation between Pes Area and Pes Duration. DeltaPes, the difference between the Max and the Min, was described by Krieger et al. as a measure of the increase in respiratory effort throughout an episode of apnea [6]. However, the DeltaPes is not suitable to represent the respiratory effort in patients with morbid obesity because, since there is no Pes reverse to the normal range, differences between the Max and the Min may be underestimated as compared to the respiratory effort.
Severity of sleep-related breathing events
It is currently a standard procedure in clinical practice and epidemiologic studies to assess the severity of OSAHS by combining the number of episodes of apnea, hypopnea, and RERA as AHI. However, not only between hypopneas and normal breathings, but also between apneas and hypopneas does interchangeable misidentification sometimes occur because of variability in the definitions of the degree of reduction of the airflow or respiratory effort when scoring breathing events [7]. On the other hand, the Pes swing at the inspiratory peak during sleep is an absolute value and rarely influenced by the differences in the scorers or methods of detection. Our previous study demonstrated that upper airway surgeries such as uvulopalatoplasty or tonsillectomy change apneas to hypopneas, reducing the Pes rather than AHI, suggesting that only AHI does not always reflect the severity of the disease in patients with OSAHS [8]. Pulse transit time is considered as a noninvasive alternative estimate of inspiratory effort; however, beat to beat measurement does not truly represent the maximum and minimum value of respiratory effort [9]. Only an increasing negativity of the Pes directly reflects the energy of respiratory effort and demonstrates the intensity of the obstructive breathing events during sleep. The detection of airflow is considered essential for assessing sleep-related obstructive breathing events; however, crescendo negative increases in the Pes values are also important in OSAHS. Not only respiratory disturbance indices but also the Pes measurement are beneficial in the evaluation of the sleep-related breathing events.
The influence of the increased negative Pes
There are several possible explanations concerning the influence of the Pes. First, Pes may affect the arousal threshold and even respiratory drive. In patients with OSAHS, apnea termination occurs coincident with arousal that is predominantly produced by the stimulation of the upper airway mechanoreceptors. Berry et al. reported that the correlation between the values of Pes and EEG delta power was found to be significant and, thus, Pes monitoring can be used to identify an arousal threshold [10]. In the case of an accelerated hypercapnic ventilatory response (HCVR), the respiratory drive will increase, making the respiratory effort stronger in patients with OSAHS [11]. Sforza et al. reported that the respiratory effort was correlated positively with the awake ventilatory response to hypoxia and hypercapnia, suggesting that Pes may be connected with the respiratory control system [12]. Second, Pes influences both the right and left ventricular functions. Because the pulmonary vasculature is exposed to pleural pressure (Ppl), increased negative Ppl during obstructive apnea could increase the pulmonary arterial transmural pressure, i.e., the difference between the pulmonary artery pressure and Ppl [13, 14]. Increased negative Ppl also increases the venous return to the right ventricle, and the resulting right ventricular distension can lead to a leftward shift of the interventricular septum during diastole, thereby impeding left ventricular (LV) filling [15]. Negative Ppl can also reduce the rate of LV relaxation, further increasing the impedance to LV filling and raising the LV pressure [16] leading to an increase in the difference between the internal and external heart pressure, i.e., the transmural pressure, which is an important determinant of the LV afterload, and decreases in the stroke volume and cardiac output [17, 18]. It is very important for understanding the pathogenesis of the OSAHS to know the influence of an exaggerated subatmospheric Pes.
Possible therapeutic application
Pes deflection, e.g., less than −10 cmH2O, can be a titration goal for continuous positive airway pressure (CPAP). A strong correlation was noted between the efficacious pressure of CPAP, the critical pharyngeal pressure, and the Pes [19, 20]. CPAP treatment contributes to not only airway patency but also cardiac and sympathetic improvement such as LV function [21], pulmonary hemodynamics [22], and sympathetic activity [22–24] for OSAHS patients both with and without cardiovascular diseases. The CPAP titration system, together with measurement of the degree of oronasal inspiratory airflow limitation in terms of the amplitude of a valid measure of breathing, flow limitation detected at an outlet of CPAP machine, and thoracoabdominal movements, has been used as the gold standard for patients with OSAHS. The optimal pressure determined by CPAP titration should be changed when the patient does not adapt to the CPAP treatment and has poor compliance. In such a case, the respiratory effort or cardiac parameters also could be good alternative titration markers. Shiomi et al. reported that the presence of pulsus paradoxus in OSAHS indicates a marked increase in Pes Nadir, and its disappearance with nasal CPAP may account for the effectiveness of OSAHS [15]. Although the present cardiac or sympathetic technologies such as Doppler echocardiography or radionuclide ventriculography are not useful for CPAP titration, Pes and cardiac parameters are more important than flow limitation in terms of the consequences and complications of OSAHS [17, 18].
In conclusion, we demonstrated that digital recording of the Pes is easy to perform for clinical sleep facilities and provides varied and detailed Pes analyses in patients with OSAHS. Pes parameters should be found to be significant in the evaluation of the severity of sleep-related obstructive breathing events as well as AHI or other PSG parameters and could be used as therapeutic objective for a biological marker.
