Abstract
Purpose
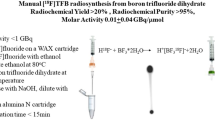
The aim of this study was the automated synthesis of the mitochondrial membrane potential sensor 4-[18F]fluorobenzyl-triphenylphosphonium ([18F]FBnTP) on a commercially available synthesizer in activity yields (AY) that allow for imaging of multiple patients.
Procedures
A three-pot, four-step synthesis was implemented on the ELIXYS FLEX/CHEM radiosynthesizer (Sofie Biosciences) and optimized for radiochemical yield (RCY), radiochemical purity (RCP) as well as chemical purity during several production runs (n = 24). The compound was purified by solid-phase extraction (SPE) with a Sep-Pak Plus Accell CM cartridge, thereby avoiding HPLC purification.
Results
Under optimized conditions, AY of 1.4–2.2 GBq of [18F]FBnTP were obtained from 9.4 to 12.0 GBq [18F]fluoride in 90–92 min (RCY = 28.6 ± 5.1 % with n = 3). Molar activities ranged from 80 to 99 GBq/μmol at the end of synthesis. RCP of final formulations was > 99 % at the end of synthesis and > 95 % after 8 h. With starting activities of 23.2–33.0 GBq, RCY decreased to 16.1 ± 0.4 % (n = 3). The main cause of the decline in RCY when high amounts of [18F]fluoride are used is radiolytic decomposition of [18F]FBnTP during SPE purification.
Conclusions
In initial attempts, the probe was synthesized with RCY < 0.6 % when starting activities up to 44.6 GBq were used. Rapid radiolysis of the intermediate 4-[18F]fluorobenzaldehyde and the final product [18F]FBnTP during purification was identified as the main cause for low yields in high-activity runs. Radiolytic decomposition was hindered by the addition of radical scavengers during synthesis, purification, and formulation, thereby improving AY and RCP. The formulated probe in injectable form was synthesized without the use of HPLC and passed all applicable quality control tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondrial metabolism provides adenosine triphosphate (ATP) as the main source of chemical energy in the cell and produces a myriad of other important co-factors as well as metabolites. Mitochondrial dysfunction has been associated with human diseases such as diabetes [1], cardiac failure [2], neurodegenerative diseases [3], and cancer [4]. There have been rapid advances in the development of tools to study mitochondrial metabolism in vitro during the last decades [5]. Among these tools, monitoring mitochondrial membrane potential (ΔΨm) with lipophilic, cationic dyes has emerged as one of the most commonly used techniques [6]. While lipophilic cations have proven good molecular markers of mitochondrial activity, in vivo study of mitochondrial potential requires the development of noninvasive molecular imaging probes.
To harness lipophilic cations for the assessment of ΔΨm in vivo, carbon-11-labeled phosphonium salts were synthesized [7] and studied as imaging probes [8] for positron emission tomography (PET) [9]. The synthesis of 4-[18F]fluorobenzyl-triphenylphosphonium ([18F]FBnTP) was later investigated to take advantage of the superior decay properties of fluorine-18 (t 1/2 = 109.8 min, maximum β + energy = 635 keV) [10]. The probe was successfully used in various animal models for imaging of paclitaxel-induced apoptotic activity [11], unstimulated brown adipose tissue [12], ischemic areas after transient coronary occlusion [13], and myocardial perfusion [14]. The original synthesis by Ravert et al. [10] was reported to proceed in four steps, affording the probe in 6 % nondecay corrected (n.d.c.) radiochemical yield (RCY) within 82 min and molar activities (A m) of 16.7 GBq/μmol. The synthesis was performed manually and no activity yields (AY) were reported. The same group later reported a microwave-facilitated synthesis using custom hardware delivering the probe in n.d.c. RCY of 8.3 % within 52.4 ± 14 min and A m of 534.5 ± 371.4 GBq/μmol [15]. Again, no AY or starting activities were disclosed. Zhang et al. recently described the formation of [18F]FBnTP in one step via copper-mediated 18F-fluorination using a pinacolyl boronate precursor [16]. Formation of the probe was reported in high RCY of 62 ± 1.4 % before purification. The synthesis was performed manually and neither AY nor A m of the purified product formulation were revealed. Even though metal-mediated 18F-fluorinations will likely be used for the clinical production of PET imaging probes in the future, their compliance with the principles of current good manufacturing practice (cGMP) has not been extensively established to this date [17].
