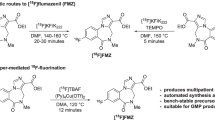
Abstract
Purpose
[18F]Flumazenil, which has the advantage of a longer half-life than [11C]flumazenil, is well known for determining of the central benzodiazepine receptor concentrations. However, [18F]flumazenil has not been widely used because fluctuating and relatively low yields render automatic production insufficient for routine and multicenter clinical trials. Here, we describe the results of a 2.5-year production study of [18F]flumazenil using an iodonium tosylate precursor, which allowed us to overcome the limitations of low and fluctuating radiochemical yields.
Procedures
We developed a clinically applicable production system by modifying a commercial synthesizer for the reliable and reproducible production of [18F]flumazenil for routine clinical studies. [18F]Flumazenil was prepared at 150 °C for 5 min in the presence of 4-methylphenyl-mazenil iodonium tosylate (4 mg), a radical scavenger (TEMPO, 1 mg), and [18F]KF/kryptofix 2.2.2 complex in N,N-dimethylformamide (1 ml). In the purification step, the final mixture was pretreated using different cartridges before performing high-performance liquid chromatography (HPLC) separation. Finally, we measured the radiochemical yield and performed quality-control assays on 94 batches.
Results
After carrying out additional purification before HPLC separation using a C18 plus Sep-Pak cartridge, the radiochemical yield of [18F]flumazenil increased from 34.4 ± 9.7 % (without the pretreatment, n = 24) to 53.4 ± 9.0 % (n = 94), and the lifetime of the semi-preparative column was five times that of the column without the C18 plus Sep-Pak cartridge. The mean-specific activity of [18F]flumazenil was 572 ± 116 GBq/μmol at the end of synthesis, and the radiochemical purity was more than 99 %, as determined by analytical HPLC and radio-TLC. [18F]Flumazenil prepared using this method satisfied all quality-control test standards and was highly stable for up to 6 h after preparation.
Conclusions
The results of the 2.5-year production study using an iodonium tosylate precursor indicate that [18F]flumazenil has commercial and routine clinical applicability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gamma-aminobutyric acid (GABAA) receptor/central benzodiazepine receptor (cBZR) complex has been implicated in various diseases [1]. Radioisotopically labeled flumazenil is an important radiopharmaceutical for the assessment of the central benzodiazepine receptor (cBZR) concentration [2]. Among the various flumazenil (FMZ) analogues labeled with different radioisotopes, [11C]flumazenil ([11C]FMZ, 1a) has been extensively studied; [11C]FMZ-positron emission tomography was one of the first methods developed to determine the changes in cBZR density which is important because low levels of cBZR have been observed in various neurological and psychiatric disorders such as epilepsy [3–5], panic disorder [6], Alzheimer’s disease [7], Huntington’s disease [8], schizophrenia [9, 10], and cortical damage in the brain after acute stroke [11, 12]. However, the disadvantage of [11C]FMZ is the short half-life (t 1/2 = 20 min) of carbon-11 label which limits its use to centers with an onsite cyclotron. Therefore, the fluorine atom of FMZ is an attractive feature in order to introduce fluorine-18 into FMZ without any structural modifications. This fluorine-18 analog, [18F]FMZ (Fig. 1), has the advantage of a longer half-life of 110 min and shows uptake patterns similar to that of [11C]FMZ [13, 14]. Although many studies have demonstrated the radiosynthesis of [18F]FMZ with radiochemical yields of 15–30 %, introduction of fluorine-18 to the native structure of FMZ remains challenging [13, 15, 16]. In recent communications, the radiochemical yield of FMZ was reported to be 3–12 % only, demonstrating the marked variation among different laboratories [14, 15, 17] and the use of nitromazenil as a precursor in a commercial automated module based on conductive heating provided insufficient yields of [18F]flumazenil for multicenter clinical trials [17, 18]. Moreover, the purification of [18F]FMZ was found to be difficult and tedious when nitromazenil (Fig. 1) was used as a labeling precursor, which results in low-specific activity and radiochemical yield.