References
Cistulli PA, Sullivan CE (1994) Pathophysiology of sleep apnea. In: Saunders NA, Sullivan CE (eds) Sleep and breathing, 2nd edn. Marcel Dekker, New York, pp 405–448
American Academy of Sleep Medicine Task Force (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22:667–689
Rechtschaffen A, Kales A (1968) A manual of standardized terminology, technique and scoring system for sleep stages of human sleep. Brain Information Service, Los Angeles
American Sleep Disorders Association (1992) EEG arousals: scoring rules and examples [ASDA Report]. Sleep 15:173–184
McCool FD, Hershenson MB, Tzelepis GE, Kikuchi Y, Leith DE (1992) Effect of fatigue on maximal inspiratory pressure-flow capacity. J Appl Physiol 73:36–43
Krieger J, Sforza E, Boudewijns A et al (1997) Respiratory effort in obstructive sleep apnea role of age and sleep state. Chest 112:875–884
Suzuki M, Saigusa H, Chiba S et al (2005) Discrepancy in polysomnography scoring for a patient with obstructive sleep apnea hypopnea syndrome. Tohoku J Exp Med 206 (in press)
Suzuki M, Ogawa H, Okabe S et al (2004) The effect of upper airway structural changes on central chemosensitivity in obstructive sleep apnea hypopnea. Sleep Breath 8:73–83
Pitson DJ, Sandell A, Van den Hout R, Stradling JR (1995) Use of pulse transit time as a measurement of inspiratory effort in patients with obstructive sleep apnea. Eur Respir J 8:1669–1674
Bery RB, Asyali MA, McNellis MI, Khoo MCK (1998) Within-night variation in respiratory effort preceding apnea termination and EEG delta power in sleep apnea. J Appl Physiol 85:1434–1441
Chapman KR, Bruce EN, Gothe B, Cherniack NS (1988) Possible mechanisms of periodic breathing during sleep. J Appl Physiol 64:1000–1008
Sforza E, Boudewijns AN, Schenedecker B, Zamagni M, Krieger J (1996) Role of chemosensitivity in intrathoracic pressure changes during obstructive sleep apnea. Am J Respir Crit Care Med 154:1741–1747
Parish JM, Shepard JW (1990) Cardiovascular effects of sleep disorders. Chest 97:1220–1226
Marrone O, Bonsignore MR, Romano S, Bonsignore G (1994) Slow and fast changes in transmural pulmonary artery pressure in obstructive sleep apnea. Eur Respir J 7:2192–2198
Shiomi T, Guilleminault C, Stoohs R, Schnittger I (1991) Leftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndrome. Chest 100:894–902
Virolainen J, Ventilä M, Turto H, Kupari M (1995) Effect of negative intrathoracic pressure on left ventricular pressure dynamics and relaxation. J Appl Physiol 79:455–460
Shamsuzzaman AS, Gersh BJ, Somers VK (2003) Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 290:1906–1914
Lorenzi-Filho G, Bradley TD (2002) Mechanism cardiac function in sleep apnea. In: Pack AI (ed) Sleep apnea pathogenesis, diagnosis, and treatment. Marcel Dekker, New York, pp 377–410
Sforza E, Krieger J, Bacon W et al (1995) Determinants of effective continuous positive airway pressure in obstructive sleep apnea. Role of respiratory effort. Am J Respir Crit Care Med 151:1852–1856
Sforza E, Petiau C, Weiss T, Thibault A, Krieger J (1999) Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med 159:149–157
Alchanatis M, Tourkohoriti G, Kosmas EN et al (2002) Evidence for left ventricular dysfunction in patients with obstructive sleep apnea syndrome. Eur Respir J 20:1239–1245
Sajkov D, Wang T, Saunders NA, Bune AJ, McEvoy D (2002) Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med 165:152–158
Roche F, Court-Fortune I, Pichot V et al (1999) Reduced cardiac sympathetic autonomic tone after long-term nasal continuous positive airway pressure in obstructive sleep apnea syndrome. Clin Physiol 19:127–134
Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K (1995) Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J 8:222–229
Nelesen RA, Yu H, Ziegler MG et al (2001) Continuous positive airway pressure normalizes cardiac autonomic and hemodynamic responses to a laboratory stressor in apneic patients. Chest 119:1092–1101
Acknowledgements
The authors express their deepest appreciation to Mr. Kenichi Suzuki, Shinichi Chida (both of Technical Service Co.), and Takeshi Hayakawa (Bio Research Center Co.) for providing technical support for the Power Lab system, and Miss Mineko Hayashi PSGT (Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine) for data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, M., Ogawa, H., Okabe, S. et al. Digital recording and analysis of esophageal pressure for patients with obstructive sleep apnea–hypopnea syndrome. Sleep Breath 9, 64–72 (2005). https://doi.org/10.1007/s11325-005-0015-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-005-0015-0