In this study, we intended to translate the original four-step synthesis into a clinically useful production procedure on a commercially available radiosynthesizer that reliably affords multiple doses ready for injection into patients. Since the three-pot design of the ELIXYS FLEX/CHEM radiosynthesizer allows for complete automation of the sequence, it was chosen for this task [18].
Materials and Methods
Materials
All commercially available reagents and materials were used as received unless otherwise specified. Acetonitrile (MeCN, extra dry, 99.9 %), dimethyl sulfoxide (DMSO, extra dry, 99.7 %), and dichloromethane (DCM, extra dry, 99.9 %) were obtained from Acros Organics. Ethanol (EtOH, 200 proof, anhydrous) was obtained from Decon. All water used was purified to 18 MΩ and passed through a 0.1-mm filter. 4-Trimethylammoniumbenzaldehyde trifluoromethanesulfonate and 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane (K222, 98 %) were purchased from ABX. Triphenylphosphine dibromide (Ph3PBr2, 96 %; stored and handled in a glovebox), sodium borohydride on alumina (NaBH4⋅(Al2O3) x ); stored and handled in a glovebox), and triphenylphosphine (PPh3, 99 %) were purchased from Sigma-Aldrich. 4-Fluorobenzyl triphenylphosphonium chloride (FBnTP, nonradioactive reference, 98 %) was purchased from Alfa Aesar. l-Ascorbic acid sodium salt (Na-ascorbate, 99 %) was purchased from Acros Organics. Potassium carbonate (K2CO3) was purchased from FisherChemical. Sodium chloride solution (saline, 0.9 %, injection, USP) was purchased from Hospira. Sep-Pak Plus Accell CM cartridges (preconditioned with EtOH (10 ml), followed by deionized water (20 ml)), Oasis WCX Plus cartridges (preconditioned with EtOH (5 ml), followed by deionized water (10 ml)), Sep-Pak Light Plus QMA cartridges (preconditioned with 0.5 N KHCO3 (3 ml), followed by deionized water (5 ml)), and Sep-Pak Classic Silica cartridges (wetted with DCM (3 ml)) were purchased from Waters. Grace™ SCX Maxi-Clean cartridges (wetted with deionized water (5 ml)) were purchased from Alltech. Millex-GV (SLGV013SL and SLGV004SL) sterile filters with a 0.22-μm pore size hydrophilic PVDF membrane were purchased from MilliporeSigma. Reactor vials (W986259NG), reagent vial septa (224100-072), and crimp caps (224177-01) were purchased from Wheaton. Reagent vials (62413P-2) were purchased from Voigt. Magnetic stir bars (14-513-65) were purchased from Fisher Scientific. Glass chromatography columns (006SCC-10-10-AA) were purchased from Omnifit Labware.
Chromatography and Analytical Methods
Radio thin layer chromatography (radio-TLC) was performed on silica plates (TLC Silica gel 60 W F254s, Merck). After spotting a small sample volume (~ 1–5 μl) using a glass capillary, the plate was developed in the mobile phase (MeCN). Chromatograms were obtained using a radio-TLC scanner (miniGita Star, Raytest). Radio high-performance liquid chromatography (radio-HPLC) chromatograms for quality control were registered using a 1200 Series HPLC system (Agilent Technologies) equipped with a GabiStar flow-through gamma detector (Raytest). Data acquisition and processing was performed using OpenLAB CDC ChemStation Edition 2016 (Agilent Technologies). Analytical HPLC was used to estimate the radiochemical purity (RCP), the A m, and the chemical purity of final formulations of [18F]FBnTP. A radioactive sample (200 μl) was taken from the final formulation and injected for HPLC analysis (5 μl injection volume). HPLC method 1: Alltech Adsorbosphere SCX 5u, 250 × 4.6 mm. Gradient: A = MeCN (+ 0.05 % TFA); B = water (+ 0.05 % TFA); flow rate = 1.8 ml/min; 0–12 min 90 % B to 5 % B, 12–16 min 5 % B, 16–17 min 5 % B to 90 % B; 254 nm detection wavelength. RCP was calculated by dividing the area under the curve (AUC) for the desired product by the sum of AUC for all peaks. To confirm the structural identity of the synthesized compound, a radioactive sample (195 μl) of the final formulation was diluted with a 10-mM solution of the nonradioactive reference in water (5 μl) and injected for HPLC analysis (5 μl injection volume). Calibration curves of UV absorbance versus molar mass were created in advance for calculating A m (Suppl. Fig. 1). Curves were obtained using a linear-least square fit of absorbance versus molar mass for six data points spanning the expected molar mass range. A m was calculated by dividing the radioactivity of the injected sample by the molar mass (as determined from the AUC for the UV peak and the calibration curve). A m reported are decay corrected at the end of the synthesis. For measurement of RCY (expressed as the percentage of the starting activity and corrected for decay) or AY (not corrected for decay), final formulations were measured in a dose calibrator (Capintec). Analytical HPLC was used to analyze radioactive samples of intermediate steps of the sequence in low-activity runs. HPLC method 2: Phenomenex Luna 5u C18 (2) 100 A, 250 × 4.6 mm, 5 μm. Gradient: A = MeCN (+ 0.05 % trifluoroacetic acid (TFA)); B = water (+ 0.05 % TFA); flow rate = 1.8 ml/min; 0–12 min 90 % B to 5 % B, 12–16 min 5 % B, 16–17 min 5 % B to 90 % B; 254 nm detection wavelength.