Recently, we described an advanced method of [18F]FMZ radiosynthesis using various diaryliodonium tosylates as precursors. Among them, 4-methylphenyl-mazenil iodonium tosylate (Fig. 1) showed an optimal radiochemical yield of 67.2 ± 2.7 % (in manual synthesis), which was three times greater than the yield obtained by conventional methods [18]. However, we observed that radiofluorination using an iodonium tosylate precursor had some limitations to be used in routine automated production of [18F]FMZ and others during high-performance liquid chromatography (HPLC) separation, such as unreliable HPLC retention time, impurities in the final product, and shorter life of the HPLC column [19, 20]. In this study, we have adapted the cartridge filtration method, namely, sample pretreatment before HPLC, which improves reproducibility and sensitivity in radioactive material separation and protects the HPLC columns. Further applications, including large-scale production in a conventional module based on conductive heating, are also well adapted. We began a regular production of [18F]FMZ under these conditions in December 2010 for multicenter clinical trials at four different hospitals [21–24]. Herein, we describe the regular production of [18F]FMZ for 2.5 years using an advanced 4-methylphenyl-mazenil iodonium tosylate.
Materials and Methods
Materials
All commercial reagents and solvents utilized were of at least analytical grade and were used without further purification unless otherwise specified. Reagents and solvents were purchased from Sigma-Aldrich (USA). 4-Methylphenyl-mazenil iodonium tosylate, prepared from commercially available isatoic anhydride in five steps based on a previously reported procedure [18], is commercially available from Bio Imaging Korea Co., Ltd., Korea. 18O-Enriched water was purchased from Taiyo Nippon Sanso Co., Japan. [18F]Fluoride was produced at Seoul National University Bundang Hospital using an 18O(p,n)18F reaction through proton irradiation using the KOTRON-13 cyclotron (Samyoung Unitech Co., Korea). Chromafix® PS-HCO3 (45 mg) cartridges were purchased from Macherey-Nagel Ins., Germany. A C18 plus Sep-Pak cartridge and an Alumina N cartridge were purchased from Waters Co., USA. All radiochemical processes, including fluorine-18 separation from 18O-enriched water, incorporation of fluorine-18 with a precursor, HPLC purification with a semi-preparative column (Xterra RP-18, 10 μm, 10 × 250 mm, Waters, USA) and a guard column (Security Guard SemiPrep Cartridge, C18, 10 × 10 mm, Phenomenex, USA), and reformulation of the end-product for clinically injectable ethanol (EtOH)/saline solution using the C18 plus Sep-Pak cartridge, were performed in the TRACERlab™ FXFN (GE Healthcare). Quality control of the final injectable [18F]FMZ solution was performed using HPLC (YMC triart C18, 5 μm, 4.6 × 250 mm) and radio-thin-layer-chromatography (radio-TLC), which was measured on a Bioscan AC-3000 scanner, USA. The specific activity of [18F]FMZ at the end of synthesis was determined using an ultraviolet (UV) calibration curve. The HPLC solvents were of HPLC grade (J.T. Baker, USA) and were used after filtration with a sterile membrane filter (Whatman; pore size, 0.22 μm). Gas chromatography analysis was performed using an Autochro-2000 (YoungLin Instrument, Korea). All radioactivity values were measured using a VDC-505 activity calibrator from Veenstra Instruments (Netherlands) and are presented as decay-corrected values unless otherwise noted.
Reagents and Module Modification
The automatic production of [18F]FMZ was evaluated in the TRACERlab™ FXFN module with a little modification. The operating profile is shown in Fig. 2. The current study includes nine reagent-supply vials (vials 1–9, V1–V9) at the upper part of the module (from left to right) and vial V10 for reformulation at the bottom right. The reagents or solvents in each vial are summarized in Table 1. For performing an additional solid-phase extraction before HPLC separation, two different ingredient cartridges (a C18 plus Sep-Pak and an Alumina N cartridge) were introduced in the TRACERlab™ FXFN module between valves v14 and v12. For the Alumina N cartridge and the cartridge without any ingredient, we used the default system of the TRACERlab™ FXFN module. For using a sample pretreatment method with C18 plus Sep-Pak cartridge, this module was modified by adding valve v27 and the waste vial, as shown by the dotted line box in Fig. 2, between valve v12 and the HPLC injector vial.
Radiofluorination
[18F]Fluoride was prepared by the 18O(p,n)18F reaction using H2 18O as the target material and delivered to the F-18 vial (left bottom, Fig. 2). It was trapped on the Chromafix PS-HCO3 cartridge (18F separation cartridge) and released by eluting with 1.0:0.1 ml of methanol (MeOH)/water containing K2.2.2/K2CO3 (11/0.46 mg, vial V1). The extracted solution in the carbon reaction vessel was dried by azeotropic distillation, without further addition of acetonitrile (CH3CN), at 90 °C under helium gas. After adding the mixture of the precursor (4-methylphenyl-mazenil iodonium tosylate: 4 mg and 2,2,6,6-tetramethylpiperidin-1-yl)oxy (TEMPO): 1 mg in 1 ml of N,N-dimethylformamide (DMF) from vial V3), the reaction mixture was stirred for 5 min at 150 °C. The crude product was cooled using compressed air.