Radiosynthesizer Setup
Dedicated cassettes for [18F]FBnTP synthesis were reused, and liquid pathways cleaned with MeCN before each experiment using an automated sequence (Suppl. section 2 and Suppl. “FBnTP_cassette-cleaning_sequence”). The cleaned cassettes were loaded into the synthesizer and locked into position. Clean reactor vials with a magnetic stir bar were placed in the reactor positions. Shortly before the start of the synthesis, reagent vials were filled with the appropriate reagents and then sealed with a septum and crimp cap. Once sealed, the reagent vials were inverted and loaded into the appropriate cassette reagent positions. Preconditioned cartridges and the glass column for the reduction step were installed on the cassettes using Luer fittings in the positions indicated in Fig. 1. Interconnections were made as indicated in Fig. 1. Finally, a mixture of EtOH (~ 50 ml) and ice was added to the vacuum trap Dewar.
Synthesis Protocol
No-carrier-added [18F]fluoride was produced by the (p,n) reaction of [18O]H2O (~ 97 % isotopic purity, Medical Isotopes) in a RDS-112 cyclotron (Siemens) at 11 MeV using a 1-ml tantalum target with Havar foil. The activity was unloaded from the target and pushed through a strong cation exchange (SCX) cartridge (if applicable) before entering the QMA cartridge. Trapped [18F]fluoride was subsequently eluted with a solution of K222 (10 mg, 27 μmol) and K2CO3 (1 mg, 7 μmol) in acetonitrile/water (3:5, 0.8 ml) into the first reaction vessel (1.5 min, 7 psig nitrogen pressure). Contents were partially evaporated while applying vacuum and a stream of nitrogen (10 psig) at 110 °C for 3.5 min with stirring. Acetonitrile (1.2 ml) was added through the QMA cartridge to wash residual activity into the vessel, and the combined contents evaporated for 3.0 min at 110 °C (7 psig nitrogen pressure). Acetonitrile (1.2 ml) was directly added to the reactor, and the contents were fully evaporated for 2.5 min at 110 °C (7 psig nitrogen pressure). The reactor was cooled to 35 °C, and a solution of 4-trimethylammoniumbenzaldehyde trifluoromethanesulfonate (5 mg) in DMSO (0.8 ml) was added. Contents were reacted at 90 °C for 5 min with stirring. A 1 % (w/v) Na-ascorbate solution (3 ml followed by 2 ml) was added to the reactor under stirring, and the resulting mixture was passed through an Oasis WCX cartridge over a period of 1.5 min (6 psig nitrogen pressure). Another batch of 1 % (w/v) Na-ascorbate solution (3 ml followed by 2 ml) was added to the reactor and passed through the cartridge. The cartridge was blow-dried for 1 min (20 psig nitrogen pressure) and eluted with DCM (3 ml). The mixture was passed through a glass chromatography column, containing NaBH4⋅(Al2O3) x (350 mg) followed by K2CO3 (2 g) and directed into the second reaction vessel (3 psig nitrogen pressure). Cartridge and column were rinsed with DCM (1 ml, containing 0.2 % (v/v) of water) (3 psig nitrogen pressure). A solution of Ph3PBr2 (100 mg) in DCM (1.1 ml) was added to the second reaction vessel and the mixture was reacted at 35 °C for 10 min. All contents of the second reaction vessel were passed through a silica cartridge into the third reaction vessel (2 psig nitrogen pressure). A solution of PPh3 (3 mg) in EtOH (0.6 ml) was added to the third reaction vessel, and all contents were partially evaporated to approximately 2 ml by applying both vacuum and a stream of nitrogen (3 psig) at 45 °C for 3.5 min with stirring. The silica cartridge was rinsed with DCM (1 ml) (1 psig nitrogen pressure), and the contents of the third reaction vessel were partially evaporated to approximately 0.5 ml by applying both vacuum and a stream of nitrogen (3 psig) at 45 °C for 6.5 min with stirring. EtOH (1 ml) was added and the contents were partially evaporated to approximately 0.5 ml by applying both vacuum and a stream of nitrogen (7 psig) at 80 °C for 2.5 min with stirring. The resulting mixture was reacted in a sealed position at 160 °C for 5 min with stirring. The reaction vessel was cooled to 35 °C and water (3 ml) was added with stirring. The resulting mixture was passed through a Sep-Pak Plus Accell CM cartridge (8 psig nitrogen pressure). The Sep-Pak was immediately washed with EtOH (20 ml) and the product released with 2 % EtOH in saline + 0.5 % (w/v) Na-ascorbate (10 ml) and directed through a sterile filter into a vented sterile vial.
Results
The aim of this study was to develop a reliable synthesis protocol for the potentiometric PET-probe [18F]FBnTP on a commercially available radiosynthesizer. For practical clinical translation, the final formulation should be injectable, i.e., pass all applicable quality control tests, and should be obtained in AY that allow for imaging of multiple patients. To achieve these goals, we adapted a four-step, three-pot synthesis [10] by assembling a sequence of “unit operations” (e.g., “trap,” “elute,” “evaporate,” “react”) that are programmed into the synthesizer-software and specifying the parameters for each (e.g., temperature, reaction time, pressure) [19]. A seamless flow of solvents was tested in abbreviated runs without activity or reagents to ensure its correctness. The sequence was optimized for RCY and RCP during several production runs (n = 24) using activity amounts that averaged around 30.8 GBq. Adjustments to the sequence were made based on careful analysis of the crude product composition as well as the residual activity retrieved from vital parts of the setup (i.a. reactors, SPE-cartridges, and waste containers). We also performed low-activity test runs starting with an average of 300 MBq of [18F]fluoride which allowed for safe withdrawal of samples for radio-HPLC as well as radio-TLC analysis between each step. The reaction vessel was made accessible between steps by using the “Move Reactor” operation (Suppl. “FBnTP_low-activity-production_sequence”). The optimized sequence consists of 33 “unit operations” and runs autonomously without user interference (Suppl. “FBnTP_production_sequence”). The synthesis includes intermediate solid-phase extraction purifications, an on-column reduction, controlled solvent evaporation steps, and reactions in sealed position at temperatures above the boiling points of the respective solvents. An overview of the synthesizer setup as well as the flow of liquids is depicted in Fig. 1. The SPE purification with a Sep-Pak Plus Accell CM cartridge is supported by two electrically actuated three-position valves that are controlled by the user from outside the hotcell.
The synthetic steps of the optimized sequence with respective reaction conditions are shown in Scheme 1.
Briefly, the sequence consists of the synthesis of 4-[18F]-fluorobenzaldehyde ([18F]FBA), subsequent reduction of the aldehyde functionality with NaBH4⋅(Al2O3) x , conversion of 4-[18F]fluorobenzyl alcohol ([18F]FBnOH) to 4-[18F]fluorobenzyl bromide ([18F]FBnBr) with Ph3PBr2, and formation of [18F]FBnTP by nucleophilic substitution with Ph3P. Following this sequence, the probe was synthesized without the use of HPLC in AY of 1.4–2.2 GBq of [18F]FBnTP from 9.4 to 12.0 GBq [18F]fluoride in 90–92 min (RCY = 28.6 ± 5.1 % with n = 3). Molar activities at the end of synthesis ranged from 80 to 99 GBq/μmol and RCP was > 99 % in all cases. The formulated probe in injectable form passed all applicable quality control tests.
Discussion
Optimization of [18F]FBA Formation
The first synthetic step consists of [18F]FBA formation with subsequent SPE purification using an Oasis WCX cartridge. In our early synthesis attempts, [18F]FBnTP was obtained in poor RCY < 0.6 % with starting activities that averaged around 41.1 GBq. We observed that up to 80 % of the starting activity had passed through the Oasis WCX cartridge during SPE purification of [18F]FBA. HPLC analysis of the waste activity showed no evidence of [18F]FBA but of an unknown, polar, impurity alongside unreacted [18F]fluoride. This implies that [18F]FBA was available in RCY < 20 % for the subsequent three steps, hampering overall RCY of [18F]FBnTP. Since numerous publications describe the synthesis of [18F]FBA in RCY ranging from 50 to 72 % [20, 21], we investigated the reason for the low formation of [18F]FBA in our hands. We considered radiolysis of [18F]FBA during SPE purification in the presence of water as a possible reason for low yields from high starting activities [22]. This possibility was explored by synthesizing [18F]FBA with escalating amounts of radioactivity and a negative correlation between starting activities and synthesis yield was indeed observed (Suppl. Table 1). We found that radiolysis during SPE purification was efficiently hindered by replacing water with a 1 % (w/v) Na-ascorbate solution. Analysis of the crude mixture in low-activity test runs showed formation of [18F]FBA in high purity (radio-HPLC = 94–99 %, radio-TLC = 92 %; Suppl. Figs. 2 and 3). After addition of water, 69–79 % of the starting activity were trapped on the Oasis WCX cartridge, indicating high yields of [18F]FBA, similar to previously reported results. The waste activities after this step amounted to 20–25 % of starting activities. Following the addition of 1 % (w/v) Na-ascorbate solution, similar percentages of waste activities were observed in high-activity [18F]FBnTP production runs, leading us to conclude that hindrance of radiolysis was key in enabling the synthesis of [18F]FBA in high AY.
During certain production runs, we observed a considerable decrease of [18F]FBA formation as indicated by SPE waste activities of up to 48 %. This coincided with reports of low 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) yields at the Biomedical Cyclotron facility at our institution. The yield drop was correlated to the release of undesired metal contaminants from the targets, rendering the [18F]fluoride inactive for the nucleophilic attack during [18F]FBA synthesis. With cleaning of the cyclotron target body and replacement of the Havar foil during cyclotron maintenance, [18F]FBA yield was restored. This led us to place an SCX cartridge in between the [18F]fluoride delivery line and the cassette of the synthesizer as indicated in Fig. 1, which equally proved effective in restoring the yield. Between 18 and 27 % of the activity delivered by the cyclotron accumulated in the SCX cartridge. Other strategies that efficiently reduce metal contaminants alongside negligible [18F]fluoride loss have been reported [23].
Optimization of [18F]FBnOH Formation
The formation of [18F]FBnOH proceeds by elution of [18F]FBA from the Oasis WCX cartridge and channeling liquids through a glass column filled with the reducing agent NaBH4⋅(Al2O3) x and subsequent drying with K2CO3. In early attempts, diethyl ether (Et2O) was used as the eluent which efficiently eluted [18F]FBA off the cartridge but turned out to be problematic during the subsequent bromination step (vide infra). Et2O was successfully replaced with 3 ml of dry DCM, but a second elution with 1 ml of DCM was necessary to sufficiently release [18F]FBA off the Oasis WCX. Since a proton source is necessary to form the OH-functionality from the alcoholate obtained in the reduction of aldehydes with hydride, up to 38 % of the starting activity remained in the NaBH4 bulk when extra dry DCM was used. This was addressed by adding a small amount of water (0.2 % v/v) to the second 1-ml elution with DCM to rinse the column. With this, only 1–3 % of the starting activity remained in the NaBH4 bulk at the end of the synthesis. Efficient reduction of the aldehyde with conversion rates of 83–98 % was confirmed by analytical radio-HPLC in low-activity runs (Suppl. Fig. 4).