Sample Pretreatment for HPLC
After radiofluorination, we evaluated three different conditions before HPLC purification. Method 1: water from vial V5 (1.5 ml) was added to the carbon reaction vessel to dilute the reaction mixture, transferred directly to the HPLC injection vial (middle upper), and the reaction vessel was rinsed with water from vial V6 (water, 1.5 ml). The combined solution in the HPLC injector vial was automatically injected into the HPLC system without the cartridge. Method 2: water from vial V5 (4.5 ml) was added to the reactor and transferred to the HPLC injector vial through the Alumina N cartridge with HPLC filter (0.45 μm) between valves v14 and v12. Method 3 (modified, see the dotted box in Fig. 2): water from vial V5 (10 ml) was added to the reactor and loaded to the C18 plus Sep-Pak cartridge between valves v14 and v12, and the cartridge was washed with water from vial V6 (10 ml). The reaction mixture was eluted using CH3CN from vial V2 (1.2 ml) into the HPLC injection vial, and water (3.3 ml) was added from vial V4. As a result, the sample solution for HPLC injection was formulated about 25 % CH3CN/water.
HPLC Purification and Reformulation
The HPLC condition for purification was as follows: 20 % CH3CN/water, 4 ml/min of flow rate, 254 nm of UV detector and γ-ray detector, and Xterra RP-18 column with a guard column. The collected [18F]FMZ (approximately 7–9 ml of the HPLC solvent) fraction was directly transferred to the replacement unit prefilled with water (50 ml) from vial V10 for the second solid-phase extraction. The diluted solution was passed through the C18 plus Sep-Pak cartridge to remove the organic solvent from the collected [18F]FMZ fraction. The [18F]FMZ trapped on the cartridge was rinsed with water (10 ml) from vial V7 and eluted with EtOH (1.2 ml) from vial V8, followed by saline (10 ml) from vial V9, into a sterile receiving vial prefilled with 10 ml of saline. Finally, the product was passed through a sterile filter (pore size, 0.22 μm; Supor AEF filter, PALL).
A 2.5-Year Production Study: Quality Control and Specific Activity
After optimizing the automatic production process, we started a regular production of [18F]FMZ. During regular production, fluorine-18 was received at approximately 22.1 ± 9.7 GBq/2.0 ml of O-18 water from the cyclotron, and [18F]FMZ was produced using the conditions described above. We measured the radiochemical yield from 94 batches of [18F]FMZ produced from December 2010 to May 2013. The radioactive peak and radiochemical purity were identified as [18F]FMZ by comparison with the authentic compound on the basis of retention time in the HPLC analysis (25 % CH3CN/water; flow rate 1 ml/min) and Rf in a radio-TLC scanner (10 % MeOH/dichloromethane), respectively. Specific activity of [18F]FMZ at the end of synthesis was calculated by relating radioactivity to the mass associated with the UV absorbance (254 nm). Visual inspection, sterility, presence of endotoxins, pH, terminal filter integrity, and measurement of residual solvents such as acetone, MeOH, CH3CN, and DMF using gas chromatography were determined by standard procedures routinely performed at the PET center, Seoul National University Bundang Hospital. The stability of the product over 6 h was assessed using analytical HPLC and a radio-TLC scanner, while maintaining the radioactivity of [18F]FMZ at approximately 370 MBq/ml.
Results and Discussion
The preparation method of [18F]FMZ is well established, and it is easily synthesized by one-step radiofluorination reaction from an iodonium salt precursor (Fig. 1). However, in the large-scale production of [18F]FMZ in the commercial synthesizer, we realized the importance of sample pretreatment prior to HPLC separation for achieving a reliable and reproducible [18F]FMZ synthesis using an iodonium salt precursor. Therefore, we modified the commercial synthesizer to introduce the C18 Sep-Pak purification system (Fig. 2, dotted box), because the default system of the commercial synthesizer could be used only with the Alumina Sep-Pak cartridge or without any ingredient cartridge. Subsequently, we investigated the large-scale production of [18F]FMZ under three different conditions described above (Methods 1–3). After performing radiofluorination in a carbon reaction vessel, the crude product was investigated with solid-phase filtration or without a cartridge under three conditions mentioned above before HPLC injection (Method 1–3), as summarized in Table 2.