Optimization of [18F]FBnBr Formation
Transformation of [18F]FBnOH into [18F]FBnBr is achieved through the use of bromination agent Ph3PBr2 with subsequent silica cartridge purification. In early attempts, we added a solution of [18F]FBnOH in Et2O directly to a solution of Ph3PBr2 in DCM, which resulted in unpredictable yields and synthesis failures. Replacing Et2O with DCM during the on-column reduction avoided the formation of a biphasic system as well as precipitation of by-products derived from Ph3PBr2. Moreover, weighing the Ph3PBr2 solution in a glovebox, and adding the dry solvent shortly before the start of the sequence to the septum-sealed vial of the reagent resulted in a more reliable bromination efficiency. Dry conditions have been reported to be a crucial requirement for high bromination yields [24] and strong fuming was observed if the brominating reagent was kept in an open vessel. To further increase the dryness of DCM coming from the preceding reduction step, the amount of K2CO3 in the column was increased from 0.5 to 2 g. With these measures in place, up to 88 % bromination of [18F]FBnOH was observed as confirmed by radio-HPLC in low-activity test runs (Suppl. Fig. 5). Less than 8 % leftover activity on the silica cartridge after purification was an additional indicator of good conversion rates in this step. The leftover activity consisted primarily of unreacted [18F]FBnOH that was predominantly adsorbed on silica whereas [18F]FBnBr passed through efficiently. The more delicate requirements in this step have been the most common cause of inconstant outcomes in our experiments.
Optimization of [18F]FBnTP Formation
The last synthetic step consists of nucleophilic substitution of bromide in [18F]FBnBr with PPh3. Adapting published procedure [10], we initially added [18F]FBnBr in DCM from the previous step to a 21-mg solution of PPh3 in toluene followed by removal of most of the DCM under reduced pressure and heating to 110 °C for 5 min. In our hand, [18F]FBnTP formation from [18F]FBnBr was 28 % using these conditions as confirmed by radio-HPLC. In a nonradioactive condition screening with different solvents and temperatures, we observed almost quantitative FBnTP formation at 160 °C for 5 min in 0.5 ml of either MeCN or EtOH (Suppl. Table 2). EtOH was used as the solvent to hinder radiolysis of [18F]FBnTP during SPE purification (vide infra). The activity fraction associated with [18F]FBnTP in the crude mixture after nucleophilic substitution was 70–81 % in low-activity runs (Suppl. Fig. 6).
Purification and Formulation of [18F]FBnTP
Initially, the crude mixture was purified by semipreparative HPLC. The bulk of unreacted Ph3PBr2 and PPh3 were removed with a Sep-Pak C18 Plus Short cartridge and the resultant mixture was injected into the HPLC system. After collection of fractions containing the desired product and removal of solvent with a rotary evaporator, the probe was reformulated in saline. Problems associated with this approach were loss of product activity to the C18 cartridge, difficulties to remove residual Ph3PBr2 that elutes shortly after [18F]FBnTP under reversed phase conditions, and stickiness of the probe to the flask after removal of the HPLC-solvent. Formulations suffered from low chemical purities and low pH, both of which can be attributed to residual Ph3PBr2. The cationic nature of [18F]FBnTP led to use an analytical SCX HPLC column for the determination of A m and resulted in a well separated UV signal of the product. UV and radio-impurities eluted at considerably earlier retention times than the cationic product activity, allowing for an accurate determination of AUC for A m measurements. This led us to consider using a cation exchange cartridge for purification instead of HPLC, similar to a previously reported use of SPE purification for a cationic probe with a Sep-Pak Plus Accell CM cartridge [25]. In several radioactive and nonradioactive test runs, we optimized conditions to trap and wash [18F]FBnTP on the Sep-Pak. The probe was efficiently trapped on the Accell CM even in the presence of large amounts of Ph3PBr2, PPh3, and other impurities, making the prepurification requirement obsolete. Trapping was achieved in a nearly quantitative manner by diluting the organic solvent in the last step (MeCN or EtOH) with water (water/solvent > 7). Water can function as an activator of the carboxylic acid functionality on the Accell CM resin to enable binding with the cationic probe. After trapping of [18F]FBnTP, the Sep-Pak was washed with EtOH to remove all noncationic radioactive and nonradioactive impurities without any notable losses (Suppl. Fig. 7). While elution with 0.9 % saline proved inadequate for complete release of [18F]FBnTP, ~ 90 % elution was achieved by increasing the ionic strength of saline with 0.5 % (w/v) Na-ascorbate which also stabilized the final formulation against radiolytic decomposition. The probe was obtained in RCP > 99 % and high chemical purity (Suppl. Fig. 9). The purification process from the end of the synthetic sequence to the final formulation took less than 4 min. Unfortunately, considerable radiolysis of the probe was observed during Sep-Pak purification when starting with 23.2–33.0 GBq [18F]fluoride. Analysis of the activity that had passed through the Accell CM revealed a radiosignal of an unidentified, nonionic radiolysis product that accounted for up to 87 % of the waste activity and was not observed in low-activity runs. Addition of radical scavengers such as Na-ascorbate, ascorbic acid, and N-tert-butyl-α-phenylnitrone (PBN) [22] or reservatrol-3-β-mono-d-glucoside (polydatin) [26] either prevented trapping of [18F]FBnTP on the Accell CM or did not hinder radiolysis. Trapping efficiencies were low in pure EtOH and water is known to facilitate radiolysis [22]. We therefore considered activation of the Sep-Pak with hydroxide or organic bases prior to purification in the absence of water but the trapping efficiencies remained low. Consequently, RCY of 28.6 ± 5.1 % (n = 3) were observed with starting activities of up to 12.0 GBq, whereas starting activities reaching 33.0 GBq resulted in lower RCY of 16.1 ± 0.4 % (n = 3). Despite the radiolytic decomposition during purification, the highest AY of 3.0 GBq was obtained from 33.0 GBq [18F]fluoride. Considerable higher AY will be achieved if radiolysis can be prevented in the final step. The probe was used in small animal imaging studies which will be reported elsewhere. Representative maximum intensity projection images of [18F]FBnTP in a wild-type mouse are shown in the Supplementary Material (Suppl. Fig. 10).
Clinical Quality Control
Formulations of the probe in 2 % (v/v) EtOH in saline + 0.5 % (w/v) Na-ascorbate were subjected to a quality control established at the UCLA Ahmanson Biomedical Cyclotron Facility in accordance with the U.S. Pharmacopeia. RCP at the end of synthesis was > 99 % and remained > 95 % for at least 8 h. Chemical purity was estimated to be high (Suppl. Fig. 9). Doses were clear, colorless, and free of particulate matter with pH ranging from 5.0 to 5.8. Half-life of doses matched that of fluorine-18 (109.8 min) and energy of gamma rays was 511 keV. No kryptofix-222 (K222) levels were observed with the spot test. Since the presence of Na-ascorbate gave false positive results, samples were spotted on a silica plate and developed in a mixture of methanol and 30 % ammonium hydroxide (9:1) prior to placing the plate in the iodine chamber. Other than the purposely added EtOH, no residual solvents were detected by gas chromatography. Sterile filters were intact after use. Doses contained < 175 endotoxin units (EU) per ml and no growth was observed at days 3, 7, and 14 as required to pass the sterility test.
Conclusion
A robust synthesis protocol for the production of multiple doses of the potentiometric PET probe [18F]FBnTP was established on the ELYXIS FLEX/CHEM radiosynthesizer. Purification and formulation was achieved in < 4 min with a Sep-Pak Accell CM cartridge. RCY of 28.6 ± 5.1 % (n = 3) were obtained which is a significant improvement to previous reports. Despite our best efforts, radiolysis was not prevented in the final purification step when starting activities exceeded 12.0 GBq [18F]fluoride. Although a possible alternative would be the use of a SCX semipreparative HPLC column and addition of radical scavengers to the mobile phase, this would add additional time and complication. Avoidance of radiolysis with the more elegant Sep-Pak purification can further improve AY in preparing multipatient doses. The reported automated synthesis of clinical grade [18F]FBnTP on a commercially available platform paves the way for the use of the probe in future clinical imaging studies.