In Method 1, although the total production time was shorter than that of other methods, [18F]FMZ showed variable retention times of 24 to 32 min during HPLC purification whenever each production; in many cases, the collected [18F]FMZ fraction also contained UV mass. In addition, the lifetime of the HPLC column was decreased to about 20 runs, despite the use of a guard column. The main reason for this may be the presence of insoluble residues from the iodonium salt precursor after heating. Therefore, we introduced the Alumina N cartridge to remove insoluble residues, as described in Method 2. Under this condition, the desired compound was collected at the same retention time without fluctuation in HPLC retention times. However, we observed a loss of [18F]FMZ (about 30 %) because of absorption in the Alumina N cartridge and HPLC filter. Finally, after modifying the commercial synthesizer, we replaced the Alumina N cartridge with the C18 plus Sep-Pak cartridge (Method 3). The production time was approximately 2 min longer than that of other methods. However, pure [18F]FMZ was obtained with high reproducibility without any loss of radioactivity after performing HPLC purification (Fig. 3). In addition, the lifetime of the HPLC column was significantly increased.
After determining optimal conditions, our center carried out routine production in the modified module with just conductive heating for a 2.5-year period (December 2010 to April 2013). The starting activity from the cyclotron ranged from 2.7 to 62.9 GBq (22.1 ± 9.7 GBq/2.0 ml of enriched O-18 water). Over 94 batches of [18F]FMZ production, the average radiochemical yield was 53.4 ± 9.0 % of decay corrected yield (including preparation failure, Fig. 4), and the total production time was approximately 61 ± 0.3 min. The amounts of residual radioactivity retained on the anion exchange resin (Chromafix® PS-HCO3), the reactor, and two C18 plus Sep-Pak cartridges were 2.2 ± 0.4, 1.9 ± 0.7, and 3.2 ± 0.9 %, respectively. In our study, one preparation failed and three preparations showed relatively low yields because of mechanical problems or other issues such as line leakage, failure to add a reagent, or moisturized TEMPO. The mean-specific activity was 450–750 GBq/μmol, and the radiochemical purity was 99.3 ± 0.3 %, as determined by HPLC and radio-TLC. The obtained [18F]FMZ showed sufficient radioactivity (7.9 ± 3.8 GBq/25 ml of 5 % EtOH/saline) for routine clinical use. The levels of the residual solvents, acetone, MeOH, CH3CN, and DMF were 18.2 ± 3.8, 4.8 ± 1.1, 22.5 ± 7.3, and 74.0 ± 12.6 ppm, respectively. The prepared [18F]FMZ satisfied all quality control test standards and was >98 % stable for up to 6 h after preparation (Table 3), and is now being used in multicenter clinical research studies in four different areas of the Republic of Korea.
Conclusion
We successfully established a convenient and reliable method for the synthesis of [18F]FMZ with a high radiochemical yield (53.4 ± 9.0 %, d.c., n = 94), purity (>99 %), and specific activity (572 ± 116 GBq/μmol) using a commercial synthesizer with a simple modification. During the 2.5-year automated [18F]flumazenil production, our system showed highly stable and reproducible radiochemical yield. This method could facilitate the routine clinical use of [18F]flumazenil for cBZR-related clinical studies.