References
Montgomery MK, Turner N (2015) Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 4:R1–R15
Dorn GW, Vega RB, Kelly DP (2015) Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev 29:1981–1991
Johri A, Beal MF (2012) Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342:619–630
Weinberg SE, Chandel NS (2015) Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11:9–15
Zhang Y, Avalos JL (2017) Traditional and novel tools to probe the mitochondrial metabolism in health and disease. WIREs Syst Biol Med 9(n/a):e1373. https://doi.org/10.1002/wsbm.1373
Ehrenberg B, Montana V, Wei MD et al (1988) Membrane potential can be determined in individual cells from the Nernstian distribution of cationic dyes. Biophys J 53:785–794
Fukuda H, Syrota P, Charbonneau P et al (1986) Use of 11C-triphenylmethylphosphonium for the evaluation of membrane potential in the heart by positron-emission tomography. Eur J Nucl Med 11:478–483
Madar I, Anderson JH, Szabo Z et al (1999) Enhanced uptake of [11C]TPMP in canine brain tumor: a PET study. J Nucl Med 40:1180–1185
Phelps ME (2000) Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci U S A 97:9226–9233
Ravert HT, Madar I, Dannals RF (2004) Radiosynthesis of 3-[18F]fluoropropyl and 4-[18F]fluorobenzyl triarylphosphonium ions. J Label Compd Radiopharm 47:469–476
Madar I, Huang Y, Ravert H et al (2009) Detection and quantification of the evolution dynamics of apoptosis using the PET voltage sensor 18F-fluorobenzyl triphenyl phosphonium. J Nucl Med 50:774–780
Madar I, Isoda T, Finley P, Angle J, Wahl R (2011) 18F-fluorobenzyl triphenyl phosphonium: a noninvasive sensor of brown adipose tissue thermogenesis. J Nucl Med 52:808–814
Higuchi T, Fukushima K, Rischpler C et al (2011) Stable delineation of the ischemic area by the PET perfusion tracer 18F-fluorobenzyl triphenyl phosphonium after transient coronary occlusion. J Nucl Med 52:965–969
Madar I, Ravert HT, Du Y et al (2006) Characterization of uptake of the new PET imaging compound 18F-fluorobenzyl triphenyl phosphonium in dog myocardium. J Nucl Med 47:1359–1366
Ravert HT, Holt DP, Dannals RF (2014) A microwave radiosynthesis of the 4-[18F]-fluorobenzyltriphenylphosphonium ion. J Label Compd Radiopharm 57:695–698
Zhang Z, Zhang C, Lau J et al (2016) One-step synthesis of 4-[18F]fluorobenzyltriphenylphosphonium cation for imaging with positron emission tomography. J Label Compd Radiopharm 59:467–471
Sanford MS, Scott PJH (2016) Moving metal-mediated 18F-fluorination from concept to clinic. ACS Cent Sci 2:128–130
Lazari M, Collins J, Shen B et al (2014) Fully automated production of diverse 18F-labeled PET tracers on the ELIXYS multireactor radiosynthesizer without hardware modification. J Nucl Med Technol 42:203–210
Claggett SB, Quinn KM, Lazari M et al (2013) Simplified programming and control of automated radiosynthesizers through unit operations. Eur J Nucl Med Mol Imaging Res 3:53
Speranza A, Ortosecco G, Castaldi E et al (2009) Fully automated synthesis procedure of 4-[18F]fluorobenzaldehyde by commercial synthesizer: amino-oxi peptide labelling prosthetic group. Appl Radiat Isot 67:1664–1669
Poethko T, Schottelius M, Thumshirn G et al (2004) Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med 45:892–902
Scott PJH, Hockley BG, Kung HF et al (2009) Studies into radiolytic decomposition of fluorine-18 labeled radiopharmaceuticals for positron emission tomography. Appl Radiat Isot 67:88–94
Schueller MJ, Alexoff DL, Schlyer DJ (2007) Separating long-lived metal ions from 18F during H2 18O recovery. Nucl Instrum Methods Phys Res Sect B 261:795–799
Iwata R, Pascali C, Bogni A et al (2000) A new, convenient method for the preparation of 4-[18F]fluorobenzyl halides. Appl Radiat Isot 52:87–92
Rodnick ME, Brooks AF, Hockley BG et al (2013) A fully-automated one-pot synthesis of [18F]fluoromethylcholine with reduced dimethylaminoethanol contamination via [18F]fluoromethyl tosylate. Appl Radiat Isot 78:26–32
Su D, Cheng Y, Liu M et al (2013) Comparison of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. PLoS One 8:e54505
Acknowledgements
We would like to thank Dr. Michael E. Phelps for support and guidance with this study; Dr. Roger Slavik, Krzysztof Bobinski, and Daniel Yeh for providing [18F]fluoride; Dr. Jason Lee, Dr. Tove Olafsen, and Charles Zamilpa for their help with the small animal imaging; and Dr. Michael van Dam, Jeffrey Collins as well as Krzysztof Bobinski for valuable technical input.
Funding
The authors gratefully acknowledge the support from NIH through program, research, and training grants (CA186842, CA208642 and CA086306) and the support from the Department of Energy (DE-SC0012353).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Waldmann, C.M., Gomez, A., Marchis, P. et al. An Automated Multidose Synthesis of the Potentiometric PET Probe 4-[18F]Fluorobenzyl-Triphenylphosphonium ([18F]FBnTP). Mol Imaging Biol 20, 205–212 (2018). https://doi.org/10.1007/s11307-017-1119-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1119-1