References
Sieghart W (1995) Structure and pharmacology of gamma-aminobutyric acid A receptor subtypes. Pharmacol Rev 47:181–234
Pike VW, Halldin C, Crouzel C et al (1993) Radioligands for PET studies of central benzodiazepine receptors and PK (peripheral benzodiazepine) binding sites—current status. Nucl Med Biol 20:503–525
Ryvlin P, Bouvard S, Le Bars D et al (1998) Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. Brain 121:2067–2081
Savic I, Thorell JO, Roland P (1995) [11C]Flumazenil positron emission tomography visualizes frontal epileptogenic regions. Epilepsia 36:1225–1232
Savic I, Svanborg E, Thorell JO (1996) Cortical benzodiazepine receptor changes are related to frequency of partial seizures: a positron emission tomography study. Epilepsia 37:236–244
Malizia AL, Cunningham VJ, Bell CJ et al (1998) Decreased brain GABAA-benzodiazepine receptor binding in panic disorder. Arch Gen Psychiatry 55:715–720
Meyer M, Koeppe RA, Frey KA et al (1995) Positron emission tomography measures of benzodiazepine binding in Alzheimer’s disease. Arch Neurol 52:314–317
Holthoff VA, Koeppe RA, Frey KA et al (1993) Positron emission tomography measures of benzodiazepine receptors in Huntington’s disease. Ann Neurol 34:76–81
Benes FM, Vincent SL, Alsterberg G et al (1992) Increased GABAA receptor binding in superficial layers of cingulated cortex in schizophrenics. J Neurosci 12:924–929
Kiuchi Y, Kobayashi T, Takeuchi J et al (1989) Benzodiazepine receptors increase in post-mortem brain of chronic schizophrenics. Eur Arch Psychiatry Neurol Sci 239:71–78
Heiss WD, Grond M, Thiel A et al (1998) Permanent cortical damage detected by flumazenil positron emission tomography in acute stroke. Stroke 29:454–461
Heiss WD, Kracht L, Grond M et al (2000) Early [11C]flumazenil/H2O positron emission tomography predicts irreversible ischemic cortical damage in stroke patients receiving acute thrombolytic therapy. Stroke 31:366–369
Ryzhikov NN, Seneca N, Krasikova RN et al (2005) Preparation of highly specific radioactivity [18F]flumazenil and its evaluation in cynomolgus monkey by positron emission tomography. Nucl Med Biol 32:109–116
Odano I, Halldin C, Karlsson P et al (2009) [18F]Flumazenil binding to central benzodiazepine receptor studies by PET—quantitative analysis and comparisons with [11C]flumazenil. NeuroImage 45:891–902
Massaweh G, Schirrmacher E, La Fougere C et al (2009) Improved work-up procedure for the production of [18F]flumazenil and first results of its use with a high-resolution research tomography in human stroke. Nucl Med Biol 36:721–727
Mandap KS, Ido T, Kiyono Y et al (2009) Development of microwave-based automated nucleophilic [18F]fluorination system and its application to the production of [18F]flumazenil. Nucl Med Biol 36:403–409
Woodcraft J, Jones C, Gaeta A et al. (2011) Automated radiosynthesis. WO/2011/042529
Moon BS, Kil HS, Park JH et al. (2011) Facile aromatic radiofluorination of [18F]flumazenil from diaryliodonium salts with evaluation of their stability and selectivity. Org Biomol Chem 9:8346–8355
Lee BC, Kim JS, Kim BS et al (2011) Aromatic radiofluorination and biological evaluation of 2-aryl-6-[18F]fluorobenzothiazoles as a potential positron emission tomography imaging probe for β-amyloid plaques. Bioorg Med Chem 19:2980–2990
Lee BC, Dence CS, Zhou H et al (2009) Fluorine-18 labeling and biodistribution studies on peroxisome proliferator-activated receptor-gamma ligands: potential positron emission tomography imaging agents. Nucl Med Biol 36:147–153
Kwon HW, Kim YK, Shin SA et al (2012) Evaluation of cerebral glucose metabolism and GABAergic system in patients with essential tremor. J Nucl Med 53(suppl 1):2006
Kim YI, Kim YK, Yoon EJ et al (2011) Age-related changes in the availability of GABAA receptors in healthy subjects: imaging study with F-18 flumazenil PET. J Nucl Med 52(suppl 1):1209
Yoon HJ, Lee HY, Kang H et al (2012) Clinical utility of flumazenil PET versus FDG PET and MRI in intractable epilepsy patients: prospective study with a statistical parametric mapping method. J Nucl Med 53(suppl 1):38
Kim YK, Yang EJ, Cho K et al (2014) Functional recovery after ischemic stroke is associated with reduced GABAergic inhibition in the cerebral cortex: A GABA PET study. Neurorehabil Neural Repair. doi:10.1177/1545968313520411
Acknowledgments
This study was supported by grants (HI09C-1444-010013, 20090078370, HI12C-0035-030013, and 2012R1A1A2005887) from the government of South Korea. In addition, this study was supported by grant no. 11-2012-003 from the SNUBH research fund. We would like to give a special thank to Bio Imaging Korea Co., Ltd., which had provided the necessary iodonium tosylate precursors.
Conflict of Interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moon, B.S., Park, J.H., Lee, H.J. et al. Routine Production of [18F]Flumazenil from Iodonium Tosylate Using a Sample Pretreatment Method: a 2.5-Year Production Report. Mol Imaging Biol 16, 619–625 (2014). https://doi.org/10.1007/s11307-014-0738-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0738-z